Bioinformatics-Based Analysis of Key Genes in Steroid-Induced Osteonecrosis of the Femoral Head That Are Associated with Copper Metabolism
Abstract
1. Introduction
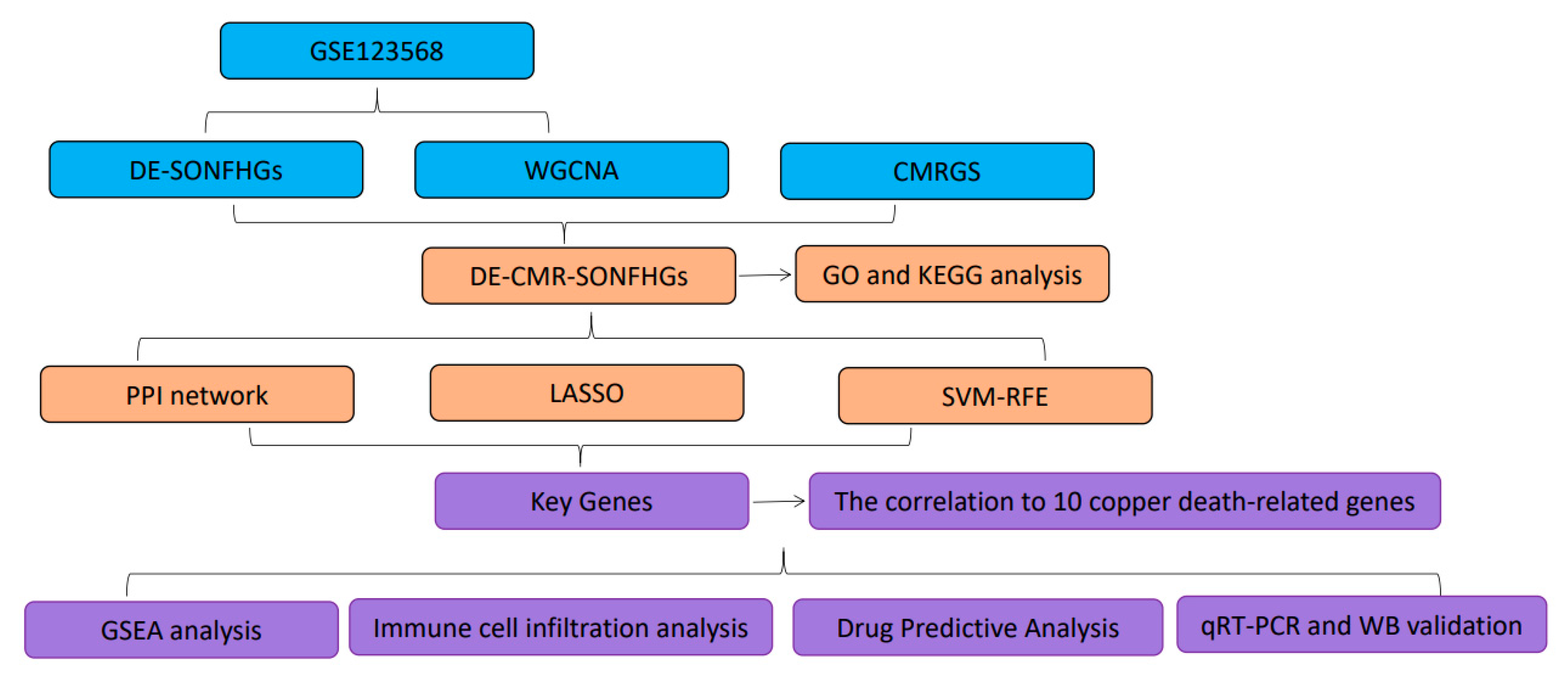
2. Materials and Methods
2.1. Data Source
2.2. Differential Gene Expression Analysis
2.3. Weighted Gene Coexpression Network Analysis (WGCNA)
2.4. Functional Enrichment Analysis of DE-CMR-ONFHGs
2.5. PPI Network Construction
2.6. Screening for Key Genes using a Machine Learning Algorithm
2.7. Single-Gene Gene Set Enrichment Analysis (GSEA)
2.8. Immune Cell Infiltration Analysis
2.9. Drug Predictive Analysis
2.10. Analysis of the Relevance of Copper Death-Related Genes and Differentially Expressed Genes in Disease
2.11. Verification of the Expression of Key Genes in Clinical Samples
2.12. Statistical Analysis
3. Results
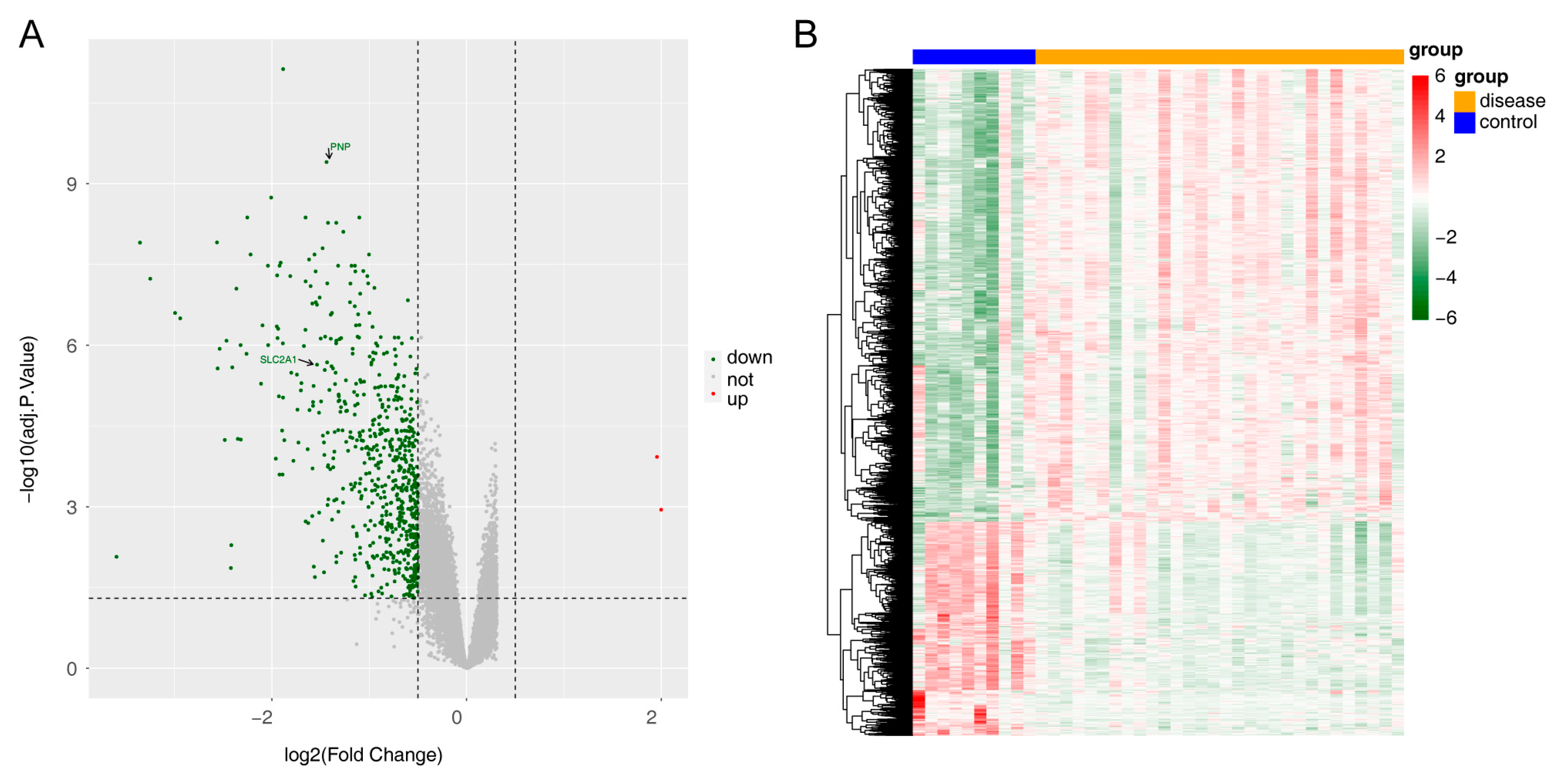
3.1. Screening of Differentially Expressed Genes
3.2. WGCNA
3.3. Functional Enrichment Analysis of DE-CMR-ONFHGs
3.4. Linkage between Proteins
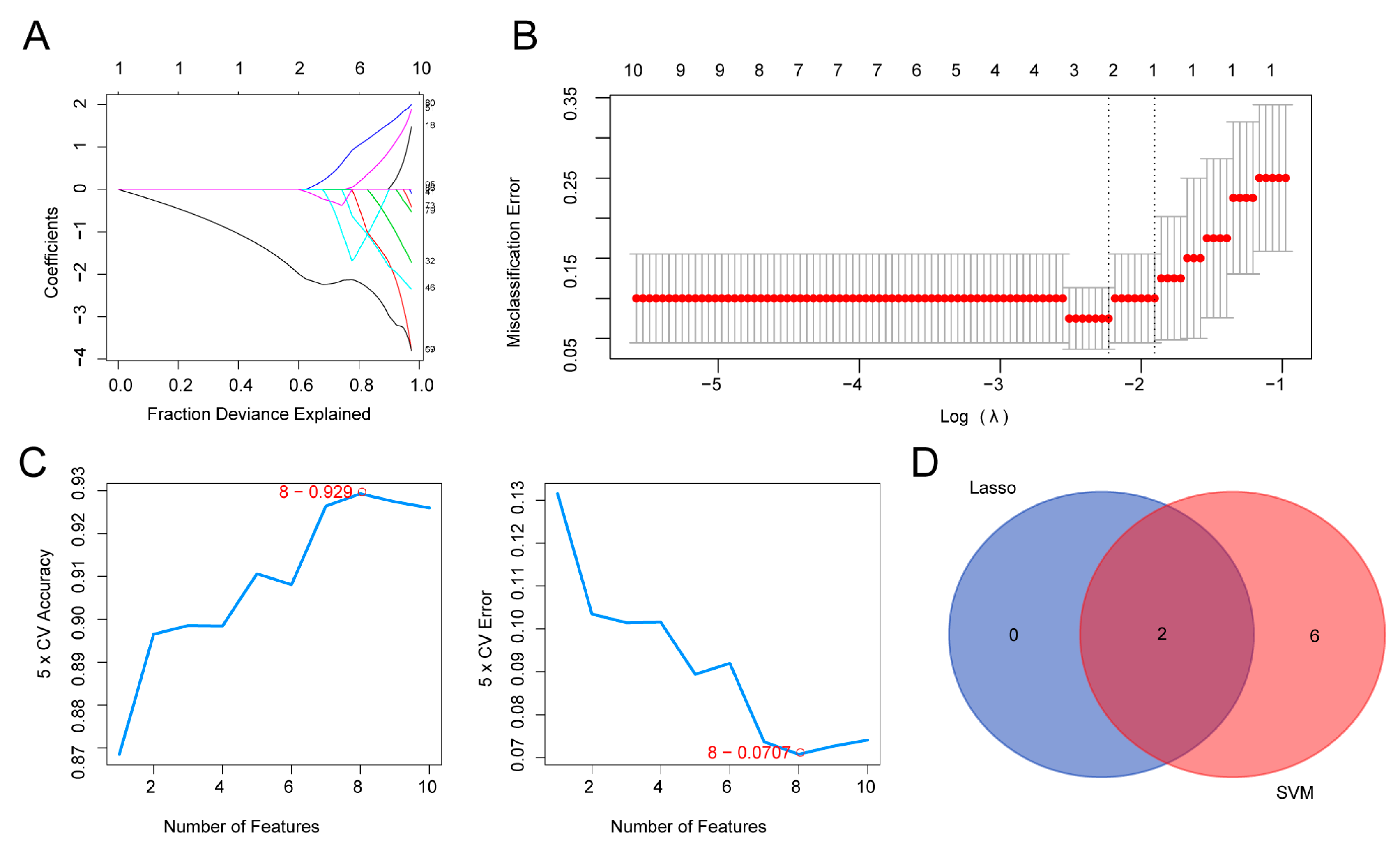
3.5. Screening for Key Genes using a Machine Learning Algorithm
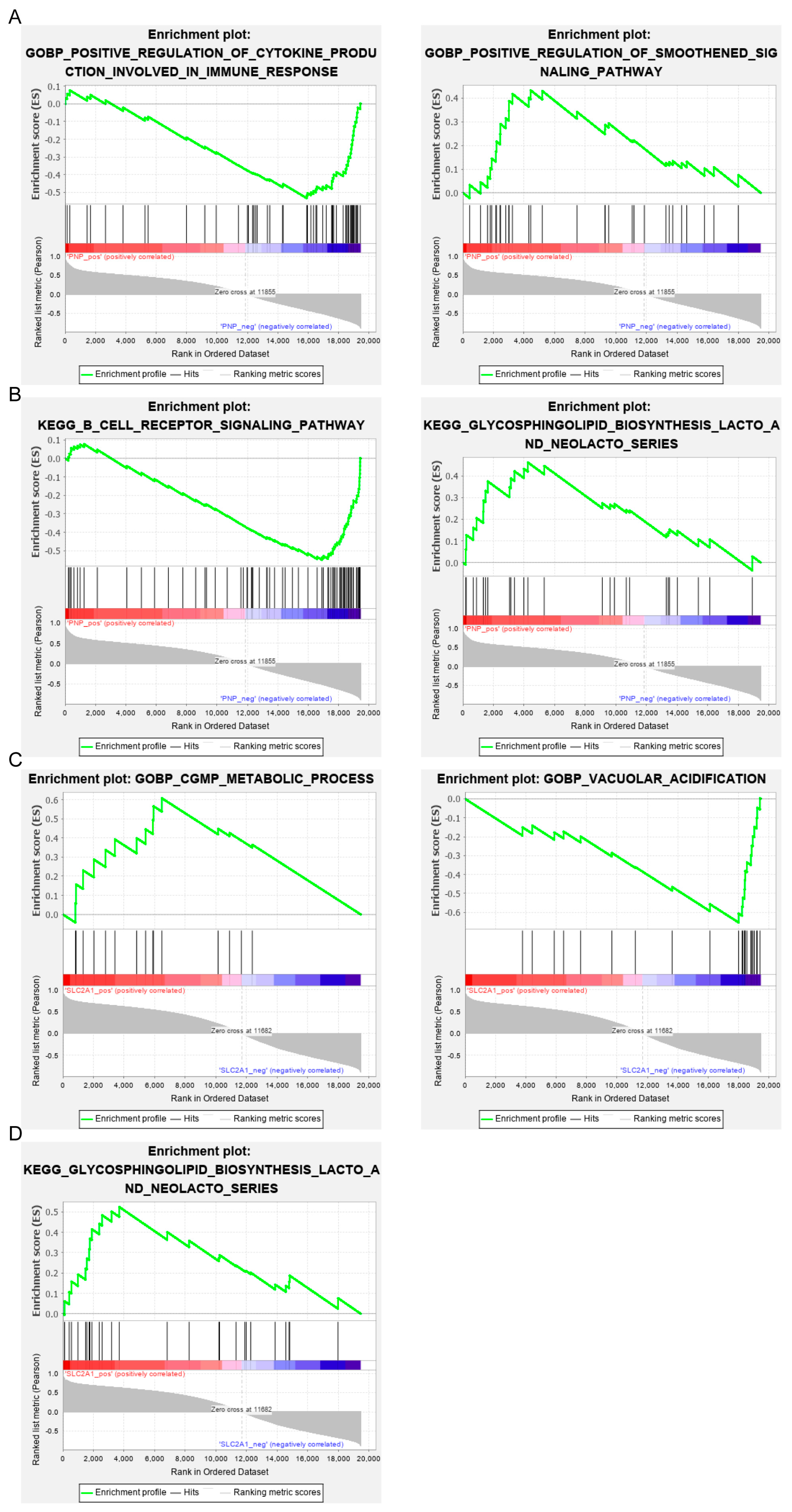
3.6. Single Gene Enrichment Analysis
3.7. Immune Cell Infiltration Analysis
3.8. Drug Prediction Analysis
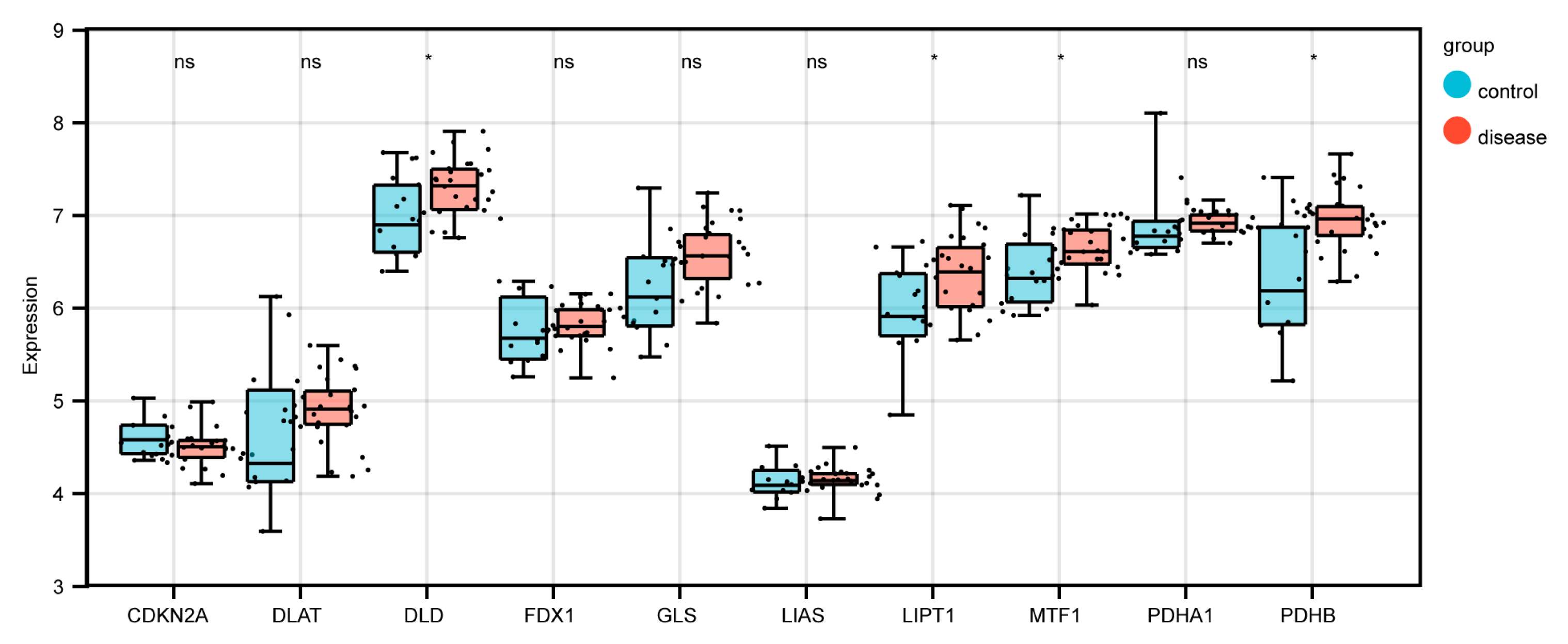
3.9. Relevance of Copper Death-Related Genes and Differentially Expressed Genes to Disease
3.10. Verification of the Expression of Key Targets through qRT–PCR and WB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPI | Protein–protein interaction |
| SONFH | Steroid-induced osteonecrosis of the femoral head |
| ONFH | Osteonecrosis of the femoral head |
| PNP | Purine nucleoside phosphorylase |
| qRT–PCR | Quantitative real-time polymerase chain reaction |
| SONFH | Steroid-induced osteonecrosis of the femoral head |
| GEO | Gene Expression Omnibus |
| DE-CMR-SONFHGs | Differentially expressed copper metabolism-related SONFH genes |
| WGCNA | Weighted gene co-expression network analysis |
| CMRGs | Copper metabolism-related genes |
| LASSO | Least-Absolute Shrinkage and Selection Operator |
| SVM | Support Vector Machine |
| ROC | Receiver operating characteristic |
| DGIdb | The Drug Gene Interaction Database |
| OA | Osteoarthritis |
| MM | Module members |
| GS | Gene significance |
| Cu | Copper |
| HIF-1α | Hypoxia-inducible factor-1α |
| VEGF | Vascular endothelial growth factor |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genome |
| STRING | Search Tool for the Retrieval of Interacting Genes |
