Unlocking the Pragmatic Potential of Regenerative Therapies in Heart Failure with Next-Generation Treatments
Abstract
:1. Introduction
2. A Brief History of Regenerative Medicine for HF
3. Double-Blind Clinical Trials of First-Generation Cell-Based Therapies
3.1. Unfractionated BM-Derived Mononuclear Cells
3.2. Mesenchymal Stem Cells
3.3. UC-Derived MSCs
3.4. Adipose-Derived Regenerative Cells/Adipose-Derived MSCs
3.5. C-Kit-Positive Cardiac Cells
3.6. Summary of First-Generation Cell Therapies
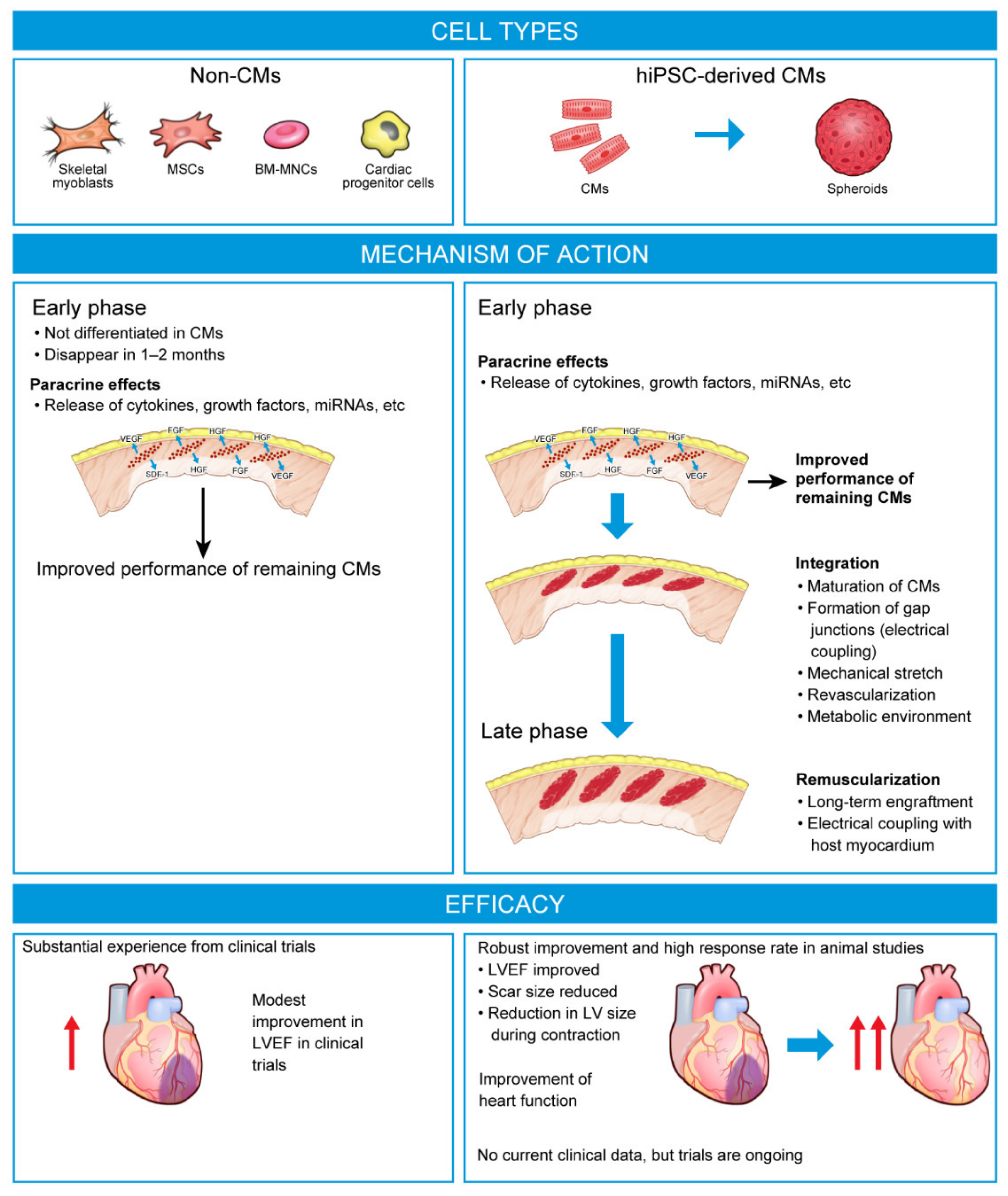
4. Next-Generation Stem Cell Therapies
- ▪
- ESCs are derived from the inner cell mass of blastocysts and can differentiate into all three embryonic germ layers.
