Natriuretic Peptide Levels and Stages of Left Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Results
2.1. Clinical and Echocardiographic Characteristics
2.2. Clinical Characteristics, Cardiac Structure and Function in HFpEF Patients with Low and High NT-proBNP Levels
2.3. Association between Myocardial Tissue Characteristics and NT-proBNP Levels
3. Discussion
3.1. Relatively Low NT-proBNP Plasma Levels in “Comorbidity-Driven” HFpEF Patients
3.2. NP Levels Mirror Stage of Myocardial Disease Progression in HFpEF
3.3. NP Levels as Selection Criteria for HFpEF Trials
4. Methods
4.1. Study Population
4.2. Echocardiography
4.3. Cardiac Magnetic Resonance
4.4. Statistical Analysis
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinberg, B.A.; Zhao, X.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Cannon, C.P.; Hernandez, A.F.; Fonarow, G.C. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012, 126, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Senni, M.; Paulus, W.J.; Gavazzi, A.; Fraser, A.G.; Díez, J.; Solomon, S.D.; Smiseth, O.A.; Guazzi, M.; Lam, C.S.P.; Maggioni, A.P.; et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur. Heart J. 2014, 35, 2797–2815. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef]
- Packer, M.; Lam, C.S.P.; Lund, L.H.; Maurer, M.S.; Borlaug, B.A. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: A hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur. J. Heart Fail. 2020, 22, 1551–1567. [Google Scholar] [CrossRef]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef]
- van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.; Laarman, G.J.; Somsen, A.; et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Anjan, V.Y.; Loftus, T.M.; Burke, M.A.; Akhter, N.; Fonarow, G.C.; Gheorghiade, M.; Shah, S.J. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am. J. Cardiol. 2012, 110, 870–876. [Google Scholar] [CrossRef]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef]
- Madamanchi, C.; Alhosaini, H.; Sumida, A.; Runge, M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: Mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 2014, 176, 611–617. [Google Scholar] [CrossRef]
- Iwanaga, Y.; Nishi, I.; Furuichi, S.; Noguchi, T.; Sase, K.; Kihara, Y.; Goto, Y.; Nonogi, H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: Comparison between systolic and diastolic heart failure. J. Am. Coll. Cardiol. 2006, 47, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.R.; Kararigas, G. Menopause-related estrogen decrease and the pathogenesis of HFpEF: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 1074–1082. [Google Scholar] [CrossRef]
