The Influence of Ultra-Low Tidal Volume Ventilation during Cardiopulmonary Resuscitation on Renal and Hepatic End-Organ Damage in a Porcine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Anesthesia and Instrumentation
2.2. Intervention
2.3. Measurements/Sample Collection
2.4. Tissue Damage Scoring Systems
2.5. Statistics
3. Results
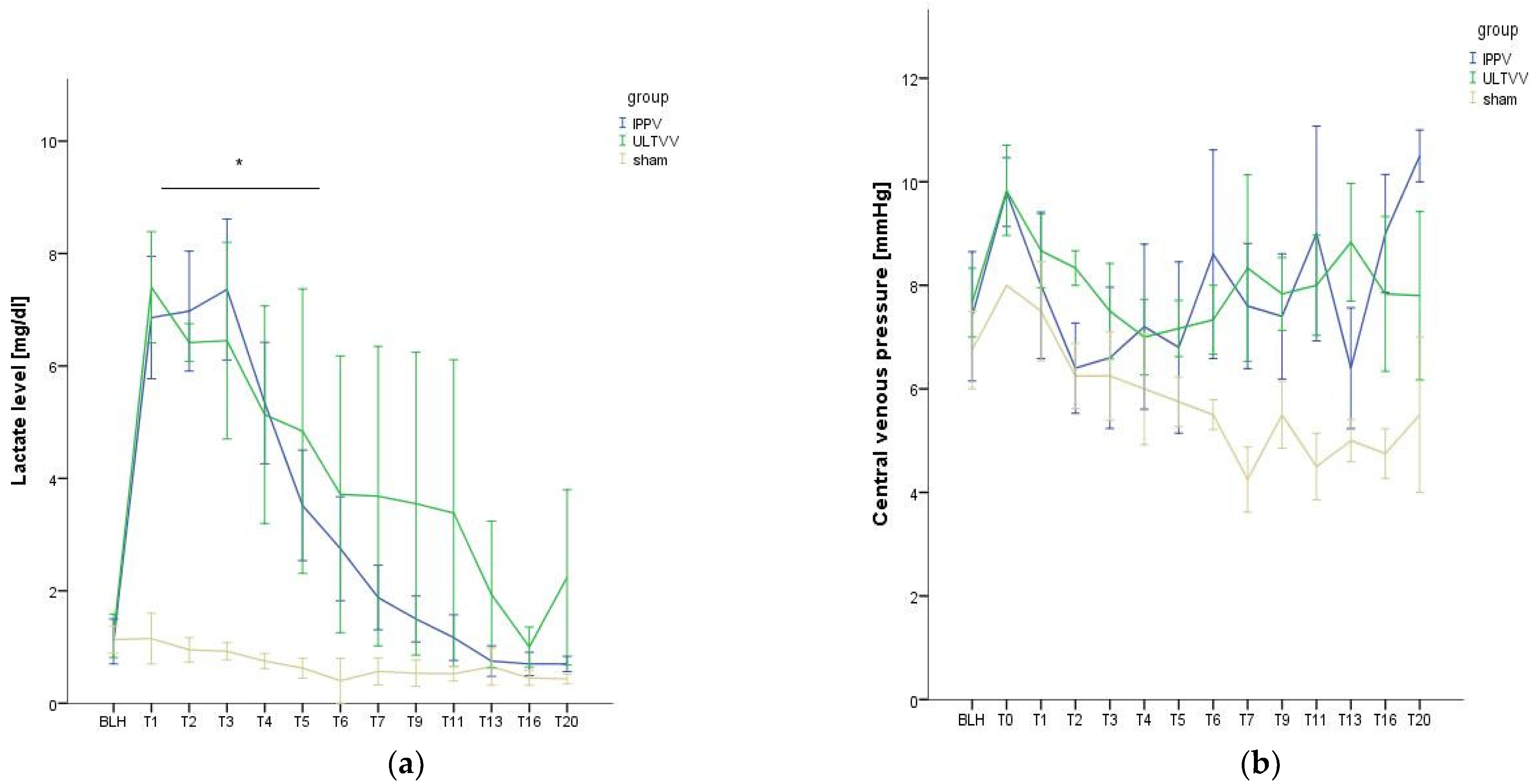
3.1. Hemodynamic Stability
3.2. Function and Histopathological Damage of the Kidney
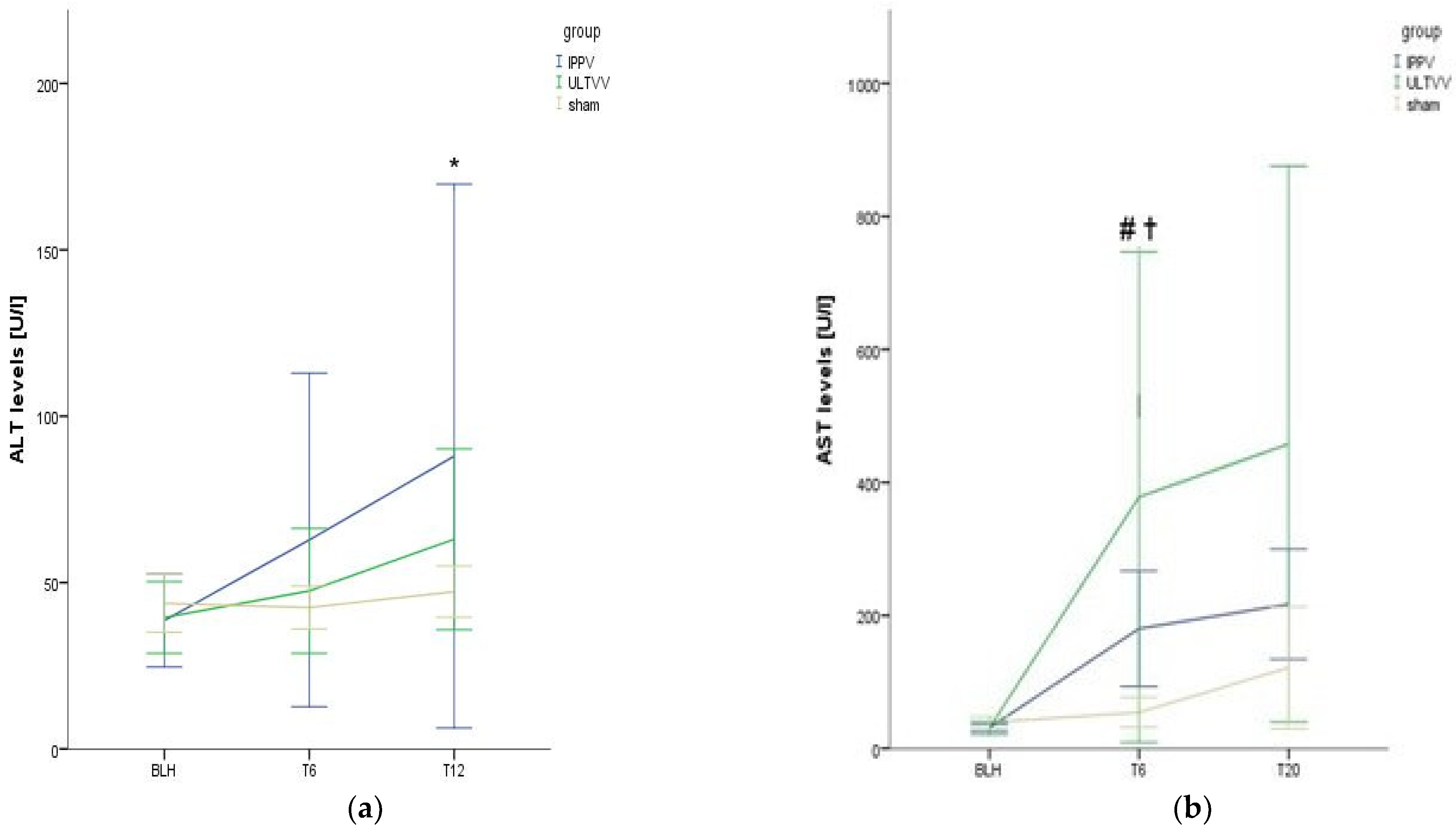
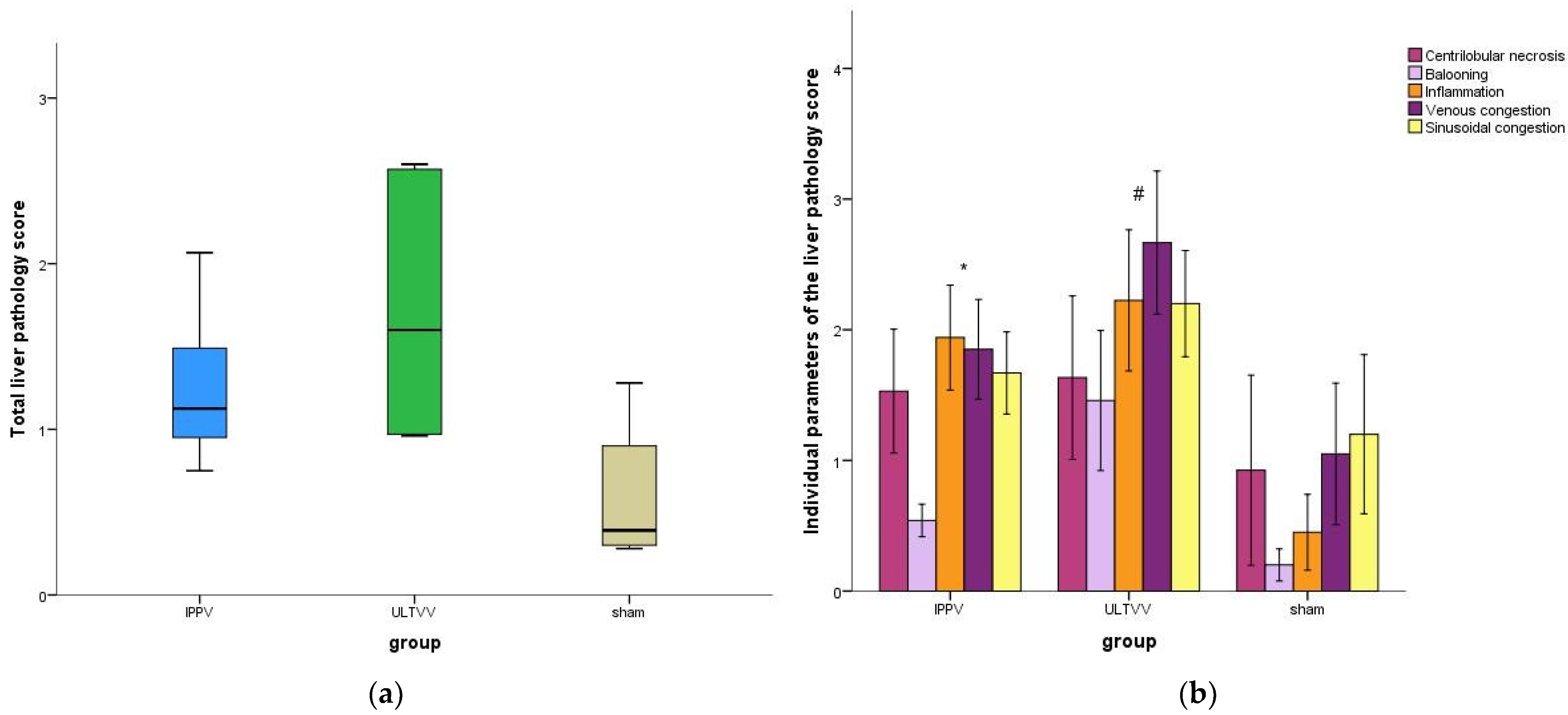
3.3. Function and Histopathological Damage of the Liver
4. Discussion
4.1. Statement of Key Findings
4.2. Explanation of the Selected Model
4.3. Connection with Previous Studies
4.4. Clinical Significance
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Cordioli, R.L.; Brochard, L.; Suppan, L.; Lyazidi, A.; Templier, F.; Khoury, A.; Delisle, S.; Savary, D.; Richard, J.C. How Ventilation Is Delivered During Cardiopulmonary Resuscitation: An International Survey. Respir. Care 2018, 63, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Ruemmler, R.; Ziebart, A.; Moellmann, C.; Garcia-Bardon, A.; Kamuf, J.; Kuropka, F.; Duenges, B.; Hartmann, E.K. Ultra-low tidal volume ventilation-A novel and effective ventilation strategy during experimental cardiopulmonary resuscitation. Resuscitation 2018, 132, 56–62. [Google Scholar] [CrossRef]
- Sandroni, C.; Dell’anna, A.M.; Tujjar, O.; Geri, G.; Cariou, A.; Taccone, F.S. Acute kidney injury after cardiac arrest: A systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016, 82, 989–999. [Google Scholar]
- Champigneulle, B.; Geri, G.; Bougouin, W.; Dumas, F.; Arnaout, M.; Zafrani, L.; Pène, F.; Charpentier, J.; Mira, J.P.; Cariou, A. Hypoxic hepatitis after out-of-hospital cardiac arrest: Incidence, determinants and prognosis. Resuscitation 2016, 103, 60–65. [Google Scholar] [CrossRef]
