Bidirectional Relationship between Glycemic Control and COVID-19 and Perspectives of Islet Organoid Models of SARS-CoV-2 Infection
Abstract
1. Introduction
2. Method
3. Human Infection and Organ Injury
3.1. Virus Entry
3.2. Organ damage
3.3. SARS-CoV-2 Infection Leading to Islet Damage
4. The Bidirectional Relationship between SARS-CoV-2 Infection and Glucose Metabolism
4.1. Diabetic Patients Are Prone to Infection by SARS-CoV-2 and Have Increased COVID-19 Severity
4.2. SARS-CoV-2 Infection Predisposes COVID-19 Patients to Hyperglycemia
5. Remaining Questions
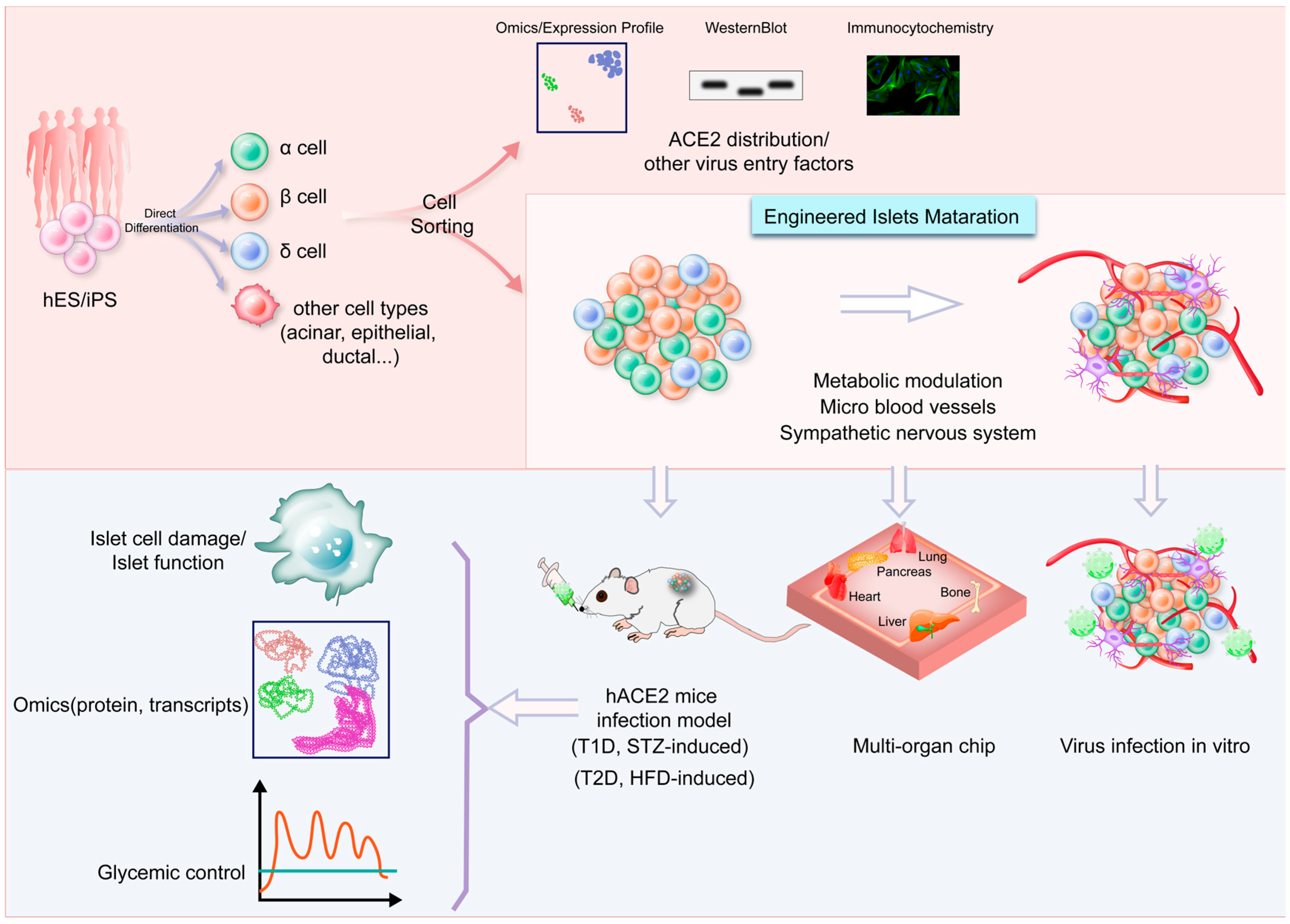
6. Strengths and Challenges of Using Islet Organoid Models
7. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.M.; Charytan, D.M.; Gasmi, B.; et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 2020, 24, 100434. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Kusmartseva, I.; Wu, W.; Syed, F.; Van Der Heide, V.; Jorgensen, M.; Joseph, P.; Tang, X.; Candelario-Jalil, E.; Yang, C.; Nick, H.; et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020, 32, 1041–1051. [Google Scholar] [CrossRef]
- Chen, L.; Hao, G. The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. Cardiovasc. Res. 2020, 116, 1932–1936. [Google Scholar] [CrossRef]
- Goldman, N.; Fink, D.; Cai, J.; Lee, Y.N.; Davies, Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res. Clin. Pract. 2020, 166, 108291. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Ahmad Mulyadi Lai, H.I.; Chou, S.J.; Chien, Y.; Tsai, P.H.; Chien, C.S.; Hsu, C.C.; Jheng, Y.C.; Wang, M.L.; Chiou, S.H.; Chou, Y.B.; et al. Expression of endogenous angiotensin-converting enzyme 2 in human induced pluripotent stem cell-derived retinal organoids. Int. J. Mol. Sci. 2021, 22, 1320. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Cell Metab. 2021, 33, 1565–1576. [Google Scholar] [CrossRef]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Smajlović, S.; Tombuloglu, H.; Ćordić, S.; Hajdarević, A.; Kudić, N.; Al Mutai, A.; Turkistani, S.A.; Al-Ahmed, S.H.; Al-Zaki, N.A.; et al. SARS-CoV-2 infection and multi-organ system damage: A review. Biomol. Biomed. 2023, 23, 37–52. [Google Scholar] [CrossRef]
- Steenblock, C.; Schwarz, P.E.H.; Ludwig, B.; Linkermann, A.; Zimmet, P.; Kulebyakin, K.; Tkachuk, V.A.; Markov, A.G.; Lehnert, H.; de Angelis, M.H.; et al. COVID-19 and metabolic disease: Mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021, 9, 786–798. [Google Scholar] [CrossRef]
- Mirani, M.; Favacchio, G.; Carrone, F.; Betella, N.; Biamonte, E.; Morenghi, E.; Mazziotti, G.; Lania, A.G. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: A case series from an academic hospital in Lombardy, Italy. Diabetes Care 2020, 43, 3042–3049. [Google Scholar] [CrossRef]
- Ceriello, A.; Schnell, O. COVID-19: Considerations of diabetes and cardiovascular disease management. J. Diabetes Sci. Technol. 2020, 14, 723–724. [Google Scholar] [CrossRef]