- Anand, I.S.; Rector, T.S.; Cleland, J.G.; Kuskowski, M.; McKelvie, R.S.; Persson, H.; McMurray, J.J.; Zile, M.R.; Komajda, M.; Massie, B.M.; et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: Findings from the I-PRESERVE trial. Circ. Heart Fail. 2011, 4, 569–577. [Google Scholar] [CrossRef]
- Anand, I.S.; Claggett, B.; Liu, J.; Shah, A.M.; Rector, T.S.; Shah, S.J.; Desai, A.S.; O’Meara, E.; Fleg, J.L.; Pfeffer, M.A.; et al. Interaction Between Spironolactone and Natriuretic Peptides in Patients With Heart Failure and Preserved Ejection Fraction: From the TOPCAT Trial. JACC Heart Fail. 2017, 5, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; Zile, M.R.; Anand, I.S.; Packer, M.; Zannad, F.; Lam, C.S.; Janssens, S.; Jhund, P.S.; et al. Effects of Sacubitril/Valsartan on N-Terminal Pro-B-Type Natriuretic Peptide in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2020, 8, 372–381. [Google Scholar] [CrossRef]
- Kasner, M.; Gaub, R.; Westermann, D.; Kaplan, H.; Akpulat, S.; Steendijk, P.; Schultheiss, H.-P.; Tschöpe, C. Simultaneous estimation of NT-proBNP on top to mitral flow Doppler echocardiography as an accurate strategy to diagnose diastolic dysfunction in HFNEF. Int. J. Cardiol. 2011, 149, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Heckbert, S.R.; Lai, S.; Ambale-Venkatesh, B.; Ostovaneh, M.R.; McClelland, R.L.; Lima, J.A.; Bluemke, D.A. Association of Elevated NT-proBNP With Myocardial Fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 2017, 70, 3102–3109. [Google Scholar] [CrossRef]
- Duca, F.; Kammerlander, A.A.; Zotter-Tufaro, C.; Aschauer, S.; Schwaiger, M.L.; Marzluf, B.A.; Bonderman, D.; Mascherbauer, J. Interstitial Fibrosis, Functional Status, and Outcomes in Heart Failure With Preserved Ejection Fraction: Insights From a Prospective Cardiac Magnetic Resonance Imaging Study. Circ. Cardiovasc. Imaging. 2016, 9, e005277. [Google Scholar] [CrossRef]
- Zile, M.R.; Jhund, P.S.; Baicu, C.F.; Claggett, B.L.; Pieske, B.; Voors, A.A.; Prescott, M.F.; Shi, V.; Lefkowitz, M.; McMurray, J.J.; et al. Plasma Biomarkers Reflecting Profibrotic Processes in Heart Failure With a Preserved Ejection Fraction: Data From the Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction Study. Circ. Heart Fail. 2016, 9, e002551. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Burnett, J.C.; Butler, J., Jr.; Camacho, A.; Felker, G.M.; Fiuzat, M.; O’Connor, C.; Solomon, S.D.; Vaduganathan, M.; Zile, M.R.; et al. Natriuretic Peptides as Inclusion Criteria in Clinical Trials: A JACC: Heart Failure Position Paper. JACC Heart Fail. 2020, 8, 347–358. [Google Scholar] [CrossRef]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Venkateshvaran, A.; Tureli, H.O.; Faxén, U.L.; Lund, L.H.; Tossavainen, E.; Lindqvist, P. Left atrial reservoir strain improves diagnostic accuracy of the 2016 ASE/EACVI diastolic algorithm in patients with preserved left ventricular ejection fraction: Insights from the KARUM haemodynamic database. Eur. Heart J. Cardiovasc. Imaging. 2022, 23, 1157–1168. [Google Scholar] [CrossRef]
- Tschöpe, C.; Kasner, M.; Westermann, D.; Gaub, R.; Poller, W.C.; Schultheiss, H.P. The role of NT-proBNP in the diagnostics of isolated diastolic dysfunction: Correlation with echocardiographic and invasive measurements. Eur. Heart J. 2005, 26, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Rommel, K.-P.; von Roeder, M.; Latuscynski, K.; Oberueck, C.; Blazek, S.; Fengler, K.; Besler, C.; Sandri, M.; Lücke, C.; Gutberlet, M.; et al. Extracellular Volume Fraction for Characterization of Patients With Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2016, 67, 1815–1825. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef]
- Sorimachi, H.; Verbrugge, F.H.; Omote, K.; Omar, M.; Obokata, M.; Reddy, Y.N.; Ye, Z.; Michelena, H.I.; Borlaug, B.A. Longitudinal evolution of cardiac dysfunction in heart failure and preserved ejection fraction with normal natriuretic peptide levels. Circulation 2022, 146, 500–502. [Google Scholar] [CrossRef]
- Kasner, M.; Westermann, D.; Lopez, B.; Gaub, R.; Escher, F.; Kühl, U.; Schultheiss, H.-P.; Tschöpe, C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 2011, 57, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.; van der Velden, J.; Papp, Z.; Bronzwaer, J.G.; Edes, I.; Stienen, G.; Paulus, W.J. Cardiomyocyte stiffness in diastolic heart failure. Circulation 2005, 111, 774–781. [Google Scholar] [CrossRef] [PubMed]
- van Heerebeek, L.; Borbély, A.; Niessen, H.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.; Linke, W.; Laarman, G.J.; Paulus, W.J. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 2006, 113, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Linke, W.A.; Hamdani, N. Gigantic business: Titin properties and function through thick and thin. Circ. Res. 2014, 114, 1052–1068. [Google Scholar] [CrossRef]