- Ruemmler, R.; Ziebart, A.; Garcia-Bardon, A.; Kamuf, J.; Hartmann, E.K. Standardized Model of Ventricular Fibrillation and Advanced Cardiac Life Support in Swine. J. Vis. Exp. 2020, 155. [Google Scholar] [CrossRef]
- Brower, R.G.; Fessler, H.E. Mechanical ventilation in acute lung injury and acute respiratory distress syndrome. Clin. Chest Med. 2000, 21, 491–510. [Google Scholar] [CrossRef]
- Khalid, U.; Pino-Chavez, G.; Nesargikar, P.; Jenkins, R.; Bowen, T.; Fraser, D.; Chávez, R. Kidney ischaemia reperfusion injury in the rat: The EGTI scoring system as a valid and reliable tool for histological assessment. J. Histol. Histopathol. 2016, 3, 1. [Google Scholar] [CrossRef]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Standish, R.A.; Cholongitas, E.; Dhillon, A.; Burroughs, A.K.; Dhillon, A.P. An appraisal of the histopathological assessment of liver fibrosis. Gut 2006, 55, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, A.M. The microcirculatory acinar concept of normal and pathological hepatic structure. Beitr. Pathol. 1976, 157, 215–243. [Google Scholar] [CrossRef]
- Kirch, W. (Ed.) Pearson’s Correlation Coefficient. In Encyclopedia of Public Health; Springer: Dordrecht, The Netherlands, 2008; pp. 1090–1091. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Hotelling, H., Eds.; Stanford University Press: Redwood City, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Bonferroni, C.E. Teoria Statistica delle Classi e Calcolo delle Probabilità; Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze: Firenze, Italy, 1936. [Google Scholar]
- Niemann, J.T.; Rosborough, J.P.; Youngquist, S.; Thomas, J.; Lewis, R.J. Is all ventricular fibrillation the same? A comparison of ischemically induced with electrically induced ventricular fibrillation in a porcine cardiac arrest and resuscitation model. Crit. Care Med. 2007, 35, 1356–1361. [Google Scholar] [CrossRef]
