Experimentally Induced Burns in Rats Treated with Innovative Polymeric Films Type Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
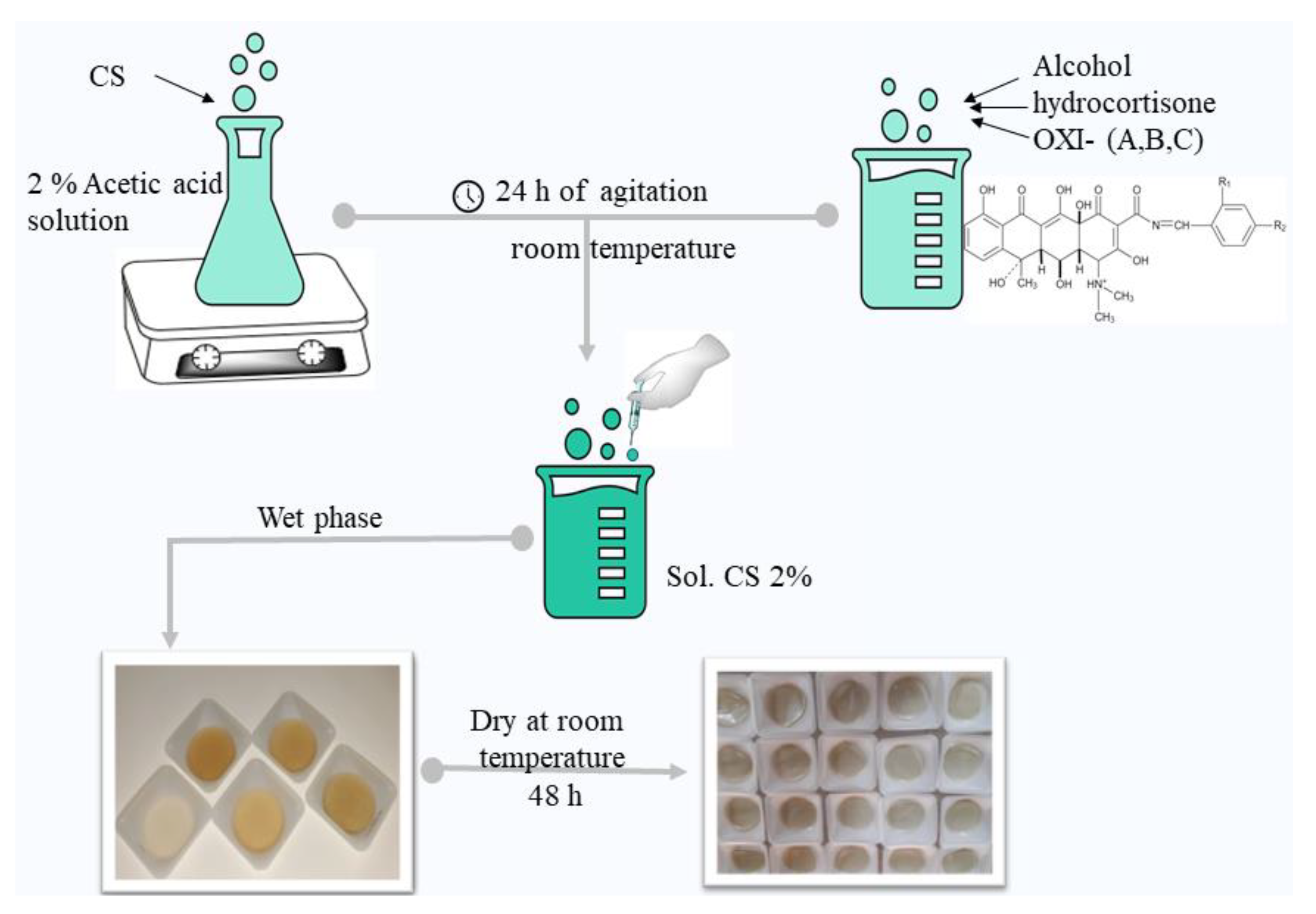
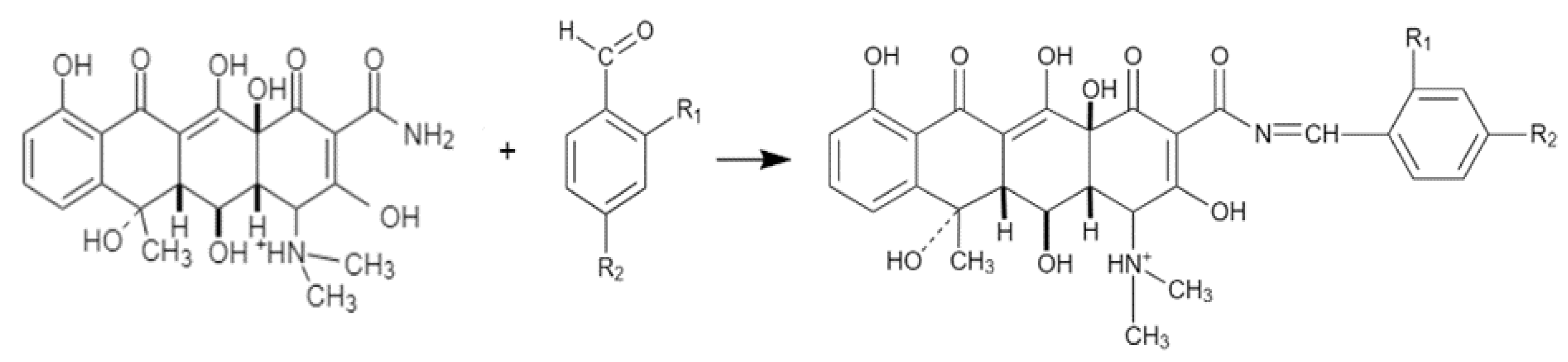
2.2. Description of the Chemical Synthesis Protocol
2.3. Physico-Chemical and Spectral Characterization
2.3.1. LC Parameters
2.3.2. MS Parameters
2.4. Obtaining of Chitosan-Oxytetracycline Membranes
2.5. Characterization of Chitosan-Oxytetracycline Membranes
2.5.1. In Vitro Swelling Ratio
2.5.2. In Vitro Biodegradation
2.6. Biological Evaluation
2.6.1. Assessment of Antimicrobial Activity
2.6.2. In Vivo Study
2.7. Statistical Analysis
3. Results
3.1. Chemistry
3.2. Characterization of Oxytetracycline Derivatives
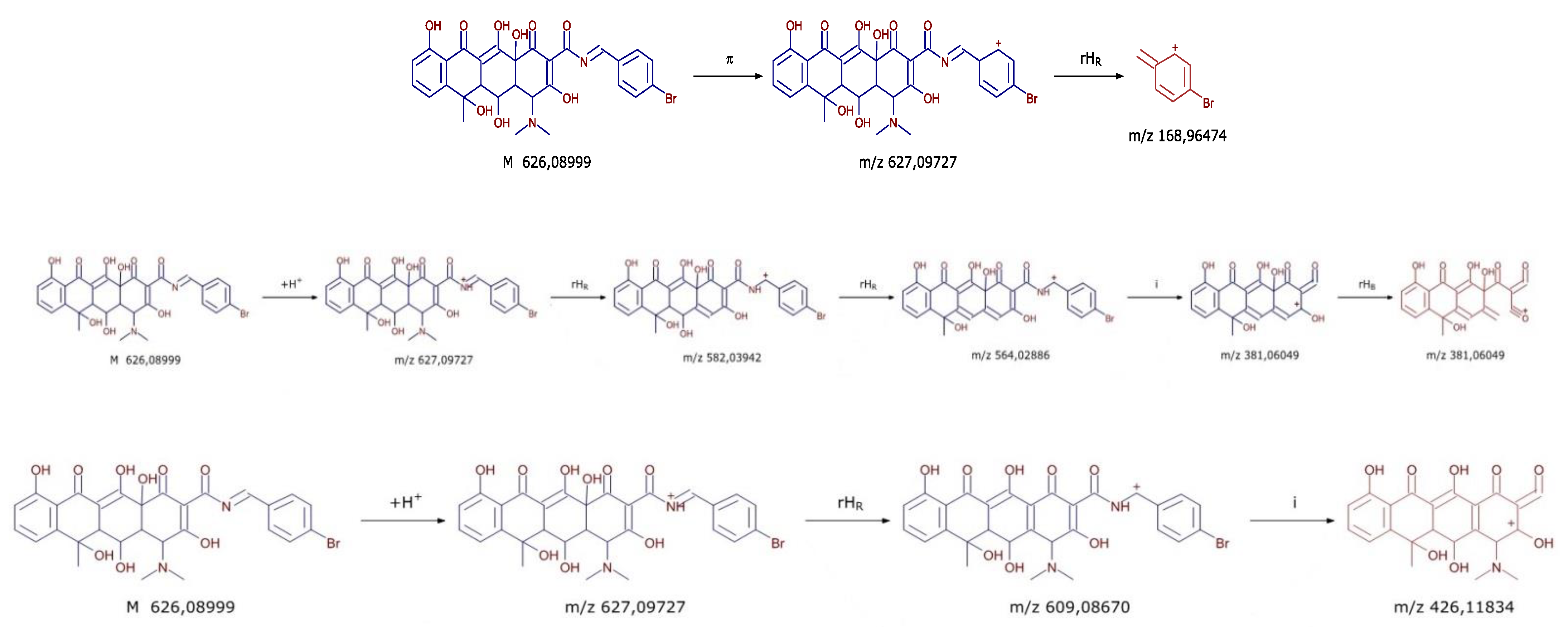
3.2.1. Spectral Data
3.2.2. Physico-Chemical Characterization
3.3. Characterization of Chitosan-Oxytetracycline Membranes
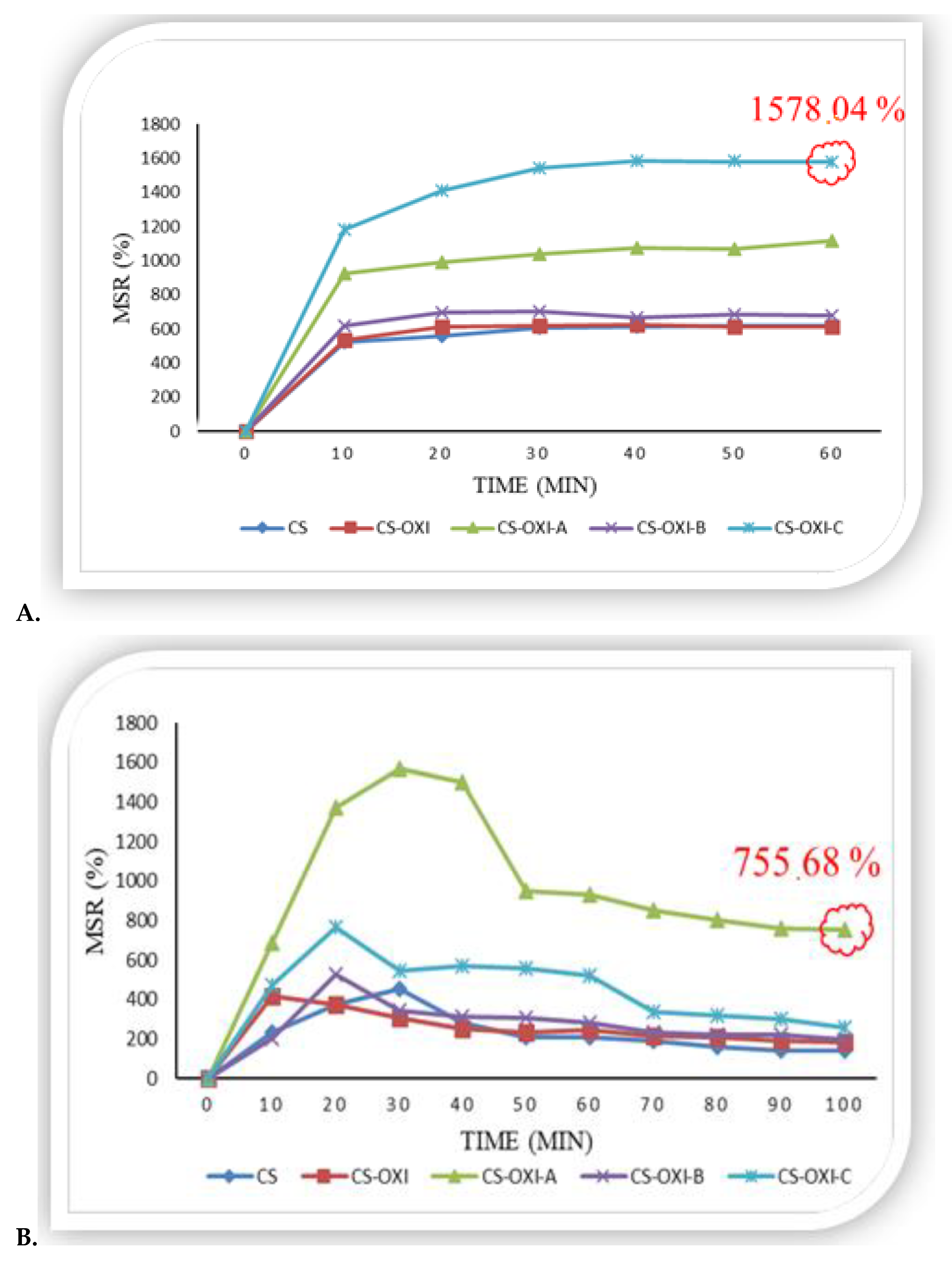
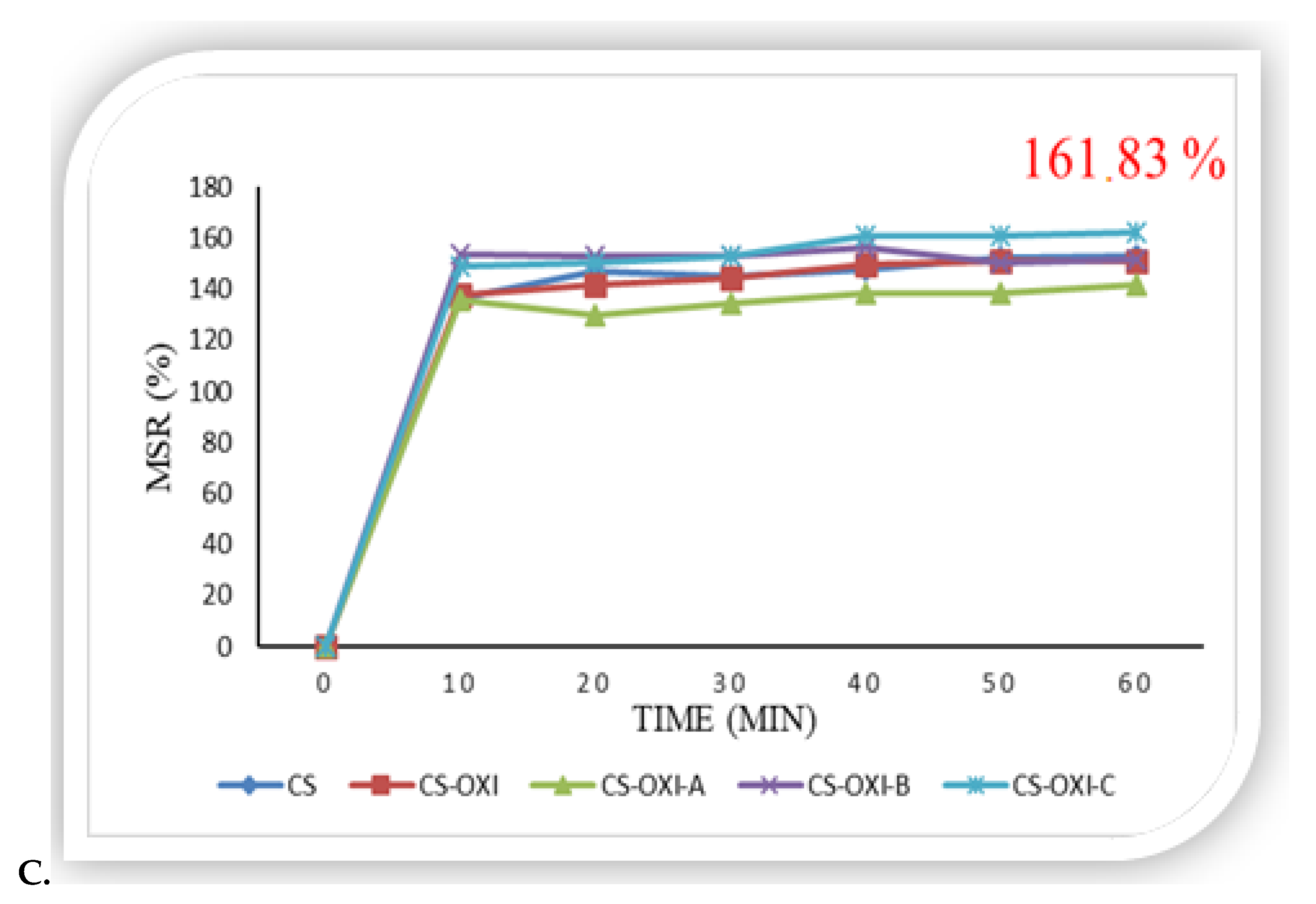
3.3.1. In Vitro Swelling Ratio
3.3.2. In Vitro Biodegradation
3.4. Biological Evaluation
3.4.1. Assessment of Antimicrobial Activity
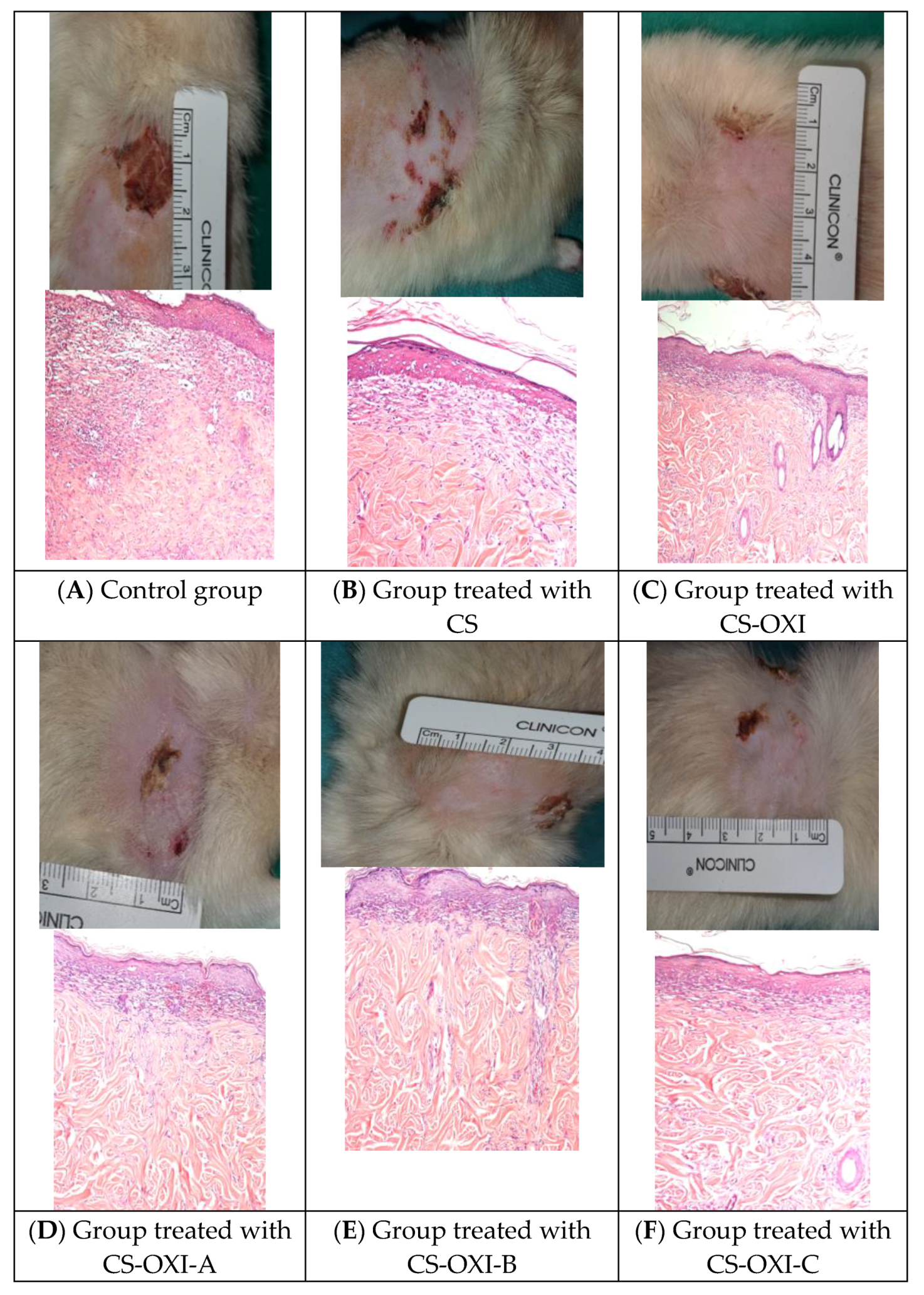
3.4.2. In Vivo Study: Wound Healing Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenhalgh, D.G. Management of burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.; Sharma, S.K.; Hashmi, J.T.; Kurup, D.B.; Hamblin, M.R. Topical antimicrobials for burn wound infections. Recent Pat. Antiinfect. Drug Discov. 