| PPI | Protein–protein interaction |
| GSEA | Gene Set Enrichment Analysis |
| ssGSEA | Single sample Gene Set Enrichment Analysis |
References
- Ikeuchi, K.; Hasegawa, Y.; Seki, T.; Takegami, Y.; Amano, T.; Ishiguro, N. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod. Rheumatol. 2015, 25, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, Z.; Ren, M.; Yang, Q.; Wang, Q.; Chen, G.; Zhao, H.; Li, Z.; Wang, J.; Zhang, G. Association of gene variants of transcription factors PPARgamma, RUNX2, Osterix genes and COL2A1, IGFBP3 genes with the development of osteonecrosis of the femoral head in Chinese population. Bone 2017, 101, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Moya-Angeler, J.; Gianakos, A.L.; Villa, J.C.; Ni, A.; Lane, J.M. Current concepts on osteonecrosis of the femoral head. World J. Orthop. 2015, 6, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Petek, D.; Hannouche, D.; Suva, D. Osteonecrosis of the femoral head: Pathophysiology and current concepts of treaI. EFORT Open Rev. 2019, 4, 85–97. [Google Scholar] [CrossRef]
- Cui, L.; Zhuang, Q.; Lin, J.; Jin, J.; Zhang, K.; Cao, L.; Lin, J.; Yan, S.; Guo, W.; He, W.; et al. Multicentric epidemiologic study on six thousand three hundred and ninety five cases of femoral head osteonecrosis in China. Int. Orthop. 2016, 40, 267–276. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflug. Arch. 2020, 472, 1415–1429. [Google Scholar] [CrossRef]
- Tulinska, J.; Mikusova, M.L.; Liskova, A.; Busova, M.; Masanova, V.; Uhnakova, I.; Rollerova, E.; Alacova, R.; Krivosikova, Z.; Wsolova, L.; et al. Copper oxide nanoparticles stimulate the immune response and decrease antioxidant defense in mice after six-week inhalation. Front. Immunol. 2022, 13, 874253. [Google Scholar] [CrossRef]
- Chen, C.H.; Chou, Y.T.; Yang, Y.W.; Lo, K.Y. High-dose copper activates p53-independent apoptosis through the induction of nucleolar stress in human cell lines. Apoptosis. 2021, 26, 612–627. [Google Scholar] [CrossRef]
- Mitra, S.; Keswani, T.; Ghosh, N.; Goswami, S.; Datta, A.; Das, S.; Maity, S.; Bhattacharyya, A. Copper induced immunotoxicity promote differential apoptotic pathways in spleen and thymus. Toxicology 2013, 306, 74–84. [Google Scholar] [CrossRef]
- Keswani, T.; Mitra, S.; Bhattacharyya, A. Copper-induced immunotoxicity involves cell cycle arrest and cell death in the liver. Environ. Toxicol. 2015, 30, 411–421. [Google Scholar] [CrossRef]
