Predictors and Outcomes of SGLT2 Inhibitor Discontinuation in a Real-World Population after Hospitalization for Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exclusion Criteria
2.3. Study Design and Data Collection
2.4. Study Endpoints
2.5. Statistical Analyses
3. Results
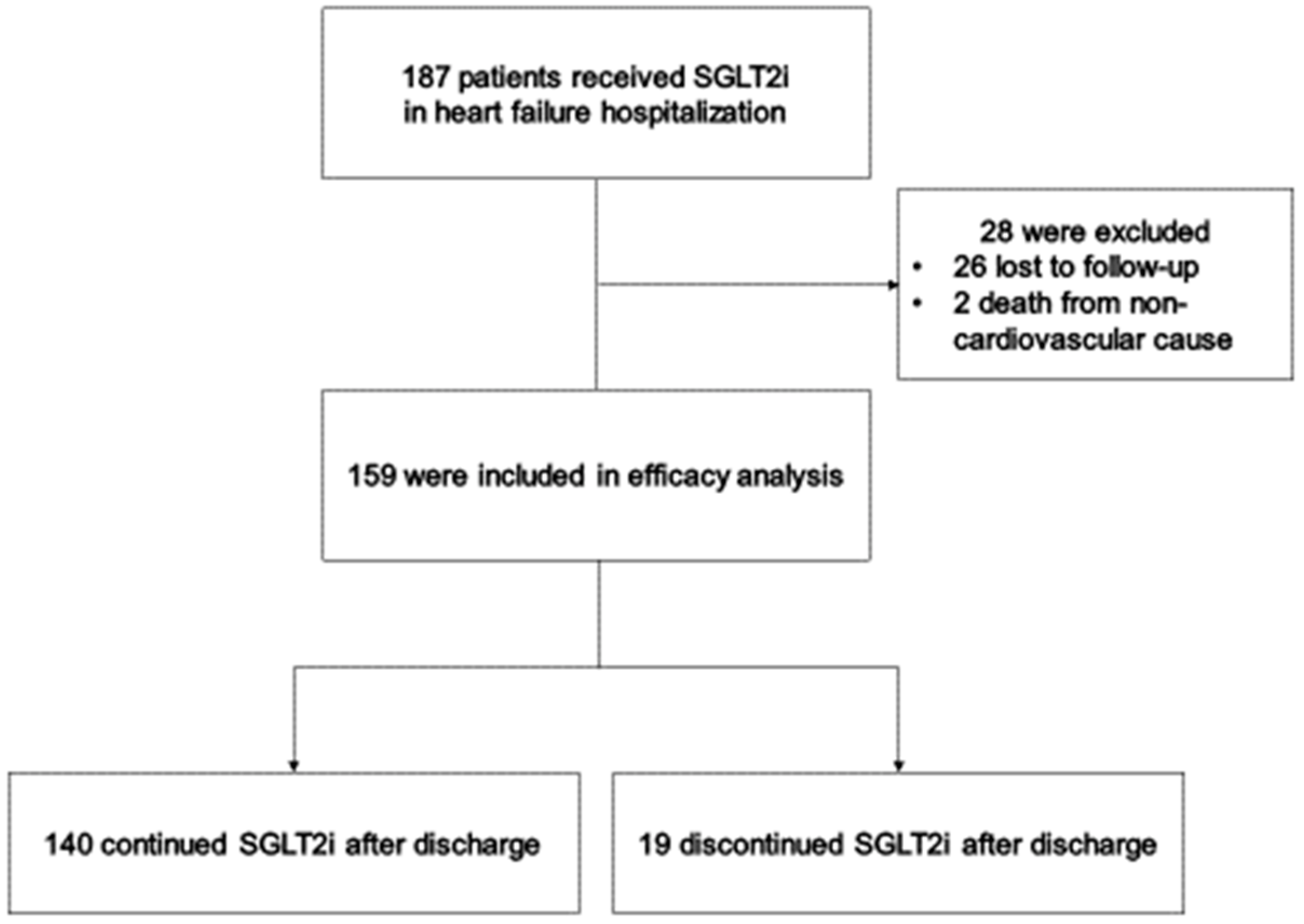
3.1. Follow-Up and Patient Characteristics
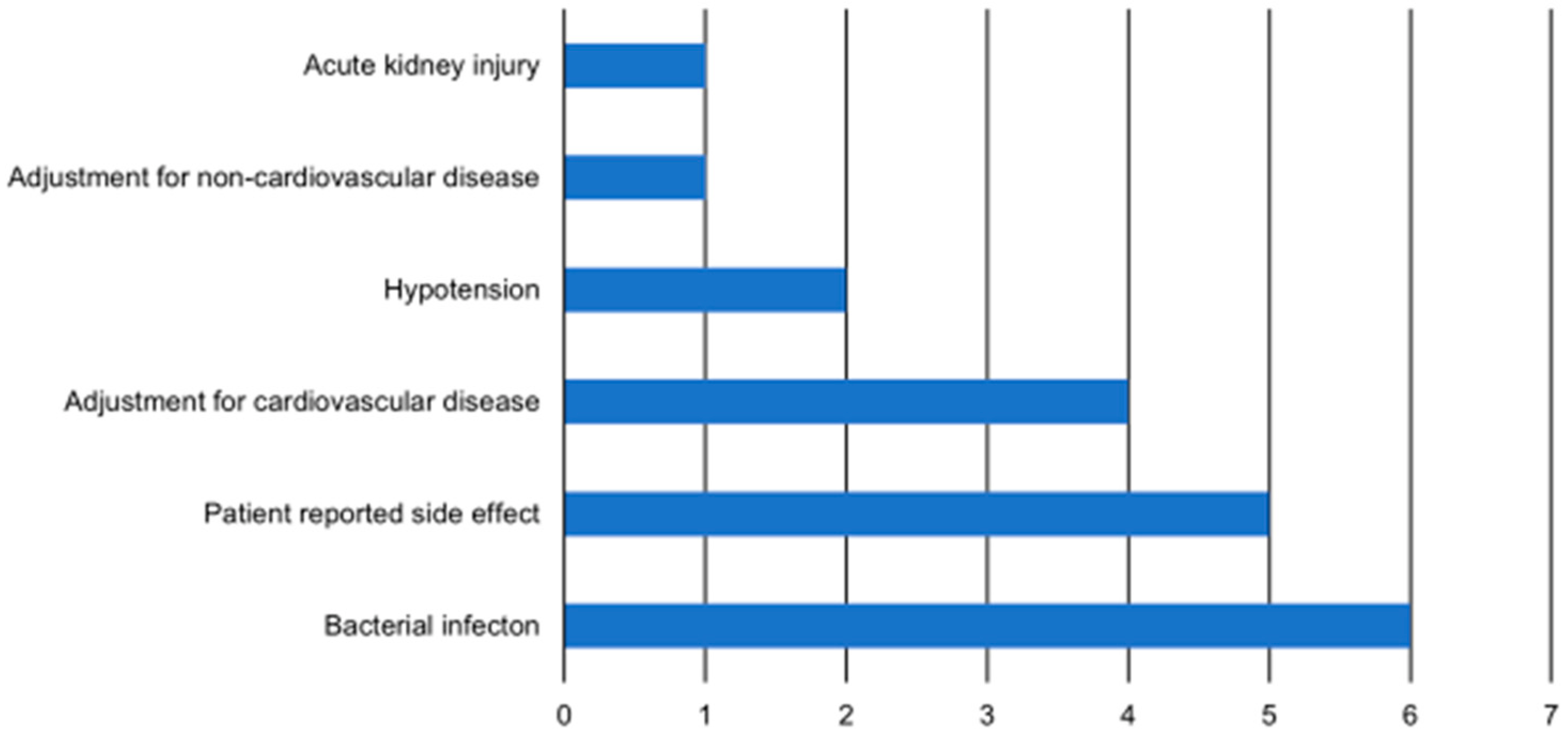
3.2. Causes of Primary Outcome
3.3. Prediction of Primary Outcome
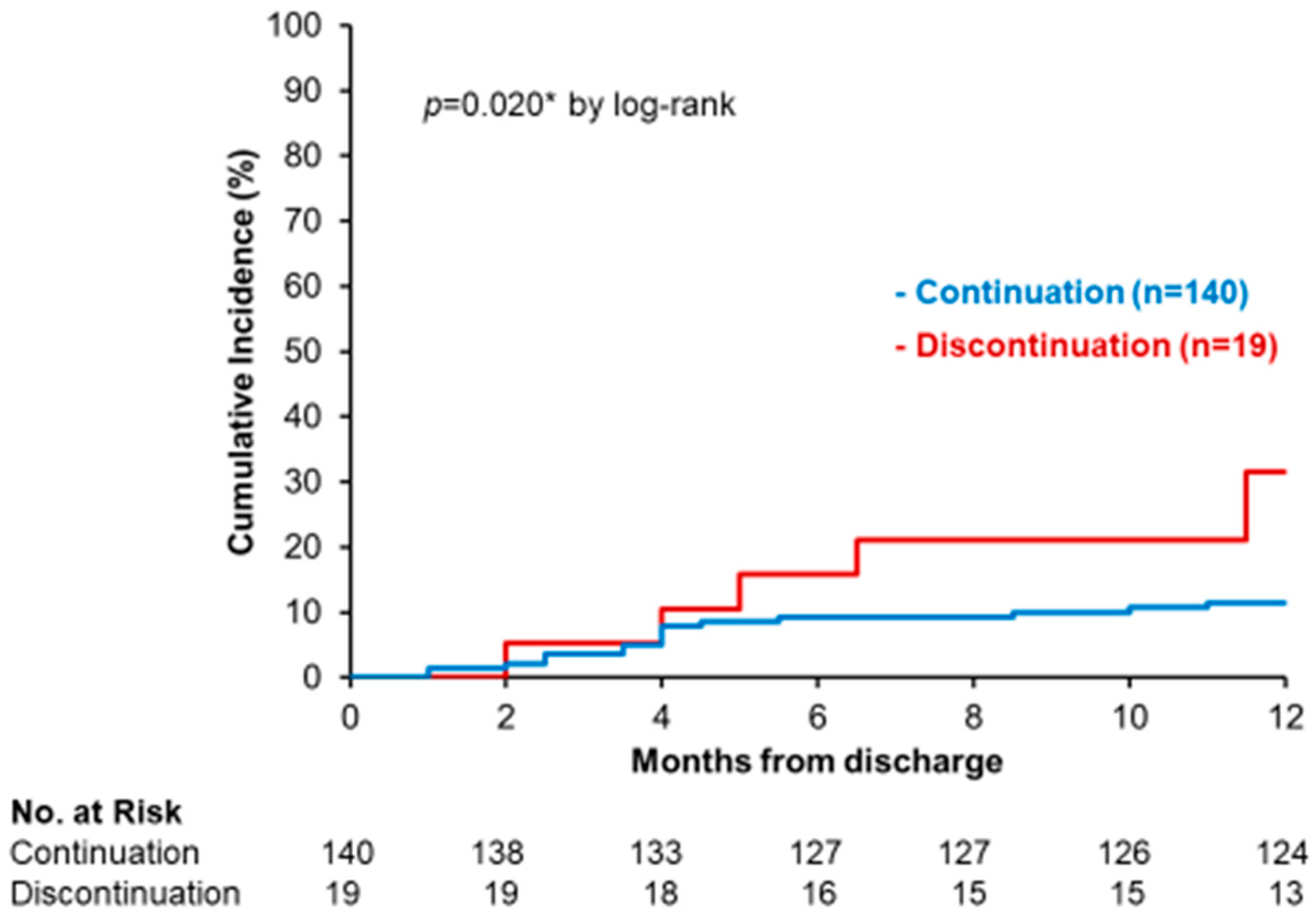
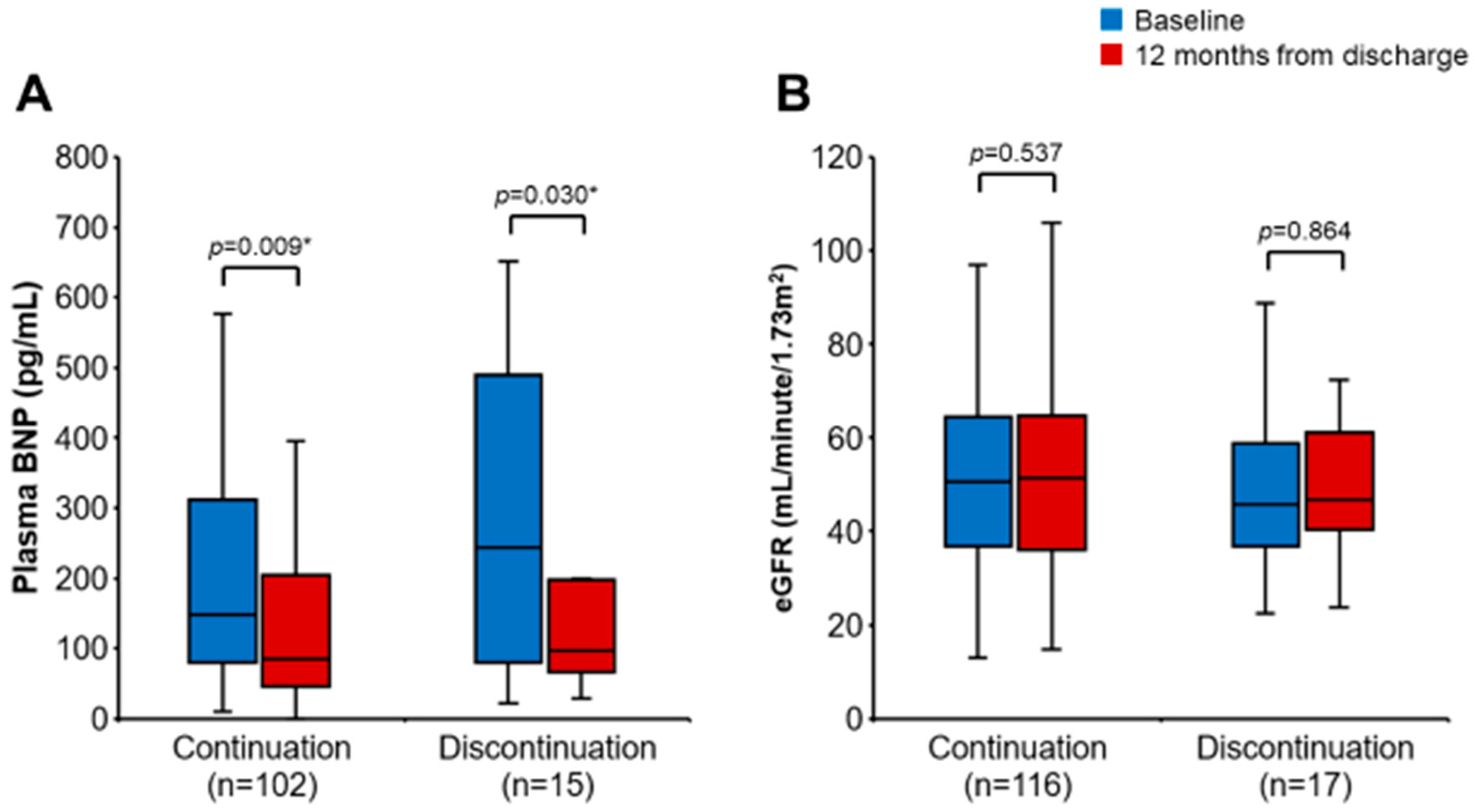
3.4. Impact of SGLT2i Discontinuation on Clinical Outcomes
4. Discussion
4.1. Discontinuation of SGLT2i
4.2. Diabetes and SGLT2i Discontinuation
4.3. Hypoalbuminemia and SGLT2i Discontinuation
4.4. High Dose of Loop Diuretics and SGLT2i Discontinuation
4.5. Outcomes of SGLT2i Discontinuation
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients with Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Nakagaito, M.; Imamura, T.; Joho, S.; Ushijima, R.; Nakamura, M.; Kinugawa, K. Factors Associated with Recurrent Heart Failure during Incorporating SGLT2 Inhibitors in Patients Hospitalized for Acute Decompensated Heart Failure. J. Clin. Med. 2022, 11, 5027. [Google Scholar] [CrossRef]
- Nakagaito, M.; Imamura, T.; Joho, S.; Ushijima, R.; Nakamura, M.; Kinugawa, K. Efficacy of Continuing SGLT2 Inhibitors on Outcomes in Patients with Acute Decompensated Heart Failure. Int. Heart J. 2021, 62, 885–890. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef]