- ▪
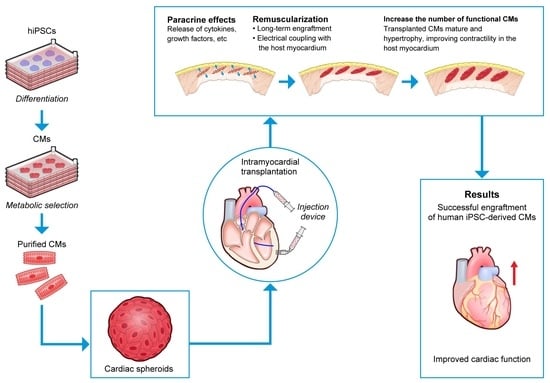
4.1. Human PSC-Derived CMs in Preclinical Models of HF
4.2. Human Pluripotent Stem Cell Cardiomyocytes in Large Animal Models of HF
5. Challenges for hPSC-Based Regenerative Therapies in HF
5.1. Teratoma Prevention
5.2. Risk Reduction of Arrhythmia after Transplantation
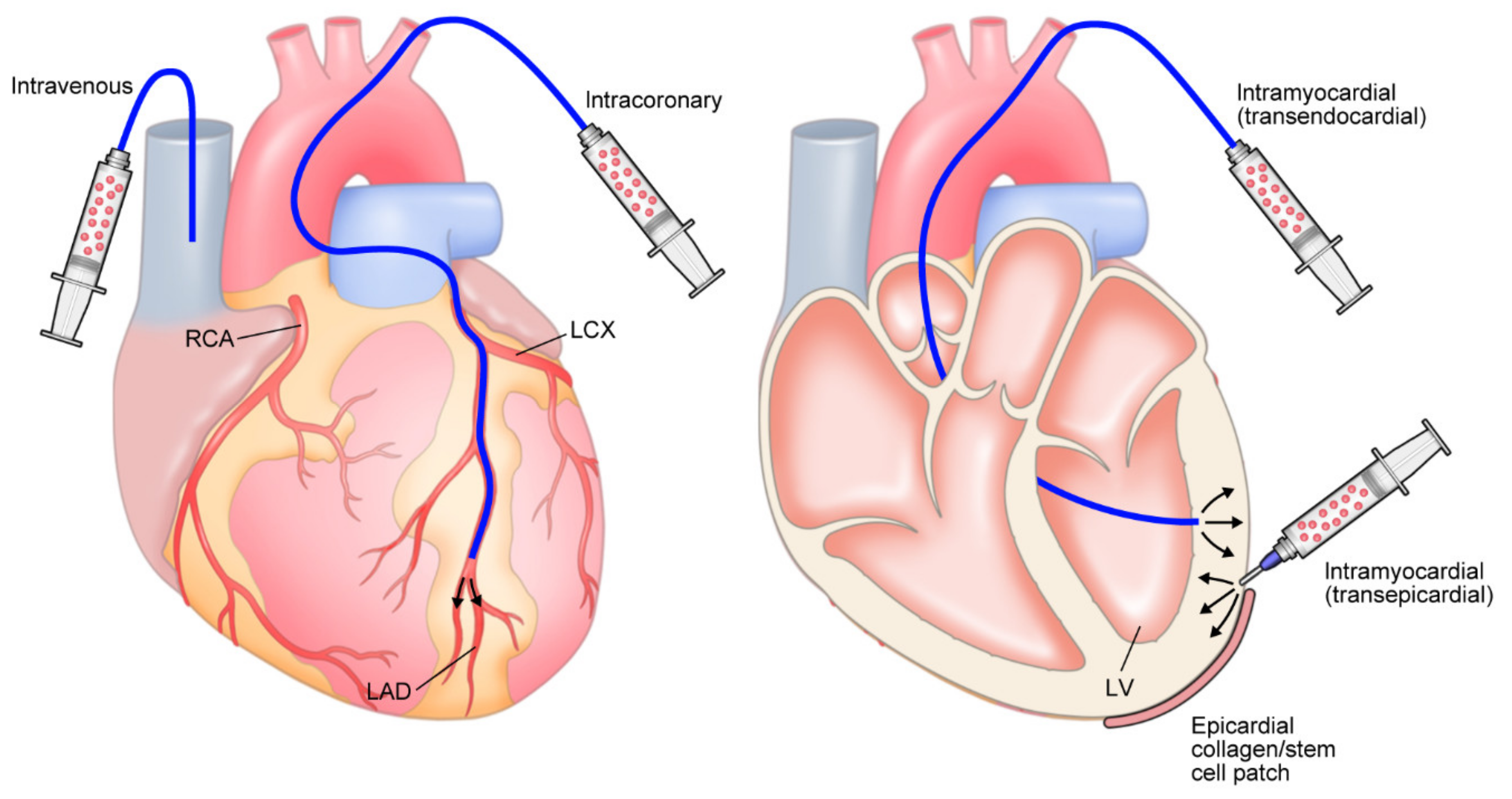
5.3. Optimizing Delivery
5.4. Further Improvement in Engraftment Rates and Longevity
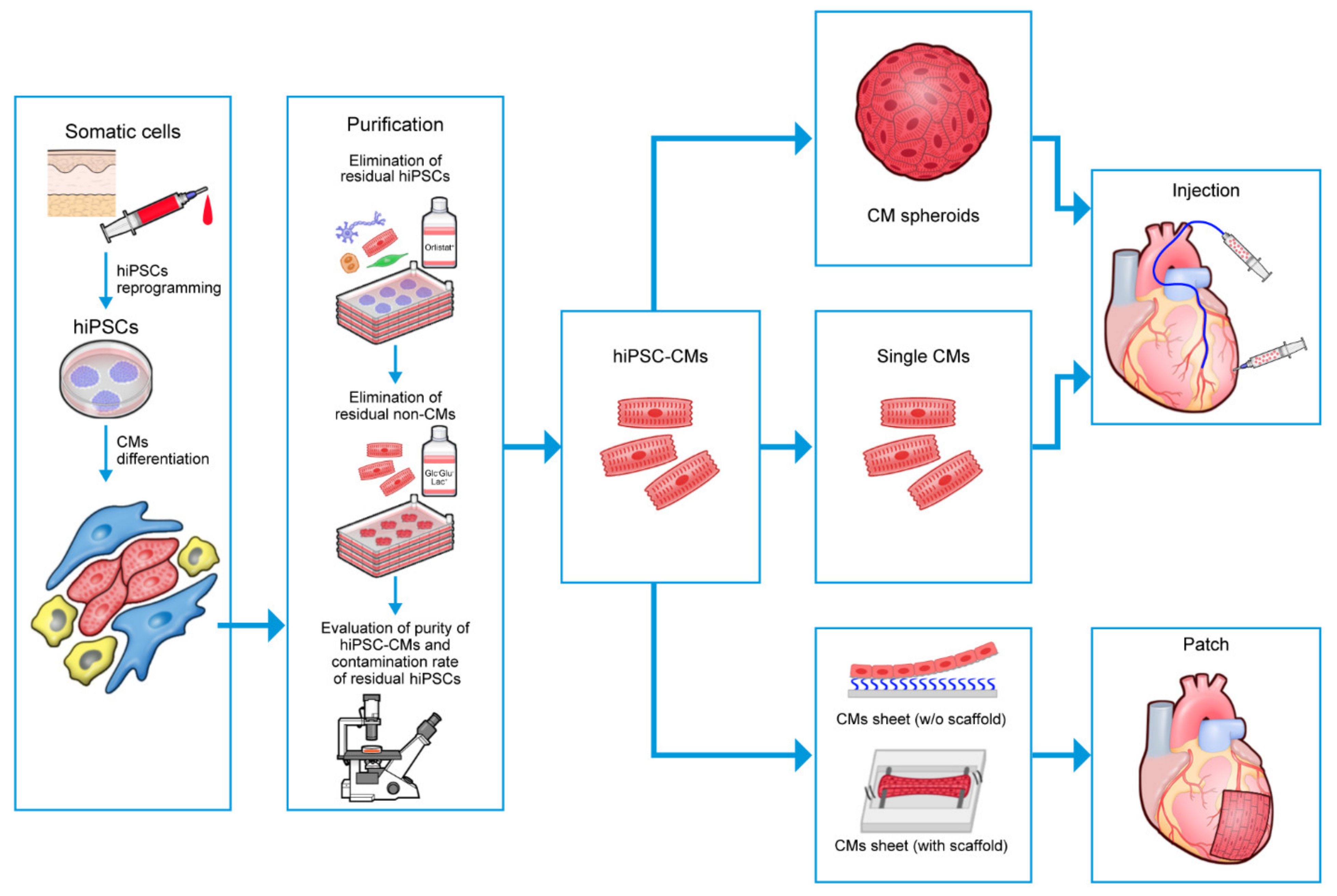
5.4.1. Cardiospheres
5.4.2. Delivering Cells via Epicardial Patches/Sheets
5.5. Economic Improvement of Production
6. Clinical Trials with hPSC-CMs
7. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef]
- Calvert, M.J.; Freemantle, N.; Cleland, J.G.F. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur. J. Heart Fail. 2005, 7, 243–251. [Google Scholar] [CrossRef]
- Mamas, M.A.; Sperrin, M.; Watson, M.C.; Coutts, A.; Wilde, K.; Burton, C.; Kadam, U.T.; Kwok, C.S.; Clark, A.B.; Murchie, P.; et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017, 19, 1095–1104. [Google Scholar] [CrossRef]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L., Jr. Heart failure with reduced ejection fraction: A review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Pastore, M.C.; Campora, A.; Lisi, M.; Mandoli, G.E. Donor shortage in heart transplantation: How can we overcome this challenge? Front. Cardiovasc. Med. 2022, 9, 1001002. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Suzuki, K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail. Rev. 2014, 20, 53–68. [Google Scholar] [CrossRef]
- Tompkins, B.A.; Balkan, W.; Winkler, J.; Gyöngyösi, M.; Goliasch, G.; Fernández-Avilés, F.; Hare, J.M. Preclinical studies of stem cell therapy for heart disease. Circ. Res. 2018, 122, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Murry, C.E. Function follows form—A review of cardiac cell therapy. Circ. J. 2019, 83, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Pan, C.-Z.; Huang, G.-Q.; Li, Y.-L.; Ge, J.-B.; Shu, X.-H. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chin. Med. J. 2005, 118, 1175–1181. [Google Scholar]
- Janssens, S.; Dubois, C.; Bogaert, J.; Theunissen, K.; Deroose, C.; Desmet, W.; Kalantzi, M.; Herbots, L.; Sinnaeve, P.; Dens, J.; et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet 2006, 367, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Rolf, A.; Erbs, S.; Elsässer, A.; Haberbosch, W.; Hambrecht, R.; Tillmanns, H.; Yu, J.; Corti, R.; Mathey, D.G.; et al. Clinical outcome 2 years after intracoronary administration of bone marrow–derived progenitor cells in acute myocardial infarction. Circ. Heart Fail. 2010, 3, 89–96. [Google Scholar] [CrossRef]