- Borbély, A.; Falcão-Pires, I.; van Heerebeek, L.; Hamdani, N.; Édes, I.; Gavina, C.; Leite-Moreira, A.; Bronzwaer, J.G.; Papp, Z.; van der Velden, J.; et al. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ. Res. 2009, 104, 780–786. [Google Scholar] [CrossRef]
- Jiao, L.; Machuki, J.O.; Wu, Q.; Shi, M.; Fu, L.; Adekunle, A.O.; Tao, X.; Xu, C.; Hu, X.; Yin, Z.; et al. Estrogen and calcium handling proteins: New discoveries and mechanisms in cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H820–H829. [Google Scholar] [CrossRef]
- Maslov, P.Z.; Kim, J.K.; Argulian, E.; Ahmadi, A.; Narula, N.; Singh, M.; Bax, J.; Narula, J. Is cardiac diastolic dysfunction a part of post-menopausal syndrome? JACC Heart Fail 2019, 7, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Pabel, S.; Wagner, S.; Bollenberg, H.; Bengel, P.; Kovács, A.; Schach, C.; Tirilomis, P.; Mustroph, J.; Renner, A.; Gummert, J.; et al. Empagliflozin directly improves diastolic function in human heart failure. Eur. J. Heart Fail. 2018, 20, 1690–1700. [Google Scholar] [CrossRef]
- Juni, R.P.; Kuster, D.W.; Goebel, M.; Helmes, M.; Musters, R.J.; van der Velden, J.; Koolwijk, P.; Paulus, W.J.; van Hinsbergh, V.W. Cardiac Microvascular Endothelial Enhancement of Cardiomyocyte Function Is Impaired by Inflammation and Restored by Empagliflozin. JACC Basic Transl. Sci. 2019, 4, 575–591. [Google Scholar] [CrossRef]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Scheffer, M.; Driessen-Waaijer, A.; Hamdani, N.; Landzaat, J.W.; Jonkman, N.H.; Paulus, W.J.; van Heerebeek, L. Stratified Treatment of Heart Failure with preserved Ejection Fraction: Rationale and design of the STADIA-HFpEF trial. ESC Heart Fail. 2020, 7, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.; Gillebert, T.; Klein, A.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]

| Parameters | HFpEF Patients (n = 152) | Low NT-proBNP (n = 78) | High NT-proBNP (n = 74) | * p-Value | † p-Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (yr) | 71.2 ± 8.8 | 69.2 ± 8.5 | 73.0 ± 8.6 | 0.008 | - |
| Sex women (%) | 141 (92.7) | 72 (92.3) | 70 (94.6) | 0.570 | - |
| BMI (kg/m2) | 31.7 ± 6.4 | 32.0 ± 5.6 | 31.2 ± 7.1 | 0.484 | - |
| Hemodynamics | |||||
| Systolic BP (mmHg) | 147 ± 22 | 142 ± 18 | 151 ± 25 | 0.008 | 0.032 |
| Diastolic BP (mmHg) | 77 ± 12 | 76 ± 10 | 77 ± 14 | 0.658 | 0.178 |
| Pulse Pressure (mmHg) | 69 ± 19 | 66 ± 17 | 72 ± 20 | 0.089 | 0.737 |
| Medical history n (%) | |||||