- Rong, X.; Zhu, Y.; Wen, B.; Liu, K.; Li, X.; Gou, Q.; Chen, X. Risk of hypovolemia associated with sodium-glucose cotransporter-2 inhibitors treatment: A meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 973129. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Wang, Y.; Qin, X.; Deng, K.; Liu, Y.; Zou, K.; Sun, X. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2020, 22, 1619–1627. [Google Scholar] [CrossRef]
- Adimadhyam, S.; Lee, T.A.; Calip, G.S.; Smith Marsh, D.E.; Layden, B.T.; Schumock, G.T. Sodium-glucose co-transporter 2 inhibitors and the risk of fractures: A propensity score-matched cohort study. Pharmacoepidemiol. Drug Saf. 2019, 28, 1629–1639. [Google Scholar] [CrossRef]
- Khouri, C.; Cracowski, J.L.; Roustit, M. SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect? Diabetes Obes. Metab. 2018, 20, 1531–1534. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; Kulac, I.J.; Desai, A.S.; Jhund, P.S.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; et al. Dapagliflozin in Patients Recently Hospitalized wth Heart Failure and Mildly Reduced or Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2022, 80, 1302–1310. [Google Scholar] [CrossRef]
- Gopal, D.M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Tang, W.W.; Methvin, A.; Smith, A.L.; Bauer, D.C.; Newman, A.B.; Kim, L.; Harris, T.B.; et al. Serum albumin concentration and heart failure risk the Health, Aging, and Body Composition Study. Am. Heart J. 2010, 160, 279–285. [Google Scholar] [CrossRef]