- Traverse, J.H.; McKenna, D.H.; Harvey, K.; Jorgenso, B.C.; Olson, R.E.; Bostrom, N.; Kadidlo, D.; Lesser, J.R.; Jagadeesan, V.; Garberich, R.; et al. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. Am. Heart J. 2010, 160, 428–434. [Google Scholar] [CrossRef]
- Hu, S.; Liu, S.; Zheng, Z.; Yuan, X.; Li, L.; Lu, M.; Shen, R.; Duan, F.; Zhang, X.; Li, J.; et al. Isolated coronary artery bypass graft combined with bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: A single-center, randomized, double-blind, placebo-controlled clinical trial. J. Am. Coll. Cardiol. 2011, 57, 2409–2415. [Google Scholar] [CrossRef]
- Beitnes, J.O.; Gjesdal, O.; Lunde, K.; Solheim, S.; Edvardsen, T.; Arnesen, H.; Forfang, K.; Aakhus, S. Left ventricular systolic and diastolic function improve after acute myocardial infarction treated with acute percutaneous coronary intervention, but are not influenced by intracoronary injection of autologous mononuclear bone marrow cells: A 3 year serial echocardiographic sub-study of the randomized-controlled ASTAMI study. Eur. J. Echocardiogr. 2010, 12, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Willerson, J.T.; Pepine, C.J.; Henry, T.D.; Ellis, S.G.; Zhao, D.X.M.; Silva, G.V.; Lai, D.; Thomas, J.D.; Kronenberg, M.W.; et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA 2012, 307, 1717–1726. [Google Scholar] [CrossRef]
- Wöhrle, J.; Von Scheidt, F.; Schauwecker, P.; Wiesneth, M.; Markovic, S.; Schrezenmeier, H.; Hombach, V.; Rottbauer, W.; Bernhardt, P. Impact of cell number and microvascular obstruction in patients with bone-marrow derived cell therapy: Final results from the randomized, double-blind, placebo controlled intracoronary Stem Cell therapy in patients with Acute Myocardial Infarction (SCAMI) trial. Clin. Res. Cardiol. 2013, 102, 765–770. [Google Scholar] [CrossRef]
- Wöhrle, J.; Merkle, N.; Mailänder, V.; Nusser, T.; Schauwecker, P.; von Scheidt, F.; Schwarz, K.; Bommer, M.; Wiesneth, M.; Schrezenmeier, H.; et al. Results of intracoronary stem cell therapy after acute myocardial infarction. Am. J. Cardiol. 2010, 105, 804–812. [Google Scholar] [CrossRef]