| Hypertension | 109 (71.7) | 56 (71.7) | 54 (73.0) | 0.973 | 0.478 |
| T2DM | 52 (34.2) | 24 (30.8) | 28 (37.8) | 0.389 | 0.970 |
| Obesity | 82 (53.9) | 47 (60.3) | 36 (48.6) | 0.151 | - |
| Atrial Fibrillation | 34 (22.4) | 11 (14.1) | 23 (31.1) | 0.014 | 0.070 |
| Coronary Artery Disease | 15 (9.9) | 7 (9.0) | 8 (10.8) | 0.724 | 0.581 |
| Laboratory values | |||||
| eGFR-EPI (mL/min/1.73 m2) | 65.8 ± 18.8 | 72.7 ± 15.6 | 59.8.1 ± 20.0 | <0.001 | - |
| Haemoglobin (mmol/l) | 8.1 ± 0.9 | 8.3 ± 0.7 | 8.0 ± 1.0 | 0.013 | 0.039 |
| NT-proBNP (pg/mL) | 194.9 (84.7–436.4) | 84.7 (57.2–144.1) | 423.7 (305.1–940.7) | - | - |
| Medications n (%) | |||||
| ACE inhibitors or ARBs | 91 (60.0) | 45 (57.7) | 48 (64.9) | 0.476 | 0.292 |
| Loop diuretics | 55 (36.2) | 15 (19.2) | 41 (55.4) | <0.001 | <0.001 |
| Thiazide diuretics | 36 (23.7) | 22 (28.2) | 14 (18.9) | 0.164 | 0.085 |
| Aldosteron antagonists | 20 (13.2) | 11 (14.1) | 9 4 (12.2) | 0.762 | 0.790 |
| Calcium channels-blockers | 58 (38.2) | 24 (30.8) | 35 (47.3) | 0.049 | 0.096 |
| Beta-blockers | 87 (57.2) | 37 (47.4) | 51 (68.9) | 0.012 | 0.021 |
| Oral antiglycaemic agents | 41 (27.0) | 18 (23.1) | 23 (31.1) | 0.285 | 0.571 |
| Insulin | 21 (13.8) | 10 (12.8) | 11 (14.9) | 0.763 | 0.819 |
| Statins | 98 (64.5) | 55 (70.5) | 45 (60.8) | 0.133 | 0.142 |
| Parameters | HFpEF Patients (n = 152) | Low NT-proBNP (n = 78) | High NT-proBNP (n = 74) | * p-Value | † p-Value |
|---|---|---|---|---|---|
| LV structure and geometry | |||||
| LVMI (g/m2) | 87.0 ± 17.6 | 84.9 ± 15.5 | 89.7 ± 19.5 | 0.096 | 0.268 |
| PWTd (mm) | 10.0 ± 1.5 | 9.9 ± 1.6 | 10.0 ± 1.4 | 0.715 | 0.673 |
| RWT | 0.42 ± 0.07 | 0.42 ± 0.07 | 0.43 ± 0.07 | 0.567 | 0.381 |
| LVEDV (mL) | 80.5 ± 20.2 | 83.1 ± 18.7 | 79.2 ± 28.4 | 0.795 | 0.970 |
| LVEDVI (mL/m2) | 42.0 ± 9.6 | 42.4 ± 8.7 | 41.8 ± 10.6 | 0.679 | 0.661 |
| LV systolic function | |||||
| EF (%) | 56.2 ± 5.6 | 56.5 ± 5.2 | 56.1 ± 6.3 | 0.659 | 0.632 |
| GLS (%) | 18.6 ± 2.9 | 18.7 ± 2.8 | 18.6 ± 3.1 | 0.856 | 0.465 |
| LV diastolic function | |||||
| DT (ms) | 206.5 ± 41.5 | 208.0 ± 39 | 204.0 ± 44 | 0.525 | 0.371 |
| Lateral E′ (cm/s) | 7.3 ± 1.9 | 7.4 ± 1.6 | 7.3 ± 2.2 | 0.584 | 0.590 |
| Septal E′ (cm/s) | 5.7 ± 1.2 | 5.8 ± 1.1 | 5.6 ± 1.3 | 0.245 | 0.941 |
| Mean E′ (cm/s) | 6.5 ± 1.4 | 6.6 ± 1.2 | 6.4 ± 1.5 | 0.287 | 0.715 |
| Mean A’ (cm/s) | 8.7 ± 2.1 | 9.3 ± 1.9 | 8.0 ± 2.0 | 0.001 | 0.030 |
| Lateral E/E′ | 12.5 (9.1–14.1) | 11.1 (8.4–12.8) | 12.1 (10.4–15.5) | 0.007 | 0.139 |