- Li, B.; Lei, Y.; Hu, Q.; Li, D.; Zhao, H.; Kang, P. Porous copper- and lithium-doped nano-hydroxyapatite composite scaffold promotes angiogenesis and bone regeneration in the repair of glucocorticoids-induced osteonecrosis of the femoral head. Biomed. Mater. 2021, 16, 065012. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Wang, J.; Wang, L.; Gao, C.; Li, B.; Wang, Y.; Wu, J.; Quan, C. Bioactive gelatin cryogels with BMP-2 biomimetic peptide and VEGF: A potential scaffold for synergistically induced osteogenesis. Chin. Chem. Lett. 2022, 33, 1956–1962. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, W.; Bal, B.S.; Rahaman, M.N. Effect of copper-doped silicate 13-93 bioactive glass scaffolds on the response of MC3T3-E1 cells in vitro and on bone regeneration and angiogenesis in rat calvarial defects in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 440–452. [Google Scholar] [CrossRef]
- Ekin, S.; Berber, I.; Kiymaz, N. Effects of dexamethasone on trace elements and serum protein patterns following brain trauma in rats. Biol. Trace Elem. Res. 2005, 107, 53–60. [Google Scholar] [CrossRef]
- Jasim, N.H.; Kareem, D.A.; Al Ali, M.F.M.; Abbas, B.A. Effect of long-term treatment with dexamethasone on the liver and kidney histopathology, as well as blood biochemistry in male rabbits (Lepus cuniculus). Arch. Razi Inst. 2022, 77, 333–343. [Google Scholar] [CrossRef]
- Kang, S.; Song, J. Robust gene selection methods using weighting schemes for microarray data analysis. BMC Bioinform. 2017, 18, 389. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, C.; Liu, Z.; Ren, S.; Shen, Z.; Han, K.; Xin, W.; He, G.; Liu, J. Screening of Potential Biomarkers in the Peripheral Serum for Steroid-Induced Osteonecrosis of the Femoral Head Based on WGCNA and Machine Learning Algorithms. Dis. Markers. 2022, 2022, 2639470. [Google Scholar] [CrossRef]
- Liang, X.Z.; Luo, D.; Chen, Y.R.; Li, J.C.; Yan, B.Z.; Guo, Y.B.; Wen, M.T.; Xu, B.; Li, G. Identification of potential autophagy-related genes in steroid-induced osteonecrosis of the femoral head via bioinformatics analysis and experimental verification. J. Orthop. Surg. Res. 2022, 17, 86. [Google Scholar] [CrossRef]
- Smyth, G.K. Limma: Linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005; pp. 397–420. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.X.; Ho, C.H.; Lin, C.J. An improved glmnet for l1-regularized logistic regression. J. Mach. Learn. Res. 2012, 13, 1999–2030. [Google Scholar]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F.; Chang, C.C.; Lin, C.C. E1071: MISC functions of the department of statistics (e1071), TU Wien. R Package Version 2014, 1, 9. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Fan, Y.; Li, Y.; Jiang, H.; Wang, J. Comprehensive Diagnostics of Diabetic Nephropathy by Transcriptome RNA Sequencing. Diabetes Metab. Syndr. Obes. 2022, 15, 3069–3080. [Google Scholar] [CrossRef]