- Steenblock, C.; Hassanein, M.; Khan, E.G.; Yaman, M.; Kamel, M.; Barbir, M.; Lorke, D.E.; Rock, J.A.; Everett, D.; Bejtullah, S.; et al. Diabetes and COVID-19: Short- and long-term consequences. Horm. Metab. Res. 2022, 54, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Del Prato, S.; Mathieu, C.; Kahn, S.E.; Gabbay, R.A.; Buse, J.B. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care 2021, 44, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Fan, J.; Zhang, Y.; Wang, H.; Zhao, Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology 2020, 159, 367–370. [Google Scholar] [CrossRef]
- Unsworth, R.; Wallace, S.; Oliver, N.S.; Yeung, S.; Kshirsagar, A.; Naidu, H.; Kwong, R.M.W.; Kumar, P.; Logan, K.M. New-onset type 1 diabetes in children during COVID-19: Multicenter regional findings in the U.K. Diabetes Care 2020, 43, e170–e171. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Zuo, X.; Zhang, H.; Deng, A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020, 22, 1935–1941. [Google Scholar] [CrossRef]
- Boddu, S.K.; Aurangabadkar, G.; Kuchay, M.S. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab. Syndr. 2020, 14, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- El Jamal, S.M.; Pujadas, E.; Ramos, I.; Bryce, C.; Grimes, Z.M.; Amanat, F.; Tsankova, N.M.; Mussa, Z.; Olson, S.; Salem, F.; et al. Tissue-based SARS-CoV-2 detection in fatal COVID-19 infections: Sustained direct viral-induced damage is not necessary to drive disease progression. Hum. Pathol. 2021, 114, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Amendola, A.; Garoffolo, G.; Songia, P.; Nardacci, R.; Ferrari, S.; Bernava, G.; Canzano, P.; Myasoedova, V.; Colavita, F.; Castilletti, C.; et al. Human cardiosphere-derived stromal cells exposed to SARS-CoV-2 evolve into hyper-inflammatory/pro-fibrotic phenotype and produce infective viral particles depending on the levels of ACE2 receptor expression. Cardiovasc. Res. 2021, 117, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zaheer, S.; Kumar, N.; Singla, T.; Ranga, S. Covid19, beyond just the lungs: A review of multisystemic involvement by Covid19. Pathol. Res. Pract. 2021, 224, 153384. [Google Scholar] [CrossRef] [PubMed]
- Christ-Crain, M.; Hoorn, E.J.; Sherlock, M.; Thompson, C.J.; Wass, J. Endocrinology in the time of COVID-19-2021 updates: The management of diabetes insipidus and hyponatraemia. Eur. J. Endocrinol. 2021, 185, G35–G42. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.E.; Siddle, K.J.; Shaw, B.M.; Loreth, C.; Schaffner, S.F.; Gladden-Young, A.; Adams, G.; Fink, T.; Tomkins-Tinch, C.H.; Krasilnikova, L.A.; et al. Phylogenetic analysis of SARS-CoV-2 in Boston highlights the impact of superspreading events. Science 2021, 371, eabe3261. [Google Scholar] [CrossRef]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Dotan, A.; David, P.; Arnheim, D.; Shoenfeld, Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun. Rev. 2022, 21, 103071. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jain, A. COVID-19 and lung pathology. Indian J. Pathol. Microbiol. 2020, 63, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Copin, M.C.; Parmentier, E.; Duburcq, T.; Poissy, J.; Mathieu, D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020, 46, 1124–1126. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Tokuyama, M.; Wei, G.; Huang, R.; Livanos, A.; Jha, D.; Levescot, A.; Irizar, H.; Kosoy, R.; Cording, S.; et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2–related disease. Gastroenterology 2021, 160, 287–301. [Google Scholar] [CrossRef]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W.; Lu, Z.; Ke, A.; Zhou, J.; Shi, G.; Fang, N.; Fan, J.; et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ye, M.; Wysocki, J.; William, J.; Soler, M.J.; Cokic, I.; Batlle, D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: Implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 2006, 17, 3067–3075. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Zhang, X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens. Res. 2020, 43, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psych. 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Mine, K.; Nagafuchi, S.; Mori, H.; Takahashi, H.; Anzai, K. SARS-CoV-2 infection and pancreatic β cell failure. Biology 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Geravandi, S.; Mahmoudi-Aznaveh, A.; Azizi, Z.; Maedler, K.; Ardestani, A. SARS-CoV-2 and pancreas: A potential pathological interaction? Trends. Endocrinol. Metab. 2021, 32, 842–845. [Google Scholar] [CrossRef]
- Coate, K.C.; Cha, J.; Shrestha, S.; Wang, W.; Gonçalves, L.M.; Almaça, J.; Kapp, M.E.; Fasolino, M.; Morgan, A.; Dai, C.; et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 2020, 32, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, V.; Jangra, S.; Cohen, P.; Rathnasinghe, R.; Aslam, S.; Aydillo, T.; Geanon, D.; Handler, D.; Kelley, G.; Lee, B.; et al. Limited extent and consequences of pancreatic SARS-CoV-2 infection. Cell Rep. 2022, 38, 110508. [Google Scholar] [CrossRef]
- Müller, J.A.; Groß, R.; Conzelmann, C.; Krüger, J.; Merle, U.; Steinhart, J.; Weil, T.; Koepke, L.; Bozzo, C.P.; Read, C.; et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021, 3, 149–165. [Google Scholar] [CrossRef]
- Hollstein, T.; Schulte, D.M.; Schulz, J.; Glück, A.; Ziegler, A.G.; Bonifacio, E.; Wendorff, M.; Franke, A.; Schreiber, S.; Bornstein, S.R.; et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: A case report. Nat. Metab. 2020, 2, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Memon, B.; Abdelalim, E.M. ACE2 function in the pancreatic islet: Implications for relationship between SARS-CoV-2 and diabetes. Acta Physiol. 2021, 233, e13733. [Google Scholar] [CrossRef]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Li, B.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R.; et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021, 33, 1577–1591. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Onodera, T.; Fukuda, S.; Kita, S.; Fukuhara, A.; Shimomura, I. SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism 2022, 133, 155236. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Donath, M.Y.; Dinarello, C.A.; Mandrup-Poulsen, T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nature Reviews Immunology 2019, 19, 734–746. [Google Scholar] [CrossRef]
- Nassar, M.; Daoud, A.; Nso, N.; Medina, L.; Ghernautan, V.; Bhangoo, H.; Nyein, A.; Mohamed, M.; Alqassieh, A.; Soliman, K.; et al. Diabetes mellitus and COVID-19: Review article. Diabetes Metab. Syndr. 2021, 15, 102268. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Hosking, F.J.; Cook, D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018, 41, 2127–2135. [Google Scholar] [CrossRef]