- Lu, M.; Liu, S.; Zheng, Z.; Yin, G.; Song, L.; Chen, H.; Chen, X.; Chen, Q.; Jiang, S.; Tian, L.; et al. A pilot trial of autologous bone marrow mononuclear cell transplantation through grafting artery: A sub-study focused on segmental left ventricular function recovery and scar reduction. Int. J. Cardiol. 2013, 168, 2221–2227. [Google Scholar] [CrossRef]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA 2014, 311, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Pätilä, T.; Lehtinen, M.; Vento, A.; Schildt, J.; Sinisalo, J.; Laine, M.; Hämmäinen, P.; Nihtinen, A.; Alitalo, R.; Nikkinen, P.; et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: A prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J. Heart Lung Transplant. 2014, 33, 567–574. [Google Scholar] [CrossRef]
- Hu, X.; Huang, X.; Yang, Q.; Wang, L.; Sun, J.; Zhan, H.; Lin, J.; Pu, Z.; Jiang, J.; Sun, Y.; et al. Safety and efficacy of intracoronary hypoxia-preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: The CHINA-AMI randomized controlled trial. Int. J. Cardiol. 2015, 184, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Choudry, F.; Hamshere, S.; Saunders, N.; Veerapen, J.; Bavnbek, K.; Knight, C.; Pellerin, D.; Locca, D.; Westwood, M.; Rakhit, R.; et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: The REGENERATE-AMI clinical trial. Eur. Heart J. 2015, 37, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Martino, H.; Brofman, P.; Greco, O.; Bueno, R.; Bodanese, L.; Clausell, N.; Maldonado, J.A.; Mill, J.; Braile, D.; Moraes, J., Jr.; et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). Eur. Heart J. 2015, 36, 2898–2904. [Google Scholar] [CrossRef]
- Wollert, K.C.; Meyer, G.P.; Müller-Ehmsen, J.; Tschöpe, C.; Bonarjee, V.; Larsen, A.I.; May, A.E.; Empen, K.; Chorianopoulos, E.; Tebbe, U.; et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: The BOOST-2 randomised placebo-controlled clinical trial. Eur. Heart J. 2017, 38, 2936–2943. [Google Scholar] [CrossRef]
- Seitz, A.; Wollert, K.C.; Meyer, G.P.; Müller-Ehmsen, J.; Tschöpe, C.; May, A.E.; Empen, K.; Chorianopoulos, E.; Ritter, B.; Pirr, J.; et al. Adenosine stress perfusion cardiac magnetic resonance imaging in patients undergoing intracoronary bone marrow cell transfer after ST-elevation myocardial infarction: The BOOST-2 perfusion substudy. Clin. Res. Cardiol. 2019, 109, 539–548. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Zhao, D.X.; Ellis, S.G.; Forder, J.R.; Anderson, R.D.; Hatzopoulos, A.K.; Penn, M.S.; et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The TIME randomized trial. JAMA 2012, 308, 2380–2389. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Chugh, A.; Yang, P.C.; Zhao, D.X.M.; Ellis, S.G.; Forder, J.R.; Perin, E.C.; et al. TIME trial: Effect of timing of stem cell delivery following ST-elevation myocardial infarction on the recovery of global and regional left ventricular function: Final 2-year analysis. Circ. Res. 2018, 122, 479–488. [Google Scholar] [CrossRef]
- Nicolau, J.C.; Furtado, R.H.; Silva, S.A.; Rochitte, C.E.; Rassi, A., Jr.; Moraes, J.B.M.C., Jr.; Quintella, E.; Costantini, C.R.; Korman, A.P.M.; Mattos, M.A.; et al. Stem-cell therapy in ST-segment elevation myocardial infarction with reduced ejection fraction: A multicenter, double-blind randomized trial. Clin. Cardiol. 2018, 41, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.H.; Madani, H.; Tafti, S.H.A.; Farahani, M.M.; Saleh, D.K.; Hosseinnejad, H.; Hosseini, S.; Hekmat, S.; Ahmadi, Z.H.; Dehghani, M.; et al. COMPARE CPM-RMI trial: Intramyocardial transplantation of autologous bone marrow-derived CD133+ cells and MNCs during CABG in patients with recent MI: A phase II/III, multicenter, placebo-controlled, randomized, double-blind clinical trial. Cell J. 2018, 20, 449. [Google Scholar] [CrossRef]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Fischer-Nielsen, A.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J. 2015, 36, 1744–1753. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020, 22, 884–892. [Google Scholar] [CrossRef]
- Chullikana, A.; Majumdar, A.S.; Gottipamula, S.; Krishnamurthy, S.; Kumar, A.S.; Prakash, V.; Gupta, P.K. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy 2015, 17, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT study). Circ. Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Terzic, A.; Davison, B.A.; Filippatos, G.S.; Radovanovic, S.; Beleslin, B.; Merkely, B.; Musialek, P.; Wojakowski, W.; Andreka, P.; et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: Results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 2017, 38, 648–660. [Google Scholar] [CrossRef]
- Bartunek, J.; Terzic, A.; Davison, B.A.; Behfar, A.; Sanz-Ruiz, R.; Wojakowski, W.; Sherman, W.; Heyndrickx, G.R.; Metra, M.; Filippatos, G.S.; et al. Cardiopoietic stem cell therapy in ischaemic heart failure: Long-term clinical outcomes. ESC Heart Fail. 2020, 7, 3345–3354. [Google Scholar] [CrossRef]