- Suarez-Farinas, M.; Lowes, M.A.; Zaba, L.C.; Krueger, J.G. Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA). PLoS ONE 2010, 5, e10247. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Wu, Y.; Zhang, B.; Wei, J.; Li, J.; Huang, Y.; Chen, L.; He, X. Bioinformatics identification and validation of biomarkers and infiltrating immune cells in endometriosis. Front. Immunol. 2022, 13, 944683. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kahlson, M.A.; Dixon, S.J. Copper-induced cell death. Science 2022, 375, 1231–1232. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Liang, X.Z.; Liu, X.C.; Li, S.; Wen, M.T.; Chen, Y.R.; Luo, D.; Xu, B.; Li, N.H.; Li, G. IRF8 and its related molecules as potential diagnostic biomarkers or therapeutic candidates and immune cell infiltration characteristics in steroid-induced osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2023, 18, 27. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, D.; Mei, R.; Qian, S.; Wang, P.; Chen, K.; Yu, X. Screening and identification of potential key biomarkers for glucocorticoid-induced osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2023, 18, 28. [Google Scholar] [CrossRef]
- Li, B.B.; Yu, S.F. In vitro study of the effects of copper ion on osteoclastic resorption in various dental mineralized tissues. Zhonghua Kou Qiang Yi Xue Za Zhi 2007, 42, 110–113. [Google Scholar]
- Milkovic, L.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Zarkovic, N. Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. A 2014, 102, 3556–3561. [Google Scholar] [CrossRef]
- Rodriguez, J.P.; Rios, S.; Gonzalez, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef]
- Milachowski, K.A. Investigation of ischaemic necrosis of the femoral head with trace elements. Int. Orthop. 1988, 12, 323–330. [Google Scholar] [CrossRef]
- Yamazaki, J. Experimental study on the development of aseptic necrosis of femoral head--with comparison of osteoarthritis of the hip in collagen metabolism. Hokkaido Igaku Zasshi 1985, 60, 544–554. [Google Scholar]