| Septal E/E′ | 15.9 ± 5.2 | 14.3 ± 4.0 | 17.4 ± 5.9 | 0.001 | 0.107 |
| Mean E/E′ | 13.9 ± 4.7 | 12.6 ± 3.7 | 15.1 ± 5.3 | 0.001 | 0.117 |
| LA structure and function $ | |||||
| Max LAVI (mL/m2) | 43.6 ± 11.8 | 39.8 ± 9.0 | 47.9 ± 13.1 | <0.001 | 0.002 |
| Pre-A LAVI (mL/m2) | 32.4 ± 9.8 | 29.5 ± 7.3 | 35.8 ± 11.1 | <0.001 | 0.001 |
| Min LAVI (mL/m2) | 25.0 ± 9.4 | 21.3 ± 6.3 | 28.7 ± 10.7 | <0.001 | <0.001 |
| LA global ef (%) | 44.6 ± 9.0 | 47.2 ± 8.4 | 42.3 ± 9.4 | 0.001 | 0.063 |
| LA passive ef (%) | 25.2 ± 7.7 | 25.2 ± 7.3 | 25.3 ± 8.0 | 0.939 | 0.255 |
| LA active ef (%) | 26.5 ± 9.4 | 29.0 ± 9.5 | 24.3 ± 9.6 | 0.005 | 0.153 |
| LA compliance | 3.2 ± 1.2 | 3.6 ± 1.2 | 2.8 ± 1.1 | 0.001 | 0.003 |
| RV and RA structure and function | |||||
| TAPSE (mm) | 22.1 ± 3.5 | 22.6 ± 3.2 | 21.7 ± 3.7 | 0.117 | 0.713 |
| RV FAC (%) | 41.9 ± 10.8 | 42.4 ± 12.1 | 41.3 ± 9.0 | 0.605 | 0.484 |
| RV strain (%) | 22.8 ± 7.1 | 23.7 ± 6.5 | 21.5 ± 7.9 | 0.306 | 0.841 |
| TR velocity (m/s) | 2.65 ± 0.40 | 2.60 ± 0.47 | 2.69 ± 0.33 | 0.330 | 0.741 |
| Max RAV (mL) | 43.2 ± 15.1 | 40.0 ± 11.2 | 46.9 ± 18.2 | 0.018 | 0.136 |
| Model 1 * β (95% CI) | p-Value | Model 2 † β (95% CI) | p-Value | |
|---|---|---|---|---|
| LVMI (g/m2) | 7.63 (1.23; 14.02) | 0.020 | 7.70 (0.57; 14.82) | 0.035 |
| PWTd (mm) | 0.03 (−0.49; 0.56) | 0.895 | −0.11 (−0.69; 0.47) | 0.699 |
| RWT | −0.01 (−0.04; 0.01) | 0.395 | −0.02 (−0.05; 0.01) | 0.139 |
| LVEDV (mL) | −1.10 (−8.36; 6.17) | 0.766 | −0.31 (−8.45; 7.82) | 0.939 |
| LVEDVI (mL/m2) | −0.12 (−4.01; 3.76) | 0.949 | 0.69 (−3.67; 5.04) | 0.755 |
| EF (%) | −2.00 (−4.12; 0.12) | 0.065 | −1.64 (−4.00; 0.71) | 0.170 |
| GLS (%) | −1.25 (−2.42; −0.08) | 0.037 | −0.63 (−1.97; 0.71) | 0.355 |
| DT (ms) | −15.6 (−31.37; 0.23) | 0.053 | −17.1 (−34.83; 0.53) | 0.057 |
| Lateral E′ (cm/s) | −0.13 (−0.91; 0.65) | 0.747 | −0.48 (−1.35; 0.39) | 0.274 |
| Septal E′ (cm/s) | −0.14 (−0.626; 0.33) | 0.551 | −0.10 (−0.63; 0.42) | 0.697 |
| Mean E′ (cm/s) | −0.23 (−0.79; 0.33) | 0.415 | −0.36 (−0.99; 0.27) | 0.256 |
| A′ mean (cm/s) | −2.04 (−2.81; −1.27) | <0.001 | −1.94 (−2.81; −1.08) | <0.001 |
| Lateral E/E′ | 3.54 (1.49; 5.59) | 0.001 | 3.72 (1.45; 6.00) | 0.002 |
| Septal E/E′ | 3.08 (1.15; 5.01) | 0.002 | 2.55 (0.44; 4.66) | 0.018 |
| Mean E/E′ | 3.11 (1.32; 4.90) | 0.001 | 3.10 (1.11; 5.09) | 0.002 |
| Max LAVI (mL/m2) | 8.82 (4.54; 13.09) | <0.001 | 9.51 (4.72; 14.30) | <0.001 |
| Pre-A LAVI (mL/m2) | 6.45 (2.86; 10.05) | 0.001 | 7.04 (2.99; 11.1) | 0.001 |