- Cherry, B.H.; Nguyen, A.Q.; Hollrah, R.A.; Olivencia-Yurvati, A.H.; Mallet, R.T. Modeling cardiac arrest and resuscitation in the domestic pig. World J. Crit. Care Med. 2015, 4, 1–12. [Google Scholar] [CrossRef]
- García-Bardon, A.; Kamuf, J.; Ziebart, A.; Liu, T.; Krebs, N.; Dünges, B.; Kelm, R.F.; Morsbach, S.; Mohr, K.; Heimann, A.; et al. Levosimendan increases brain tissue oxygen levels after cardiopulmonary resuscitation independent of cardiac function and cerebral perfusion. Sci. Rep. 2021, 11, 14220. [Google Scholar] [CrossRef] [PubMed]
- Renz, M.; Müllejans, L.; Riedel, J.; Mohnke, K.; Rissel, R.; Ziebart, A.; Duenges, B.; Hartmann, E.K.; Ruemmler, R. High PEEP Levels during CPR Improve Ventilation without Deleterious Haemodynamic Effects in Pigs. J. Clin. Med. 2022, 11, 4921. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Hishikawa, S.; Teratani, T.; Lefor, A.T. The pig as a model for translational research: Overview of porcine animal models at Jichi Medical University. Transplant. Res. 2012, 1, 8. [Google Scholar] [CrossRef]
- Judge, E.P.; Hughes, J.M.; Egan, J.J.; Maguire, M.; Molloy, E.L.; O’Dea, S. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am. J. Respir. Cell Mol. Biol. 2014, 51, 334–343. [Google Scholar] [CrossRef]
- Meybohm, P.; Cavus, E.; Dörges, V.; Steinfath, M.; Sibbert, L.; Wenzel, V.; Scholz, J.; Bein, B. Revised resuscitation guidelines: Adrenaline versus adrenaline/vasopressin in a pig model of cardiopulmonary resuscitation—A randomised, controlled trial. Resuscitation 2007, 75, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Papathanassoglou, E.; Xanthos, T. Systematic review of the mechanisms driving effective blood flow during adult CPR. Resuscitation 2014, 85, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Li, H.; Zhai, X.; Ding, Y.; Zhou, H.; Ouyang, Z.; Yang, Z.; Jiang, L.; Tang, W.; Yu, T. Establishment of porcine model of prolonged cardiac arrest and cardiopulmonary resuscitation electrically induced by ventricular fibrillation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 536–541. [Google Scholar] [CrossRef]