- Uthamalingam, S.; Kandala, J.; Daley, M.; Patvardhan, E.; Capodilupo, R.; Moore, S.A.; Januzzi, J.L., Jr. Serum albumin and mortality in acutely decompensated heart failure. Am. Heart J. 2010, 160, 1149–1155. [Google Scholar] [CrossRef]
- Hasselblad, V.; Gattis Stough, W.; Shah, M.R.; Lokhnygina, Y.; O’Connor, C.M.; Califf, R.M.; Adams, K.F., Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the ESCAPE trial. Eur. J. Heart Fail. 2007, 9, 1064–1069. [Google Scholar] [CrossRef]
- Butt, J.H.; Dewan, M.P.; Merkely, B.; Belohlávek, J.; Drożdż, J.; Kitakaze, M.; Inzucchi, S.E.; Kosiborod, M.N.; Martinez, F.A.; Tereshchenko, S.; et al. Efficacy and Safety of Dapagliflozin According to Frailty in Heart Failure with Reduced Ejection Fraction: A Post Hoc Analysis of the DAPA-HF Trial. Ann. Intern. Med. 2022, 175, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; Jhund, P.S.; Belohlávek, J.; de Boer, R.A.; Chiang, C.-E.; Desai, A.S.; Drożdzż, J.; Hernandez, A.F.; Inzucchi, S.E.; Katova, T.; et al. Efficacy and Safety of Dapagliflozin According to Frailty in Patients with Heart Failure: A Prespecified Analysis of the DELIVER Trial. Circulation 2022, 146, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Rao, V.S.; Ivey-Miranda, J.; Fleming, J.; Mahoney, D.; Maulion, C.; Suda, N.; Siwakoti, K.; Ahmad, T.; Jacoby, D.; et al. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation 2020, 142, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kaneko, H.; Okada, A.; Itoh, H.; Matsuoka, S.; Fujiu, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Comparison of cardiovascular outcomes between SGLT2 inhibitors in diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 67. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 159) | Continuation (n = 140) | Discontinuation (n = 19) | p Value | |
|---|---|---|---|---|
| Age, years | 73 (64–81) | 72 (63–81) | 75 (69–83) | 0.281 |
| Male, n (%) | 102 (64) | 93 (66) | 9 (47) | 0.104 |
| Body weight, kg | 57.3 (50.0–66.8) | 57.3 (50.2–67.5) | 58.7 (47.0–64.1) | 0.547 |
| Body mass index, kg/m2 | 22.6 (19.8–24.9) | 22.6 (19.9–24.9) | 22.2 (19.8–23.7) | 0.720 |
| Systolic blood pressure, mmHg | 105 (96–117) | 104 (95–117) | 108 (98–118) | 0.227 |
| Heart rate, beats per minutes | 70 (63–78) | 70 (63–78) | 71 (65–78) | 0.655 |
| Diabetes mellitus, n (%) | 123 (77) | 105 (75) | 18 (95) | 0.054 |
| Ischemic etiology, n (%) | 66 (42) | 58 (41) | 8 (42) | 0.955 |
| Atrial fibrillation, n (%) | 45 (28) | 37 (26) | 8 (42) | 0.155 |
| Implantable cardioverter-defibrillator, n (%) | 23 (13) | 22 (16) | 1 (5) | 0.224 |
| Cardiac resynchronization therapy, n (%) | 16 (10) | 2 (11) | 14 (10) | 0.943 |
| New York Heart Association class III–IV, n (%) | 36 (23) | 29 (21) | 7 (37) | 0.115 |
| Left ventricular ejection fraction, % | 43 (33–55) | 43 (33–55) | 43 (31–60) | 0.744 |
| Value of <40% (HFrEF), n (%) | 62 (39) | 54 (39) | 8 (42) | 0.767 |
| Value of 40–49% (HFmrEF), n (%) | 42 (26) | 37 (26) | 5 (26) | 0.992 |
| Value of ≥50% (HFpEF), n (%) | 55 (35) | 49 (35) | 6 (32) | 0.769 |