2010, 5, 124–151. [Google Scholar] [CrossRef] [Green Version]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn wound healing: Clinical complications, medical care, treatment and dressing types: The current state of knowledge for clinical practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Campbell, K.; Levek, C.; Recicar, J.; Moulton, S. Antibiotic ointment versus a silver-base dressing for children with extremity burns: A randomized controlled study. J. Pediatr. Surg. 2019, 54, 1391–1396. [Google Scholar] [CrossRef]
- Holmes Iv, J.H.; Molnar, J.A.; Carter, J.E.; Hwang, J.; Cairns, B.A.; King, B.T.; Smith, D.J.; Cruse, C.W.; Foster, K.N.; Peck, M.D.; et al. A comparative study of ReCell device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J. Burn. Care Res. 2018, 39, 694–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remedio, L.N.; dos Santos, J.W.; Maciel, V.B.; Yoshida, C.M.; de Carvalho, R.A. Characterization of active chitosan films as a vehicle of potassium sorbate or nisin antimicrobial agents. Food Hydrocoll. 2019, 87, 830–838. [Google Scholar] [CrossRef]
- Fan, X.; Xue, R.; Li, N.; Yang, Q.; Yu, C.; Dong, Y.; Li, Y.; Tang, K.; Wana, G. A rapid hemostasis for incompressible wounds by microwave-assisting construction of cellulose sponge with chitosan. Mater. Des. 2022, 221, 111009. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieczonka, A.M.; Strzelczyk, A.; Sadowska, B.; Mlostoń, G.; Stączek, P. Synthesis and evaluation of antimicrobial activity of hydrazones derived from 3-oxido-1H-imidazole-4-carbohydrazides. Eur. J. Med. Chem. 2013, 64, 389–395. [Google Scholar] [CrossRef]