- Gonzalez-Reimers, E.; Santolaria-Fernandez, F.; Garrido-Benedicto, P.; Duran-Castellon, M.C.; Galindo-Martin, L.; Martinez-Riera, A.; Vina-Rodriguez, J.; de la Vega-Prieto, M.J. Combined effects of steroids, ethanol and protein deficiency on tissue content and urinary and faecal excretion of zinc, copper and iron. Alcohol Alcohol. 2002, 37, 132–137. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Dorschner, J.M.; Vsetecka, D.M.; Amin, S.; Makol, A.; Ernste, F.; Osborn, T.; Moder, K.; Chowdhary, V.; Eliopoulos, E.; et al. Lupus-associated functional polymorphism in PNP causes cell cycle abnormalities and interferon pathway activation in human immune cells. Arthritis Rheumatol. 2017, 69, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.; Biro, J.; Chan, J.; Min, W.; Dobbs, K.; Notarangelo, L.D.; Grunebaum, E. Purine nucleoside phosphorylase deficiency induces p53-mediated intrinsic apoptosis in human induced pluripotent stem cell-derived neurons. Sci. Rep. 2022, 12, 9084. [Google Scholar] [CrossRef] [PubMed]
- Uldry, M.; Thorens, B. The SLC2 family of facilitated hexose and polyol transporters. Pflug. Arch. 2004, 447, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, D.; Pupo, E.; Ducano, N.; Isella, C.; Bertalot, G.; Luise, C.; Pece, S.; Bruna, A.; Rueda, O.M.; Caldas, C.; et al. High USP6NL levels in breast cancer sustain chronic AKT phosphorylation and GLUT1 stability fueling aerobic glycolysis. Cancer Res. 2018, 78, 3432–3444. [Google Scholar] [CrossRef]
- Shen, C.; Xuan, B.; Yan, T.; Ma, Y.; Xu, P.; Tian, X.; Zhang, X.; Cao, Y.; Ma, D.; Zhu, X.; et al. m6A-dependent glycolysis enhances colorectal cancer progression. Mol. Cancer 2020, 19, 72. [Google Scholar] [CrossRef]
- Min, K.W.; Kim, D.H.; Son, B.K.; Moon, K.M.; Kim, S.M.; Intazur Rahaman, M.; Kim, S.W.; Kim, E.K.; Kwon, M.J.; Koh, Y.W.; et al. High SLC2A1 expression associated with suppressing CD8 T cells and B cells promoted cancer survival in gastric cancer. PLoS ONE 2021, 16, e0245075. [Google Scholar] [CrossRef]
- Chen, H.; Ji, X.; Lee, W.C.; Shi, Y.; Li, B.; Abel, E.D.; Jiang, D.; Huang, W.; Long, F. Increased glycolysis mediates Wnt7b-induced bone formation. FASEB J. 2019, 33, 7810–7821. [Google Scholar] [CrossRef]
- Yang, M.; Li, H.; Rong, M.; Zhang, H.; Hou, L.; Zhang, C. Dysregulated GLUT1 may be involved in the pathogenesis of preeclampsia by impairing decidualization. Mol. Cell. Endocrinol. 2022, 540, 111509. [Google Scholar] [CrossRef]
- Chen, N.; Xiao, B.; Wang, S.; Wei, B. Bioinformatics analysis of microRNA linked to ubiquitin proteasome system in traumatic osteonecrosis of the femoral head. Medicine 2020, 99, e21706. [Google Scholar] [CrossRef]