| HbA1c, % | 6.7 (6.4–7.6) | 6.7 (6.3–7.6) | 6.9 (6.5–7.6) | 0.404 |
| Fasting blood sugar, mg/dL | 110 (95–132) | 110 (95–130) | 121 (93–152) | 0.230 |
| Hemoglobin, g/dL | 12.5 (11.2–13.9) | 12.6 (11.2–14.1) | 11.9 (10.6–13.5) | 0.089 |
| Hematocrit, % | 37.8 (34.1–41.4) | 38.0 (34.2–41.6) | 35.5 (32.2–40.6) | 0.135 |
| Serum albumin, g/dL | 3.6 (3.4–3.9) | 3.7 (3.4–4.0) | 3.4 (3.2–3.6) | 0.006 * |
| Serum sodium, mEq/L | 138 (136–140) | 138 (137–140) | 136 (135–140) | 0.258 |
| Serum potassium, mEq/L | 4.3 (4.1–4.6) | 4.3 (4.1–4.6) | 4.5 (3.9–4.8) | 0.641 |
| eGFR, mL/minute/1.73 m2 | 50.5 (36.9–64.4) | 51.2 (36.9–66.6) | 41.2 (36.9–58.8) | 0.342 |
| Uric acid, mg/dL | 5.7 (4.8–6.9) | 5.7 (4.8–7.0) | 6.0 (5.6–6.7) | 0.418 |
| Plasma BNP, pg/mL | 142 (69–284) | 142 (69–269) | 182 (68–477) | 0.237 |
| Heart failure therapies | ||||
| Beta-blockers, n (%) | 142 (89) | 124 (89) | 18 (95) | 0.415 |
| ACEI/ARB/ARNI, n (%) | 149 (94) | 130 (93) | 19 (100) | 0.229 |
| Loop diuretics, n (%) | 102 (64) | 88 (63) | 14 (74) | 0.356 |
| Dose of furosemide, mg/day | 10 (0–20) | 10 (0–20) | 20 (0–20) | 0.223 |
| MRA, n (%) | 54 (34) | 44 (31) | 10 (53) | 0.067 |
| Thiazides, n (%) | 3 (2) | 3 (2) | 0 (0) | 0.520 |
| Anti-diabetic agents | ||||
| Sulfonylureas, n (%) | 6 (4) | 6 (4) | 0 (0) | 0.358 |
| DPP-4i, n (%) | 62 (39) | 53 (38) | 9 (47) | 0.425 |
| Biguanides, n (%) | 22 (14) | 21 (15) | 1 (5) | 0.249 |
| Insulin, n (%) | 13 (8) | 10 (7) | 3 (16) | 0.197 |
| Sodium–glucose cotransporter 2 inhibitors | ||||