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of infection in type 1 and type 2 diabetes compared with the general population: A matched cohort study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. COVID-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Dennis, J.M.; Mateen, B.A.; Sonabend, R.; Thomas, N.J.; Patel, K.A.; Hattersley, A.T.; Denaxas, S.; McGovern, A.P.; Vollmer, S.J. Type 2 diabetes and COVID-19–related mortality in the critical care setting: A national cohort study in England, March–July 2020. Diabetes Care 2021, 44, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat Rev Endocrinol 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Cai, Y.; Shi, S.; Yang, F.; Yi, B.; Chen, X.; Li, J.; Wen, Z. Fasting blood glucose level is a predictor of mortality in patients with COVID-19 independent of diabetes history. Diabetes Res. Clin. Pract. 2020, 169, 108437. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Morieri, M.L.; Boscari, F.; Fioretto, P.; Maran, A.; Busetto, L.; Bonora, B.M.; Selmin, E.; Arcidiacono, G.; Pinelli, S.; et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res. Clin. Pract. 2020, 168, 108374. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Messler, J.C.; Umpierrez, G.E.; Peng, L.; Booth, R.; Crowe, J.; Garrett, V.; McFarland, R.; Pasquel, F.J. Association between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19: A multicenter, retrospective hospital-based analysis. Diabetes Care 2021, 44, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Su, Y. Remdesivir attenuates high fat diet (HFD)-induced NAFLD by regulating hepatocyte dyslipidemia and inflammation via the suppression of STING. Biochem. Biophys Res Commun 2020, 526, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Wasko, M.C.; McClure, C.K.; Kelsey, S.F.; Huber, K.; Orchard, T.; Toledo, F.G. Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: A randomised trial. Diabetologia 2015, 58, 2336–2343. [Google Scholar] [CrossRef]
- Denina, M.; Trada, M.; Tinti, D.; Funiciello, E.; Novara, C.; Moretto, M.; Rosati, S.; Garazzino, S.; Bondone, C.; De Sanctis, L. Increase in newly diagnosed type 1 diabetes and serological evidence of recent SARS-CoV-2 infection: Is there a connection? Front. Med. 2022, 9, 927099. [Google Scholar] [CrossRef]
- Jedrzejak, A.P.; Urbaniak, E.K.; Wasko, J.A.; Ziojla, N.; Borowiak, M. Diabetes and SARS-CoV-2-is there a mutual connection? Front. Cell Dev. Biol. 2022, 10, 913305. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Rhee, E.J.; Jung, J.H.; Han, K.D.; Kim, S.R.; Lee, W.Y.; Yoon, K.H. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: A nationwide cohort study. Diabetes Metab. J. 2020, 44, 737–746. [Google Scholar] [CrossRef]