- Lisa, E.L.; Dragostin, O.M.; Petroaie, A.D.; Gurau, G.; Cristea, A.; Pavel, A.; Bonifate, F.; Popa, P.S.; Matei, M. The Effect of the New Imidazole Derivatives Complexation with Betacyclodextrin, on the Antifungal Activity in Oropharyngeal Infections. Processes 2022, 10, 2697. [Google Scholar] [CrossRef]
- Géhin, C.; Holman, S.W. Advances in high-resolution mass spectrometry applied to pharmaceuticals in 2020: A whole new age of information. Sci. Adv. 2021, 2, 142–156. [Google Scholar] [CrossRef]
- Floros, D.J.; Petras, D.; Kapono, C.A.; Melnik, A.V.; Ling, T.J.; Knight, R.; Dorrestein, P.C. Mass spectrometry based molecular 3D-cartography of plant metabolites. Plant Sci. 2017, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef] [Green Version]
- Hasmann, A.; Wehrschuetz-Sigl, E.; Kanzler, G.; Gewessler, U.; Hulla, E.; Schneider, K.P.; Binder, B.; Schintler, M.; Guebitz, G.M. Novel peptidoglycan-based diagnostic for detection of wound infection. Diagn. Microbiol. Infect. Dis. 2011, 71, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Baran, E.T.; Tuzlakoglu, K.; Mano, J.F.; Reis, R.L. Enzymatic degradationbehavior and cytocompatibility of silk fibroin–starch–chitosan conjugate membranes. Mat. Sci. Eng. 2012, 32, 1314–1322. [Google Scholar] [CrossRef] [Green Version]
- CLSI Supplement M100. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; Clinical and Laboratory Standard Institute: Wayne, PE, USA, 2019. [Google Scholar]
- Clinical and Laboratory Standard Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. In Approved Guideline, 2nd ed.; M44-A2; Clinical and Laboratory Standard Institute: Wayne, PE, USA, 2009. [Google Scholar]