| Canagliflozin, n (%) | 37 (23) | 32 (23) | 5 (26) | 0.738 |
| Dapagliflozin, n (%) | 82 (52) | 75 (54) | 7 (37) | 0.171 |
| Empagliflozin, n (%) | 40 (25) | 33 (24) | 7 (37) | 0.211 |
| All Patients (n = 159) | ||||||
|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | |||||
| Variables | Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value |
| Age, years | 1.03 | 0.98–1.08 | 0.224 | |||
| Male, yes | 0.46 | 0.17–1.20 | 0.110 | |||
| Body mass index, kg/m2 | 0.97 | 0.86–1.10 | 0.657 | |||
| Systolic blood pressure, mmHg | 1.02 | 0.99–1.05 | 0.136 | |||
| Heart rate, bpm | 1.01 | 0.97–1.06 | 0.578 | |||
| Ischemic etiology, yes | 1.03 | 0.39–2.71 | 0.955 | |||
| Atrial fibrillation, yes | 2.03 | 0.73–5.40 | 0.161 | |||
| NYHA class III–IV, n (%) | 2.23 | 0.81–6.18 | 0.122 | |||
| HFrEF, yes | 1.16 | 0.44–3.06 | 0.767 | |||
| Diabetes mellitus, yes | 6.00 | 0.77–46.59 | 0.087 | |||
| Fasting blood sugar, mg/dL | 1.01 | 1.00–1.02 | 0.219 | |||
| Hematocrit, % | 0.93 | 0.85–1.03 | 0.157 | |||
| Serum albumin, g/dL | 0.19 | 0.06–0.62 | 0.006 * | 0.23 | 0.07–0.76 | 0.016 * |
| Serum sodium, mEq/L | 0.96 | 0.84–1.10 | 0.551 | |||
| Serum potassium, mEq/L | 1.24 | 0.47–3.29 | 0.670 | |||
| eGFR, mL/min/1.73 m2 | 0.99 | 0.97–1.02 | 0.605 | |||
| Uric acid, mg/dL | 1.07 | 0.81–1.42 | 0.638 | |||
| ln BNP | 1.41 | 0.85–2.33 | 0.187 | |||
| Beta-blockers, yes | 2.32 | 0.29–18.59 | 0.427 | |||
| ACEI/ARB/ARNI, yes | NA | NA | 0.993 | |||
| Loop diuretics, yes | 1.66 | 0.56–4.86 | 0.360 | |||