- Altonen, B.L.; Arreglado, T.M.; Leroux, O.; Murray-Ramcharan, M.; Engdahl, R. Characteristics, comorbidities and survival analysis of young adults hospitalized with COVID-19 in New York City. PLoS ONE 2020, 15, e0243343. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Castilla-Bancayán, J.P. Diabetes and mortality among 1.6 million adult patients screened for SARS-CoV-2 in Mexico. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020, 31, 1068–1077. [Google Scholar] [CrossRef]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Wargny, M.; Gourdy, P.; Ludwig, L.; Seret-Bégué, D.; Bourron, O.; Darmon, P.; Amadou, C.; Pichelin, M.; Potier, L.; Thivolet, C.; et al. Type 1 diabetes in people hospitalized for COVID-19: New insights from the Coronado study. Diabetes Care 2020, 43, e174–e177. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct. Target 2021, 6, 427. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Marchand, L.; Pecquet, M.; Luyton, C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020, 57, 1265–1266. [Google Scholar] [CrossRef] [PubMed]
- Zippi, M.; Hong, W.; Traversa, G.; Maccioni, F.; De Biase, D.; Gallo, C.; Fiorino, S. Involvement of the exocrine pancreas during COVID-19 infection and possible pathogenetic hypothesis: A concise review. Infez. Med. 2020, 28, 507–515. [Google Scholar] [PubMed]
- Cure, E.; Cumhur Cure, M. COVID-19 may affect the endocrine pancreas by activating Na(+)/H(+) exchanger 2 and increasing lactate levels. J. Endocrinol. Investig. 2020, 43, 1167–1168. [Google Scholar] [CrossRef]
- Abramczyk, U.; Nowaczyński, M.; Słomczyński, A.; Wojnicz, P.; Zatyka, P.; Kuzan, A. Consequences of COVID-19 for the pancreas. Int. J. Mol. Sci. 2022, 23, 864. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; Longo, M.; Esposito, K.; Maiorino, M.I. Type 1 diabetes triggered by COVID-19 pandemic: A potential outbreak? Diabetes Res. Clin. Pract. 2020, 164, 108219. [Google Scholar] [CrossRef]
- Ruiz, P.L.D.; Tapia, G.; Bakken, I.J.; Håberg, S.E.; Hungnes, O.; Gulseth, H.L.; Stene, L.C. Pandemic influenza and subsequent risk of type 1 diabetes: A nationwide cohort study. Diabetologia 2018, 61, 1996–2004. [Google Scholar] [CrossRef]
- Tao, T.; Deng, P.; Wang, Y.; Zhang, X.; Guo, Y.; Chen, W.; Qin, J. Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet axis in normal and type 2 diabetes. Adv. Sci. 2022, 9, 2103495. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Song, E.; Xu, T. Islet organoid as a promising model for diabetes. Protein Cell 2022, 13, 239–257. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Lacko, L.A.; Chen, S. Human organoid models to study SARS-CoV-2 infection. Nat. Methods 2022, 19, 418–428. [Google Scholar] [CrossRef]
- Imai, Y.; Soleimanpour, S.A.; Tessem, J.S. Editorial: Study of pancreatic islets based on human models to understand pathogenesis of diabetes. Front. Endocrinol. 2022, 13, 1128653. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, F.; Toivonen, S.; Schiavo, A.A.; Chae, H.; Tariq, M.; Sawatani, T.; Pachera, N.; Cai, Y.; Vinci, C.; Virgilio, E.; et al. In depth functional characterization of human induced pluripotent stem cell-derived beta cells in vitro and in vivo. Front. Cell Dev. Biol. 2022, 10, 967765. [Google Scholar] [CrossRef] [PubMed]