- Zhou, S.; Wang, W.; Zhou, S.; Zhang, G.; He, J.; Li, Q. A Novel Model for Cutaneous Wound Healing and Scarring in Rat. Plast. Reconstr. Surg. 2019, 143, 468–477. [Google Scholar] [CrossRef]
- Domergue, S.; Jorgensen, C.; Noël, D. Advances in Research in Animal Models of Burn-Related Hypertrophic Scars. J. Burn. Care Res. 2015, 36, e259–e266. [Google Scholar] [CrossRef]
- Khalid, F.A.; Mehrose, M.Y.; Saleem, M.; Yousaf, M.A.; Mujahid, A.M.; Rehman, S.U.; Ahmad, S.; Tarar, M.N. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: Randomised control trial. Burns 2019, 1, 69–75. [Google Scholar] [CrossRef]
- Lee, H.J.; Jang, Y.J. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef] [Green Version]
- Tort, S.; Demiröz, F.T.; Cevher, Ş.C.; Sarıbaş, S.; Özoğul, C.; Acartürk, F. The effect of a new wound dressing on wound healing: Biochemical and histopathological evaluation. Burns 2020, 46, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Salihović, M.; Pazalja, M.; Halilović, S.Š.; Veljović, E.; Mahmutović-Dizdarević, I.; Roca, S.; Novaković, I.; Trifunović, S. Synthesis, characterization, antimicrobial activity and DFT study of some novel Schiff bases. J. Mol. Struct. 2021, 1241, 130670. [Google Scholar] [CrossRef]
- Kumar Giri, T.; Thakur, D.; Alexander, A.; Badwaik, H.; Krishna Tripathi, D. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: Present status and applications. Curr. Drug Deliv. 2012, 9, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Pulicharla, R.; Hegde, K.; Brar, S.K.; Surampalli, R.Y. Tetracyclines metal complexation: Significance and fate of mutual existence in the environment. Environ. Pollut. 