- Wu, Z.; Wen, Y.; Fan, G.; He, H.; Zhou, S.; Chen, L. HEMGN and SLC2A1 might be potential diagnostic biomarkers of steroid-induced osteonecrosis of femoral head: STUDY based on WGCNA and DEGs screening. BMC Musculoskelet Disord. 2021, 22, 85. [Google Scholar] [CrossRef]
- Nonokawa, M.; Shimizu, T.; Yoshinari, M.; Hashimoto, Y.; Nakamura, Y.; Takahashi, D.; Asano, T.; Nishibata, Y.; Masuda, S.; Nakazawa, D.; et al. Association of neutrophil extracellular traps with the development of idiopathic osteonecrosis of the femoral head. Am. J. Pathol. 2020, 190, 2282–2289. [Google Scholar] [CrossRef]
- Tian, G.; Liu, C.; Gong, Q.; Yu, Z.; Wang, H.; Zhang, D.; Cong, H. Human umbilical cord mesenchymal stem cells improve the necrosis and osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head model through reducing the macrophage polarization. Int. J. Stem Cells. 2022, 15, 195–202. [Google Scholar] [CrossRef]
- Zheng, L.W.; Wang, W.C.; Mao, X.Z.; Luo, Y.H.; Tong, Z.Y.; Li, D. TNF-alpha regulates the early development of avascular necrosis of the femoral head by mediating osteoblast autophagy and apoptosis via the p38 MAPK/NF-kappaB signaling pathway. Cell Biol. Int. 2020, 44, 1881–1889. [Google Scholar] [CrossRef]
- Han, J.; Chai, Y.; Zhang, X.Y.; Chen, F.; Xu, Z.W.; Feng, Z.; Yan, Q.; Wen, S.B.; Wu, Y.K. Gujiansan ameliorates avascular necrosis of the femoral head by regulating autophagy via the HIF-1alpha/BNIP3 pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 6683007. [Google Scholar] [CrossRef]
- Focarelli, F.; Giachino, A.; Waldron, K.J. Copper microenvironments in the human body define patterns of copper adaptation in pathogenic bacteria. PLoS Pathog. 2022, 18, e1010617. [Google Scholar] [CrossRef]
- Garcia, E.; Hernandez-Ayvar, F.; Rodriguez-Barrera, R.; Flores-Romero, A.; Borlongan, C.; Ibarra, A. Supplementation with vitamin E, Zinc, Selenium, and copper re-establishes T-cell function and improves motor recovery in a rat model of spinal cord injury. Cell Transplant. 2022, 31, 9636897221109884. [Google Scholar] [CrossRef]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Guan, J.; Su, Y.; Jiang, J. Identification of key biomarkers in steroid-induced osteonecrosis of the femoral head and their correlation with immune infiltration by bioinformatics analysis. BMC Musculoskelet. Disord. 2022, 23, 67. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, J.; Zhuo, Y.; Hong, X.; Ye, J.; Tang, S.; Liu, N.; Zhang, Y. ARG2, MAP4K5 and TSTA3 as Diagnostic Markers of Steroid-Induced Osteonecrosis of the Femoral Head and Their Correlation with Immune Infiltration. Front. Genet. 2021, 12, 691465. [Google Scholar] [CrossRef]