| Min LAVI (mL/m2) | 7.83 (4.78; 10.87) | <0.001 | 8.12 (4.79; 11.46) | <0.001 |
| LA global ef (%) | −6.93 (−10.12; −3.74) | <0.001 | −6.39 (−9.84; −2.93) | <0.001 |
| LA passive ef (%) | −1.58 (−4.80; 1.64) | 0.334 | −0.38 (−4.01; 3.25) | 0.837 |
| LA active ef (%) | −5.66 (−9.47; −1.85) | 0.004 | −5.08 (−9.30; −0.87) | 0.019 |
| LA compliance | −0.89 (−1.31; −0.46) | <0.001 | −0.87 (−1.34; −0.40) | <0.001 |
| TAPSE (mm) | −1.04 (−2.36; 0.28) | 0.121 | −0.63 (−2.06; 0.79) | 0.382 |
| RV FAC (%) | −3.53 (−8.16; 1.10) | 0.134 | −3.32 (−8.64; 2.01) | 0.219 |
| RV strain | −2.22 (−6.28; 1.84) | 0.275 | −0.05 (−4.52; 4.42) | 0.983 |
| TR velocity (m/s) | 0.03 (−0.19; 0.26) | 0.769 | −0.02 (−0.31; 0.27) | 0.876 |
| RAV max (mL) | 11.70 (5.73; 17.67) | <0.001 | 9.76 (2.75; 16.77) | 0.007 |
| Median (IQR Range) | Model 1 * β (95% CI) | p-Value | Model 2 † β (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Myocardial T1 pre-contrast, ms | 1005 (980–1046) | −0.21 (−3.68; 3.62) | 0.908 | −1.00 (−4.18; 2.17) | 0.530 |
| Myocardial T1 post-contrast, ms | 354 (328–385) | −0.12 (−0.02; 0.26) | 0.913 | 0.004 (−2.03; 2.04) | 0.997 |
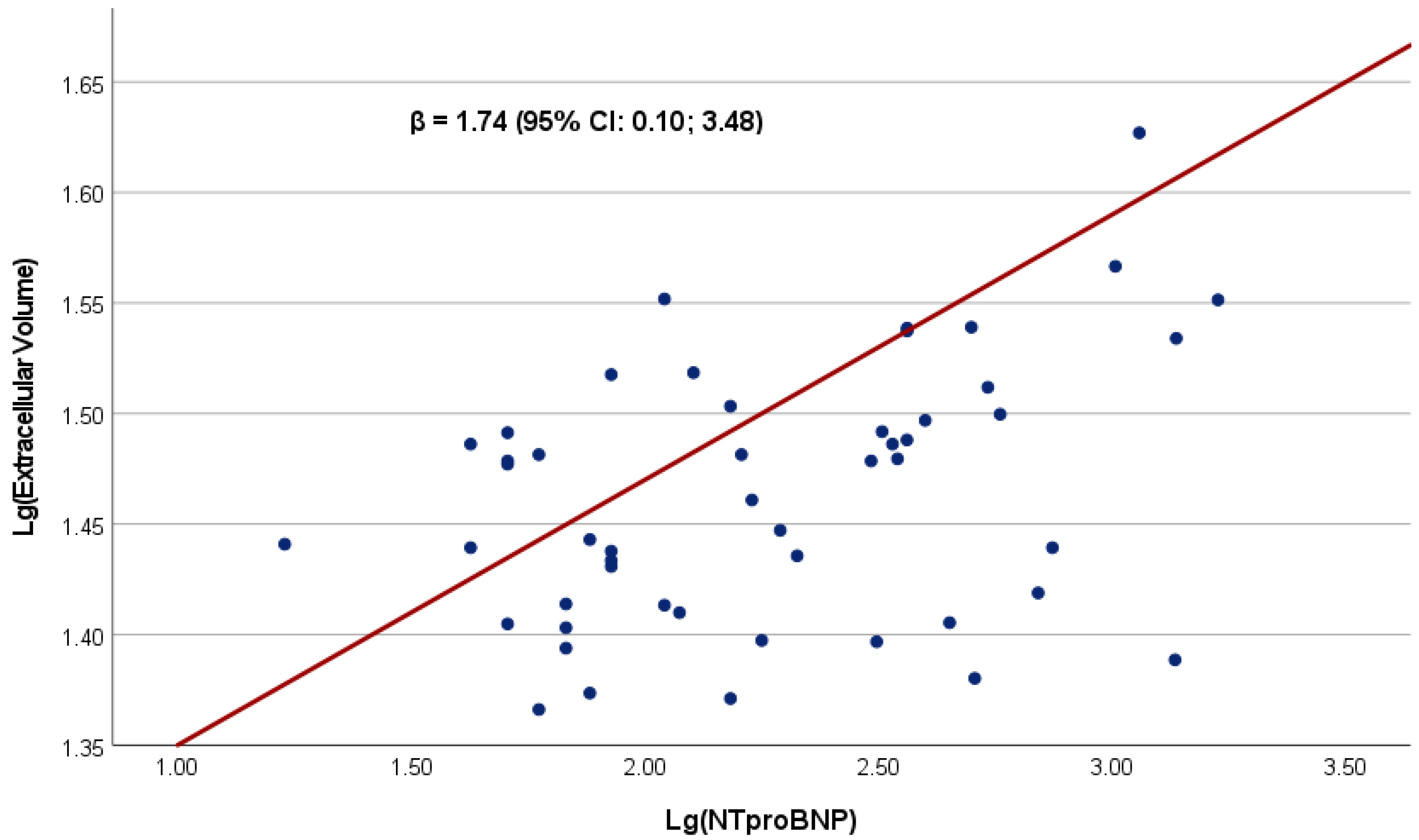
| Extracellular volume fraction, % | 27.6 (25.0–31.4) | 1.74 (0.10; 3.48) | 0.049 | 1.82 (0.19; 3.44) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Canto, E.; Scheffer, M.; Kortekaas, K.; Driessen-Waaijer, A.; Paulus, W.J.; van Heerebeek, L. Natriuretic Peptide Levels and Stages of Left Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction. Biomedicines 2023, 11, 867. https://doi.org/10.3390/biomedicines11030867
Dal Canto E, Scheffer M, Kortekaas K, Driessen-Waaijer A, Paulus WJ, van Heerebeek L. Natriuretic Peptide Levels and Stages of Left Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction. Biomedicines. 2023; 11(3):867. https://doi.org/10.3390/biomedicines11030867
Chicago/Turabian StyleDal Canto, Elisa, Marielle Scheffer, Kirsten Kortekaas, Annet Driessen-Waaijer, Walter J. Paulus, and Loek van Heerebeek. 2023. "Natriuretic Peptide Levels and Stages of Left Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction" Biomedicines 11, no. 3: 867. https://doi.org/10.3390/biomedicines11030867
APA StyleDal Canto, E., Scheffer, M., Kortekaas, K., Driessen-Waaijer, A., Paulus, W. J., & van Heerebeek, L. (2023). Natriuretic Peptide Levels and Stages of Left Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction. Biomedicines, 11(3), 867. https://doi.org/10.3390/biomedicines11030867