2017, 221, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guerra, W.; Silva-Caldeira, P.P.; Terenzi, H.; Pereira-Maia, E.C. Impact of metal coordination on the antibiotic and non-antibiotic activities of tetracycline-based drugs. Coord. Chem. Rev. 2016, 327–328, 188–199. [Google Scholar] [CrossRef]
- Romano, J.E.; Barbarossa, A.; Pagliuca, G.; Villadóniga, G.B.; Gazzotti, T.; Mislei, B.; Zironi, E.; Mari, G. Pharmacokinetics of oxytetracycline long-acting on plasma and semen of beef bulls. Theriogenology 2022, 186, 21–26. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Senchani, G.; Banerjee, D. Newer tetracycline derivatives: Synthesis, anti-HIV, antimycobacterial activities and inhibition of HIV-1 integrase. Bioorg. Med. Chem. 2007, 17, 2372–2375. [Google Scholar] [CrossRef]
- Bugatti, V.; Sorrentino, A.; Gorrasi, G. Encapsulation of Lysozyme into halloysite nanotubes and dispersion in PLA: Structural and physical properties and controlled release analysis. Eur. Polym. J. 2017, 93, 495–506. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sorlie, M.; Vrum, K.M.; Eijsink, V.G. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [Green Version]
- Guarino, V.; Caputo, T.; Altobelli, R.; Ambrosio, L. Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mat. Sci. 2015, 2, 497–502. [Google Scholar] [CrossRef]
- Dragostin, I.; Dragostin, O.M.; Dragan, M.; Stan, C.D.; Zamfir, C.L. Drug Hypersensitivity Reduction Using Encapsulation Method with Chitosan- Cetirizine Derivatives. Rev. Chim. 2018, 69, 6830. [Google Scholar] [CrossRef]