- Huang, F.; Wong, P.; Li, J.; Lv, Z.; Xu, L.; Zhu, G.; He, M.; Luo, Y. Osteoimmunology, The correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J. Cell. Mol. Med. 2022, 26, 3591–3597. [Google Scholar] [CrossRef]
- Zhu, L.; Hua, F.; Ding, W.; Ding, K.; Zhang, Y.; Xu, C. The correlation between the Th17/Treg cell balance and bone health. Immun. Ageing 2020, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.E.; Lien, J.C.; Tsai, C.W.; Wu, C.R. Therapeutic Potential and Mechanisms of Novel Simple O-Substituted Isoflavones against Cerebral Ischemia Reperfusion. Int. J. Mol. Sci. 2022, 23, 10394. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.; Sosic-Jurjevic, B.; Ajdzanovic, V.; Zivanovic, J.; Manojlovic-Stojanoski, M.; Nestorovic, N.; Ristic, N.; Trifunovic, S.; Milosevic, V. The phytoestrogen genistein prevents trabecular bone loss and affects thyroid follicular cells in a male rat model of osteoporosis. J. Anat. 2018, 233, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Burnett, B.; Levy, R.; Di Stefano, V.; Armbruster, M.A.; Marini, H.; Minutoli, L.; Altavilla, D.; Squadrito, F. Protective effect of genistein aglycone on the development of osteonecrosis of the femoral head and secondary osteoporosis induced by methylprednisolone in rats. J. Endocrinol. 2009, 201, 321–328. [Google Scholar] [CrossRef]
- Staretz-Chacham, O.; Pode-Shakked, B.; Kristal, E.; Abraham, S.Y.; Porper, K.; Wormser, O.; Shelef, I.; Anikster, Y. The effects of a ketogenic diet on patients with dihydrolipoamide dehydrogenase deficiency. Nutrients 2021, 13, 3523. [Google Scholar] [CrossRef]
- Shin, D.; Lee, J.; You, J.H.; Kim, D.; Roh, J.L. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020, 30, 101418. [Google Scholar] [CrossRef]
- DeBrosse, S.D.; Okajima, K.; Zhang, S.; Nakouzi, G.; Schmotzer, C.L.; Lusk-Kopp, M.; Frohnapfel, M.B.; Grahame, G.; Kerr, D.S. Spectrum of neurological and survival outcomes in pyruvate dehydrogenase complex (PDC) deficiency: Lack of correlation with genotype. Mol. Genet. Metab. 2012, 107, 394–402. [Google Scholar] [CrossRef]
- Chen, X.; Hua, H.; Balamurugan, K.; Kong, X.; Zhang, L.; George, G.N.; Georgiev, O.; Schaffner, W.; Giedroc, D.P. Copper sensing function of Drosophila metal-responsive transcription factor-1 is mediated by a tetranuclear Cu(I) cluster. Nucleic Acids Res. 2008, 36, 3128–3138. [Google Scholar] [CrossRef]