| Dose of furosemide, mg/day | 1.03 | 1.01–1.05 | 0.013 * | 1.02 | 1.00–1.05 | 0.046 * |
| MRA, yes | 2.42 | 0.92–6.39 | 0.073 | |||
| Thiazides, yes | NA | NA | 0.991 | |||
| Sulfonylureas, yes | NA | NA | 0.992 | |||
| DPP-4i, yes | 1.48 | 0.56–3.87 | 0.427 | |||
| Biguanides, yes | 0.32 | 0.04–2.49 | 0.273 | |||
| Insulin, yes | 2.44 | 0.61–9.79 | 0.209 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagaito, M.; Imamura, T.; Ushijima, R.; Nakamura, M.; Kinugawa, K. Predictors and Outcomes of SGLT2 Inhibitor Discontinuation in a Real-World Population after Hospitalization for Heart Failure. Biomedicines 2023, 11, 876. https://doi.org/10.3390/biomedicines11030876
Nakagaito M, Imamura T, Ushijima R, Nakamura M, Kinugawa K. Predictors and Outcomes of SGLT2 Inhibitor Discontinuation in a Real-World Population after Hospitalization for Heart Failure. Biomedicines. 2023; 11(3):876. https://doi.org/10.3390/biomedicines11030876
Chicago/Turabian StyleNakagaito, Masaki, Teruhiko Imamura, Ryuichi Ushijima, Makiko Nakamura, and Koichiro Kinugawa. 2023. "Predictors and Outcomes of SGLT2 Inhibitor Discontinuation in a Real-World Population after Hospitalization for Heart Failure" Biomedicines 11, no. 3: 876. https://doi.org/10.3390/biomedicines11030876
APA StyleNakagaito, M., Imamura, T., Ushijima, R., Nakamura, M., & Kinugawa, K. (2023). Predictors and Outcomes of SGLT2 Inhibitor Discontinuation in a Real-World Population after Hospitalization for Heart Failure. Biomedicines, 11(3), 876. https://doi.org/10.3390/biomedicines11030876