- Ray, M.; Pal, K.; Anis, A.; Banthia, A.K. Development and Characterization of Chitosan-Based Polymeric Hydrogel Membranes. Des. Monomers Polym. 2010, 13, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Gethin, G. The significance of surface Ph in chronic wounds. Wounds 2007, 3, 52–56. [Google Scholar]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. Adv. Polym. Sci. 2011, 244, 19–44. [Google Scholar]
- Castro, K.C.; Nogueira Campos, M.G.; Innocentini Mei, L.H. Hyaluronic acid electrospinning: Challenges, applications in wound dressings and new perspectives. Int. J. Biol. Macromol. 2021, 173, 251–266. [Google Scholar] [CrossRef]
- Akkaya, N.E.; Ergun, C.; Saygun, A.; Yesilcubuk, N.; Akel-Sadoglu, N.; Kavakli, I.H.; Turkmen, H.S.; Catalgil-Giz, H. New biocompatible antibacterial wound dressing candidates; agar-locust bean gum and agar-salep films. Int. J. Biol. Macromol. 2020, 155, 430–438. [Google Scholar] [CrossRef]
- Ridgway, R.; Neary, J.; Turner, A.; Barrett, D.C.; Gillespie, A. Evaluation of Horn Bud Wound Healing Following Cautery Disbudding of Dairy Calves with and without the Use of Oxytetracycline Aerosol Spray. Front. Vet. Sci. 2022, 9, 745632. [Google Scholar] [CrossRef]





| Compound | Formula | Monitored Ion [MH]+ | RT (min) | MS2 Fragments (m/z) |
|---|---|---|---|---|
| OXI | C22H24N2O9 | 461.15546 | 6.12 | 443.1448; 426.1183; 381.0605; 201.0546 |
| OXI-A | C29H27N3O11 | 594.17183 | 8.65 | 426.1183; 201.0546; 381.0605; 151.0502 * |
| OXI-B | C29H28N2O9 | 549.18673 | 7.86 | 426.1183; 381.0604; 201.0546; 91.0542 |