- Borow, K.M.; Yaroshinsky, A.; Greenberg, B.; Perin, E.C. Phase 3 DREAM-HF trial of mesenchymal precursor cells in chronic heart failure. Circ. Res. 2019, 125, 265–281. [Google Scholar] [CrossRef]
- Perin, E.C.; Greenberg, B.; Borow, K.M.; Henry, T.D.; Mendelsohn, F.O.; Miller, L.R.; Swiggum, E.; Adler, E.D.; James, C.A.; Itescu, S. Randomized trial of targeted transendocardial delivery of mesenchymal precursor cells in high-risk chronic heart failure patients with heart failure. J. Am. Coll. Cardiol. 2023, 81, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Haddad, K.; Potter, B.J.; Matteau, A.; Reeves, F.; Leclerc, G.; Rivard, A.; Gobeil, F.; Roy, D.-C.; Noiseux, N.; Mansour, S. Analysis of the COMPARE-AMI trial: First report of long-term safety of CD133+ cells. Int. J. Cardiol. 2020, 319, 32–35. [Google Scholar] [CrossRef]
- Bolli, R.; Mitrani, R.D.; Hare, J.M.; Pepine, C.J.; Perin, E.C.; Willerson, J.T.; Traverse, J.H.; Henry, T.D.; Yang, P.C.; Murphy, M.P.; et al. A phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: The CCTRN CONCERT-HF trial. Eur. J. Heart Fail. 2021, 23, 661–674. [Google Scholar] [CrossRef]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: A phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Q.; Zhao, Y.; Zhang, H.; Wang, B.; Pan, J.; Li, J.; Yu, H.; Wang, L.; Dai, J.; et al. Effect of intramyocardial grafting collagen scaffold with mesenchymal stromal cells in patients with chronic ischemic heart disease: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2016236. [Google Scholar] [CrossRef]
- Perin, E.C.; Sanz-Ruiz, R.; Sánchez, P.L.; Lasso, J.; Pérez-Cano, R.; Alonso-Farto, J.C.; Pérez-David, E.; Fernández-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE trial. Am. Heart J. 2014, 168, 88–95.e2. [Google Scholar] [CrossRef]
- Henry, T.D.; Pepine, C.J.; Lambert, C.R.; Traverse, J.H.; Schatz, R.; Costa, M.; Povsic, T.J.; Anderson, R.D.; Willerson, J.T.; Kesten, S.; et al. The Athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter. Cardiovasc. Interv. 2016, 89, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.T.; Marolleau, J.P.; Seymour, B.; Larghero, J.; et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef]