- Rittenberger, J.C.; Menegazzi, J.J.; Callaway, C.W. Association of delay to first intervention with return of spontaneous circulation in a swine model of cardiac arrest. Resuscitation 2007, 73, 154–160. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Bedja, D.; Thoburn, C.; Gabrielson, K.; Racusen, L.; Rabb, H. Acute renal venous obstruction is more detrimental to the kidney than arterial occlusion: Implication for murine models of acute kidney injury. Am. J. Physiol.-Ren. Physiol. 2012, 302, F519–F525. [Google Scholar] [CrossRef]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Böttiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008, 79, 350–379. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Adib-Conquy, M.; Laurent, I.; Monchi, M.; Vinsonneau, C.; Fitting, C.; Fraisse, F.; Dinh-Xuan, A.T.; Carli, P.; Spaulding, C.; et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 2002, 106, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Burne-Taney, M.J.; Kofler, J.; Yokota, N.; Weisfeldt, M.; Traystman, R.J.; Rabb, H. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am. J. Physiol.-Ren. Physiol. 2003, 285, F87–F94. [Google Scholar] [CrossRef]
- Hasper, D.; von Haehling, S.; Storm, C.; Jörres, A.; Schefold, J.C. Changes in serum creatinine in the first 24 hours after cardiac arrest indicate prognosis: An observational cohort study. Crit. Care 2009, 13, R168. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Wu, Z.J.; Zheng, J.H.; Qin, T.; Yang, Y.G.; Chen, M.H. The incidence of acute kidney injury following cardiac arrest and cardiopulmonary resuscitation in a rat model. Ren. Fail. 2019, 41, 278–283. [Google Scholar] [CrossRef]