| OXI-C | C29H27N2O9Br | 627.09724 | 9.62 | 426.1183; 381.0604; 201.0546; 168.9647 |
| OXI-D | C29H28N2O10 | 565.18165 | 10.7 | 426.1183; 381.0604; 201.0546; 107.0491 |
| Comp. | Chemical Formula | Theoretical Molecular Weight | M.P. (°C) | Ƞ (%) |
|---|---|---|---|---|
| OXI | C22H24N2O9 | 496.9 | 198 | - |
| OXI-A | C29H27N3O11 | 630.02 | 176 | 76.80 |
| OXI-B | C29H28N2O9 | 585.02 | 146 | 83.85 |
| OXI-C | C29H27N2O9Br | 663.92 | 127 | 69.23 |
| OXI-D | C29H28N2O11 | 601.02 | 115 | 70.33 |
| Tested Compound | Diameter of the Inhibition Area (mm) | |||
|---|---|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | C. albicans ATCC 90028 | |
| OXI | 33.16 ± 0.15 | 28.06 ± 0.15 | 18.16 ± 0.15 | 15.03 ± 0.15 |
| OXI-A | 25.2 ± 0.26 | 22.1 ± 0.1 | 12.03 ± 0.15 | 13.1 ± 0.1 |
| OXI-B | 33.1 ± 0.3 | 25.1 ± 0.1 | 20.11 ± 0.10 | 13.15 ± 0.15 |
| OXI-C | 31.2 ± 0.2 | 25.03 ± 0.15 | 17.06 ± 0.15 | 12.03 ± 0.15 |
| OXI-D | 31.23 ± 0.05 | 25.06 ± 0.05 | 19.1 ± 0.1 | 14.1 ± 0.2 |
| Tetracycline 30 µg/disc | 31.06 ± 0.15 | 25.16 ± 0.15 | NT | NT |
| Ciprofloxacin 5 µg/disc | NT | 34.1 ± 0.1 | 28.03 ± 0.15 | NT |
| Fluconazole 25 µg/disc | NT | NT | NT | 33.01 ± 0.2 |
| Voriconazole 1 µg/disc | NT | NT | NT | 30.15 ± 0.05 |
| DMSO | 0 | 0 | 0 | 0 |
| Tested Compounds | S. aureus ATCC 25923 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | |||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| OXI | 0.009 0.0095 ± 0.0002 | 0.036 0.0362 ± 0.0003 | 0.036 0.0361 ± 0.0006 | 0.14 0.141 ± 0.003 | 0.073 0.0731 ± 0.0004 | 0.58 0.58 ± 0.005 |