- Lorberbaum, D.S.; Sarbaugh, D.; Sussel, L. Leveraging the strengths of mice, human stem cells, and organoids to model pancreas development and diabetes. Front. Endocrinol. 2022, 13, 1042611. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.L.; Deng, K.; Tran, H.A.; Singh, R.; Rnjak-Kovacina, J.; Thorn, P. Glucose-dependent insulin secretion from β Cell spheroids is enhanced by embedding into softer alginate hydrogels functionalised with RGD peptide. Bioengineering 2022, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffre, F.; et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem. Cell 2020, 27, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ni, C.; Gao, R.; Wang, Y.; Yang, L.; Wei, J.; Lv, T.; Liang, J.; Zhang, Q.; Xu, W.; et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 2020, 11, 771–775. [Google Scholar] [CrossRef]
- Han, Y.; Duan, X.; Yang, L.; Nilsson-Payant, B.E.; Wang, P.; Duan, F.; Tang, X.; Yaron, T.M.; Zhang, T.; Uhl, S.; et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 2021, 589, 270–275. [Google Scholar] [CrossRef]
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.S.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem. Cell 2022, 29, 217–231. [Google Scholar] [CrossRef]
- Wagner, L.E.; Melnyk, O.; Duffett, B.E.; Linnemann, A.K. Mouse models and human islet transplantation sites for intravital imaging. Front. Endocrinol. 2022, 13, 992540. [Google Scholar] [CrossRef] [PubMed]
- Hogrebe, N.J.; Maxwell, K.G.; Augsornworawat, P.; Millman, J.R. Generation of insulin-producing pancreatic β cells from multiple human stem cell lines. Nat. Protoc. 2021, 16, 4109–4143. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic β cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef]
- Shirasawa, S.; Yoshie, S.; Yokoyama, T.; Tomotsune, D.; Yue, F.; Sasaki, K. A novel stepwise differentiation of functional pancreatic exocrine cells from embryonic stem cells. Stem. Cells Dev. 2011, 20, 1071–1078. [Google Scholar] [CrossRef]
- Veres, A.; Faust, A.L.; Bushnell, H.L.; Engquist, E.N.; Kenty, J.H.; Harb, G.; Poh, Y.C.; Sintov, E.; Gürtler, M.; Pagliuca, F.W.; et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 2019, 569, 368–373. [Google Scholar] [CrossRef]
- Kropp, P.A.; Zhu, X.; Gannon, M. Regulation of the pancreatic exocrine differentiation program and morphogenesis by onecut 1/Hnf6. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 841–856. [Google Scholar] [CrossRef]
- Pierreux, C.E.; Poll, A.V.; Kemp, C.R.; Clotman, F.; Maestro, M.A.; Cordi, S.; Ferrer, J.; Leyns, L.; Rousseau, G.G.; Lemaigre, F.P. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology 2006, 130, 532–541. [Google Scholar] [CrossRef]
- Zhang, H.; Ables, E.T.; Pope, C.F.; Washington, M.K.; Hipkens, S.; Means, A.L.; Path, G.; Seufert, J.; Costa, R.H.; Leiter, A.B.; et al. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech. Dev. 2009, 126, 958–973. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Honarpisheh, M.; Kemter, E.; Wolf, E.; Seissler, J. Butyrate and class I histone deacetylase inhibitors promote differentiation of neonatal porcine islet cells into beta cells. Cells 2021, 10, 3249. [Google Scholar] [CrossRef] [PubMed]
- Haumaitre, C.; Lenoir, O.; Scharfmann, R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol. Cell Biol. 2008, 28, 6373–6383. [Google Scholar] [CrossRef]