- Tsivilika, M.; Kavvadas, D.; Karachrysafi, S.; Kotzampassi, K.; Grosomanidis, V.; Doumaki, E.; Meditskou, S.; Sioga, A.; Papamitsou, T. Renal Injuries after Cardiac Arrest: A Morphological Ultrastructural Study. Int. J. Mol. Sci. 2022, 23, 6147. [Google Scholar] [CrossRef]
- Iesu, E.; Franchi, F.; Zama Cavicchi, F.; Pozzebon, S.; Fontana, V.; Mendoza, M.; Nobile, L.; Scolletta, S.; Vincent, J.L.; Creteur, J.; et al. Acute liver dysfunction after cardiac arrest. PLoS ONE 2018, 13, e0206655. [Google Scholar] [CrossRef]
- Roedl, K.; Spiel, A.O.; Nürnberger, A.; Horvatits, T.; Drolz, A.; Hubner, P.; Warenits, A.M.; Sterz, F.; Herkner, H.; Fuhrmann, V. Hypoxic liver injury after in- and out-of-hospital cardiac arrest: Risk factors and neurological outcome. Resuscitation 2019, 137, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ruemmler, R.; Stein, J.; Duenges, B.; Renz, M.; Hartmann, E.K. Standardized post-resuscitation damage assessment of two mechanical chest compression devices: A prospective randomized large animal trial. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 79. [Google Scholar] [CrossRef]
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care 2020, 24, 61. [Google Scholar] [CrossRef]
- Gräsner, J.T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after out-of-hospital cardiac arrest in Europe—Results of the EuReCa TWO study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef] [PubMed]


| Ventilation Mode | Tidal Volume [mL kg−1] | PEEP [mbar] | FiO2 | Respiratory Rate [Breaths min−1] | n |
|---|---|---|---|---|---|
| IPPV | 8–10 | 5 | 1.0 | 10 | 5 |
| ULTVV | 2–3 | 5 | 1.0 | 50 | 6 |
| Parameter | Group | Baseline | T6 | T20 |
|---|---|---|---|---|
| HR | IPPV | 63 ± 12 | 85 ± 17 | 84 ± 50 |
| ULTVV | 63 ± 15 | 93 ± 27 | 111 ± 59 | |
| sham | 62 ± 8 | 70 ± 18 | 65 ± 17 | |
| MAP | IPPV | 67 ± 9 | 64 ± 6 | 77 ± 14 |
| ULTVV | 73 ± 7 | 60 ± 6 | 57 ± 5 | |
| sham | 80 ± 5 | 80 ± 18 * | 101 ± 8 * | |