- Tavera-Montanez, C.; Hainer, S.J.; Cangussu, D.; Gordon, S.J.V.; Xiao, Y.; Reyes-Gutierrez, P.; Imbalzano, A.N.; Navea, J.G.; Fazzio, T.G.; Padilla-Benavides, T. The classic metal-sensing transcription factor MTF1 promotes myogenesis in response to copper. FASEB J. 2019, 33, 14556–14574. [Google Scholar] [CrossRef]
- Lyu, Z.; Yang, M.; Yang, T.; Ma, M.; Yang, Z. Metal-regulatory transcription factor-1 targeted by miR-148a-3p is implicated in human hepatocellular carcinoma progression. Front. Oncol. 2021, 11, 700649. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.; Li, T.; Bian, Y.; Weng, X. MicroRNA expression in bone marrow mesenchymal stem cells from mice with steroid-induced osteonecrosis of the femoral head. Mol. Med. Rep. 2015, 12, 7447–7454. [Google Scholar] [CrossRef]






| Gene | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| GAPDH | CCCATCACCATCTTCCAGG | CATCACGCCACAGTTTCCC |
| SLC2A1 | CTCATCAACCGCAACGA | AGTATGGCACAACCCGC |
| PNP | TCTCACACTAAGCACCGAC | ACCTCCTAATCCAGAACCA |
| LIPT1 | GGAGAAGAAGTGGAGGAGGA | CCGTTGGGTTTATTAGGTGA |
| DLD | GCCGACGACCCTTTACTA | GCCTTCATCCTCTGCTTT |
| MTF1 | TAATAATCCCACAATAACCAT | TAAAAAACACCTTCTCAACTT |
| PDHB | TGGAAAAGCCAAAATAGAAAG | CATAAGGCATAGGGACATCAG |
| Feature Name | Feature ID | Avg Rank |
|---|---|---|
| RNASET2 | 80 | 6.6 |
| PNP | 67 | 7 |
| SLC2A1 | 87 | 9.6 |
| REXO2 | 79 | 10.2 |
| CYBA | 32 | 12 |
| SOAT1 | 89 | 12 |
| TFDP1 | 19 | 13.4 |
| LYZ | 51 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, B.; Li, C.; Cai, X.; Pu, L.; Guo, M.; Tang, Z.; Bu, P.; Xu, Y. Bioinformatics-Based Analysis of Key Genes in Steroid-Induced Osteonecrosis of the Femoral Head That Are Associated with Copper Metabolism. Biomedicines 2023, 11, 873. https://doi.org/10.3390/biomedicines11030873
Qi B, Li C, Cai X, Pu L, Guo M, Tang Z, Bu P, Xu Y. Bioinformatics-Based Analysis of Key Genes in Steroid-Induced Osteonecrosis of the Femoral Head That Are Associated with Copper Metabolism. Biomedicines. 2023; 11(3):873. https://doi.org/10.3390/biomedicines11030873
Chicago/Turabian StyleQi, Baochuang, Chuan Li, Xingbo Cai, Luqiao Pu, Minzheng Guo, Zhifang Tang, Pengfei Bu, and Yongqing Xu. 2023. "Bioinformatics-Based Analysis of Key Genes in Steroid-Induced Osteonecrosis of the Femoral Head That Are Associated with Copper Metabolism" Biomedicines 11, no. 3: 873. https://doi.org/10.3390/biomedicines11030873
APA StyleQi, B., Li, C., Cai, X., Pu, L., Guo, M., Tang, Z., Bu, P., & Xu, Y. (2023). Bioinformatics-Based Analysis of Key Genes in Steroid-Induced Osteonecrosis of the Femoral Head That Are Associated with Copper Metabolism. Biomedicines, 11(3), 873. https://doi.org/10.3390/biomedicines11030873