- Ameri, J.; Borup, R.; Prawiro, C.; Ramond, C.; Schachter, K.A.; Scharfmann, R.; Semb, H. Efficient generation of glucose-responsive beta cells from isolated GP2(+) human pancreatic progenitors. Cell Rep. 2017, 19, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Augstein, P.; Naselli, G.; Loudovaris, T.; Hawthorne, W.J.; Campbell, P.; Bandala-Sanchez, E.; Rogers, K.; Heinke, P.; Thomas, H.E.; Kay, T.W.; et al. Localization of dipeptidyl peptidase-4 (CD26) to human pancreatic ducts and islet alpha cells. Diabetes Res. Clin. Pract. 2015, 110, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Chen, Q.; Gu, H.J.; Yang, G.; Wang, Y.X.; Huang, X.Y.; Liu, S.S.; Zhang, N.N.; Li, X.F.; Xiong, R.; et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 2020, 28, 124–133. [Google Scholar] [CrossRef]
- Israelow, B.; Song, E.; Mao, T.; Lu, P.; Meir, A.; Liu, F.; Alfajaro, M.M.; Wei, J.; Dong, H.; Homer, R.J.; et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J. Exp. Med. 2020, 217, 2547–2562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, F.; Lv, X.; Wang, S.; Liao, X.; Yu, Y.; Dai, Q.; Zhang, Y.; Meng, J.; Hu, G.; et al. Mefunidone ameliorates diabetic kidney disease in STZ and db/db mice. FASEB J. 2021, 35, e21198. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Song, L.; Strange, C.; Dong, X.; Wang, H. Therapeutic effects of adipose stem cells from diabetic mice for the treatment of type 2 diabetes. Mol. Ther. 2018, 26, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Teles, D.; Yeager, K.; Tavakol, D.N.; Zhao, Y.; Chramiec, A.; Tagore, S.; Summers, M.; Stylianos, S.; Tamargo, M.; et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 2022, 6, 351–371. [Google Scholar] [CrossRef] [PubMed]
| Region | Source of Data | Main Findings | Reference | |
|---|---|---|---|---|
| STUDY 1 | England | 13,809 patients admitted for COVID-19 to the HDU/5447 admitted to the ICU Mean age: 70/58 | 34.7% mortality with T2D; 25.5% mortality without T2D | [60] |
| STUDY 2 | England | 23,698 COVID-19-related deaths (with and without diabetes) | 31.4% mortality with T2D; 1.5% mortality with T1D | [18] |
| STUDY 3 | Korea | 5307 people with COVID-19 | Increased severity and higher mortality in 14.5% individuals with T2D | [74] |
| STUDY 4 | USA | 395 patients with COVID-19 Age: 18–35 | 3.8% mortality without comorbidity; 13.6% mortality with diabetes (deceased); 18.5% mortality with diabetes (diagnosed) | [75] |
| STUDY 5 | Mexico | 757,210 patients with COVID-19 | Patients with diabetes had a 49% risk of death higher than those without diabetes; Diagnosis of T2D as COVID-19 outcome in both young and old | [76] |
| STUDY 6 | USA | 1544 patients with COVID-19 | Hyperglycemia and hypoglycemia both contribute to poor outcomes of COVID-19 | [64] |
| STUDY 7 | China | 7337 with COVID-19 Ages: 18–75 | 13% of patients with T2D; Death rate is 1.49-fold higher in the T2D cohort | [77] |
| STUDY 8 | China | 1099 patients 639 male/460 female Mean age: 47 | Individuals with diabetes are more susceptible to SARS-CoV-2 and more easily develop a severe course of COVID-19 | [53] |
| STUDY 9 | England | Population-based cohort study | COVID-19-related mortality increases in people with a higher glycosylated hemoglobin level | [78] |