| OXI-A | 0.036 0.0363 ± 0.0003 | 0.073 0.0734 ± 0.0003 | 0.073 0.0732 ± 0.0003 | 0.58 0.58 ± 0.004 | 0.29 0.291 ± 0.004 | 1,17 1.173 ± 0.02 |
| OXI-B | 0.009 0.0094 ± 0.0002 | 0.036 0.0363 ± 0.0005 | 0.036 0.0361 ± 0.0004 | 0.073 0.0733 ± 0.0006 | 0.073 0.0734 ± 0.0003 | 0.58 0.58 ± 0.003 |
| OXI-C | 0.009 0.0091 ± 0.0002 | 0.036 0.0361 ± 0.0003 | 0.073 0.0732 ± 0.0004 | 0.29 0.291 ± 0.004 | 0.14 0.141 ± 0.003 | 0.58 0.58 ± 0.006 |
| OXI-D | 0.009 0.0093 ± 0.0002 | 0.036 0.0362 ± 0.0005 | 0.036 0.0361 ± 0.0005 | 0.14 0.142 ± 0.003 | 0.073 0.0733 ± 0.0006 | 0.58 0.581 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, O.-M.; Dragostin, O.-M.; Gardikiotis, I.; Chitescu, C.L.; Lisa, E.L.; Zamfir, A.-S.; Confederat, L.; Dragostin, I.; Dragan, M.; Stan, C.D.; et al. Experimentally Induced Burns in Rats Treated with Innovative Polymeric Films Type Therapies. Biomedicines 2023, 11, 852. https://doi.org/10.3390/biomedicines11030852
Grosu O-M, Dragostin O-M, Gardikiotis I, Chitescu CL, Lisa EL, Zamfir A-S, Confederat L, Dragostin I, Dragan M, Stan CD, et al. Experimentally Induced Burns in Rats Treated with Innovative Polymeric Films Type Therapies. Biomedicines. 2023; 11(3):852. https://doi.org/10.3390/biomedicines11030852
Chicago/Turabian StyleGrosu, Oxana-Madalina, Oana-Maria Dragostin, Ioannis Gardikiotis, Carmen Lidia Chitescu, Elena Lacramioara Lisa, Alexandra-Simona Zamfir, Luminita Confederat, Ionut Dragostin, Maria Dragan, Catalina Daniela Stan, and et al. 2023. "Experimentally Induced Burns in Rats Treated with Innovative Polymeric Films Type Therapies" Biomedicines 11, no. 3: 852. https://doi.org/10.3390/biomedicines11030852