| CVP | IPPV | 7 ± 3 | 9 ± 5 | 11 ± 1 |
| ULTVV | 8 ± 2 | 7 ± 2 | 8 ± 4 | |
| sham | 7 ± 2 | 5 ± 1 | 5 ± 2 | |
| NA 1 | IPPV | 0 ± 0 | 0.069 ± 0.05 | 0.044 ± 0.07 |
| ULTVV | 0 ± 0 | 0.187 ± 0.21 | 0.71 ± 1.17 | |
| sham | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| CI 2 | IPPV | 3.93 ± 1.87 | 2.81 ± 0.44 | 3.85 ± 1.64 |
| ULTVV | 3.80 ± 1.13 | 3.63 ± 1.14 | 3.86 ± 1.08 | |
| sham | 3.50 ± 0.79 | 3.08 ± 0.82 | 3.97 ± 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohnke, K.; Buschmann, V.; Baller, T.; Riedel, J.; Renz, M.; Rissel, R.; Ziebart, A.; Hartmann, E.K.; Ruemmler, R. The Influence of Ultra-Low Tidal Volume Ventilation during Cardiopulmonary Resuscitation on Renal and Hepatic End-Organ Damage in a Porcine Model. Biomedicines 2023, 11, 899. https://doi.org/10.3390/biomedicines11030899
Mohnke K, Buschmann V, Baller T, Riedel J, Renz M, Rissel R, Ziebart A, Hartmann EK, Ruemmler R. The Influence of Ultra-Low Tidal Volume Ventilation during Cardiopulmonary Resuscitation on Renal and Hepatic End-Organ Damage in a Porcine Model. Biomedicines. 2023; 11(3):899. https://doi.org/10.3390/biomedicines11030899
Chicago/Turabian StyleMohnke, Katja, Victoria Buschmann, Thomas Baller, Julian Riedel, Miriam Renz, René Rissel, Alexander Ziebart, Erik K. Hartmann, and Robert Ruemmler. 2023. "The Influence of Ultra-Low Tidal Volume Ventilation during Cardiopulmonary Resuscitation on Renal and Hepatic End-Organ Damage in a Porcine Model" Biomedicines 11, no. 3: 899. https://doi.org/10.3390/biomedicines11030899
APA StyleMohnke, K., Buschmann, V., Baller, T., Riedel, J., Renz, M., Rissel, R., Ziebart, A., Hartmann, E. K., & Ruemmler, R. (2023). The Influence of Ultra-Low Tidal Volume Ventilation during Cardiopulmonary Resuscitation on Renal and Hepatic End-Organ Damage in a Porcine Model. Biomedicines, 11(3), 899. https://doi.org/10.3390/biomedicines11030899