| STUDY 10 | France | 2,608 patients with COVID-19 Age: 56.0 (±16.4) | Patients with T1D (age > 65–75) had higher rates of COVID-19-related mortality | [79] |
| STUDY 11 | China | 92 patients with COVID-19 without metabolic-related diseases | New-onset insulin resistance, hyperglycemia, and decreased HDL-C in these patients | [80] |
| STUDY 12 | Italy | 551 patients with COVID-19 344 male/207 female Age: 61 ± 0.7 | 46% overt hyperglycemia; 12% new-onset diabetes; glycemic abnormalities last for 2 months after recovery | [81] |
| STUDY 13 | UK | 30 children with new-onset T1D Age: 23 months–16 years | The number of children with new-onset T1D increased since the COVID-19 pandemic. Some of these patients had been infected/exposed to SARS-CoV-2 | [20] |
| STUDY 14 | France | 1 woman with COVID-19 and a history of gastric bypass Age 29 | The COVID-19 patient was diagnosed with new-onset diabetes after 1.5 months | [82] |
| STUDY 15 | Italy | 413 patients with COVID-19 | 21 of 413 (5.1%) had new-onset diabetes; Patients with new-onset diabetes reported higher severity and mortality than those with pre-existing diabetes | [63] |
| Remaining Questions | Proposed Solutions/Progress * | |
|---|---|---|
| 1 | The distribution and abundance of ACE2 in endocrine and non-endocrine cells | Generating missing pancreatic cell types, biochemical analyses |
| 2 | The dynamics of ACE2 during the development of the pancreas | Biochemical analyses |
| 3 | The mechanisms of how SARS-CoV-2 infection influences the pancreatic endocrine cells’ function | Transplantation of islet organoids Activation of the Na+/H+ exchanger * Inflammatory cytokines * |
| 4 | Whether infection results in T1D or T2D | More cohort studies and experimental research based on disease models |
| 5 | The correlation between impaired glycemic control and the virulence of SARS-CoV-2 variants | Infection model of islet organoids |
| 6 | Multi-organ interactions after infection? | Multi-organoid systems, co-culture systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, N.; Zhu, L.; Chen, L.; Liu, H. Bidirectional Relationship between Glycemic Control and COVID-19 and Perspectives of Islet Organoid Models of SARS-CoV-2 Infection. Biomedicines 2023, 11, 856. https://doi.org/10.3390/biomedicines11030856
Zhang T, Wang N, Zhu L, Chen L, Liu H. Bidirectional Relationship between Glycemic Control and COVID-19 and Perspectives of Islet Organoid Models of SARS-CoV-2 Infection. Biomedicines. 2023; 11(3):856. https://doi.org/10.3390/biomedicines11030856
Chicago/Turabian StyleZhang, Tongran, Nannan Wang, Lingqiang Zhu, Lihua Chen, and Huisheng Liu. 2023. "Bidirectional Relationship between Glycemic Control and COVID-19 and Perspectives of Islet Organoid Models of SARS-CoV-2 Infection" Biomedicines 11, no. 3: 856. https://doi.org/10.3390/biomedicines11030856
APA StyleZhang, T., Wang, N., Zhu, L., Chen, L., & Liu, H. (2023). Bidirectional Relationship between Glycemic Control and COVID-19 and Perspectives of Islet Organoid Models of SARS-CoV-2 Infection. Biomedicines, 11(3), 856. https://doi.org/10.3390/biomedicines11030856