- Povsic, T.J.; O’Connor, C.M.; Henry, T.; Taussig, A.; Kereiakes, D.J.; Fortuin, F.D.; Niederman, A.; Schatz, R.; Spencer, R., 4th; Owens, D.; et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am. Heart J. 2011, 162, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Kereiakes, D.J.; Aguirre, F.; Kowalchuk, G.; Chakravarty, T.; Malliaras, K.; Francis, G.S.; Povsic, T.J.; Schatz, R.; Traverse, J.H.; et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. Eur. Heart J. 2020, 41, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Bogaert, J.; Plasencia, A.C.; Gilaberte, I.; Belmans, A.; Fernández-Santos, M.E.; Charron, D.; Mulet, M.; Yotti, R.; et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST-segment elevation myocardial infarction and left ventricular dysfunction. Circ. Res. 2018, 123, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov (NCT02438306). CardiAMP™ Cell Therapy Heart Failure Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT02438306?term=NCT02438306 (accessed on 22 November 2022).
- Raval, A.N.; Johnston, P.V.; Duckers, H.J.; Cook, T.D.; Traverse, J.H.; Altman, P.A.; Dhingra, R.; Hematti, P.; Borrello, I.; Anderson, R.D.; et al. Point of care, bone marrow mononuclear cell therapy in ischemic heart failure patients personalized for cell potency: 12-month feasibility results from CardiAMP heart failure roll-in cohort. Int. J. Cardiol. 2020, 326, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.V.; Anderson, R.D.; Raval, A.N.; Holmes-Higgin, D.; Pepine, C.J. Autologous cell therapy for HFrEF: Efficacy outcomes at two years for the roll-in cohort of a phase III pivotal trial. In Proceedings of the Heart Failure Society of America Annual Meeting, Washington, DC, USA, 30 September–3 October 2022. [Google Scholar]
- Paitazoglou, C.; Bergmann, M.W.; Vrtovec, B.; Chamuleau, S.A.J.; van Klarenbosch, B.; Wojakowski, W.; Michalewska-Włudarczyk, A.; Gyöngyösi, M.; Ekblond, A.; Haack-Sørensen, M.; et al. Rationale and design of the European multicentre study on Stem Cell therapy in IschEmic Non-treatable Cardiac diseasE (SCIENCE). Eur. J. Heart Fail. 2019, 21, 1032–1041. [Google Scholar] [CrossRef]
- Clinicaltrials.gov (NCT03092284). Allogeneic Stem Cell Therapy in Heart Failure (CSCC_ASCII). Available online: https://clinicaltrials.gov/ct2/show/NCT03092284 (accessed on 22 November 2022).
- Perin, E.C.; Dohmann, H.F.R.; Borojevic, R.; Silva, S.A.; Sousa, A.L.S.; Mesquita, C.T.; Rossi, M.I.D.; de Carvalho, A.C.; Dutra, H.S.; Dohmann, H.J.F.; et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003, 107, 2294–2302. [Google Scholar] [CrossRef]
- Perin, E.C.; Dohmann, H.F.R.; Borojevic, R.; Silva, S.A.; Sousa, A.L.S.; Silva, G.V.; Mesquita, C.T.; Belém, L.; Vaughn, W.K.; Rangel, F.O.D.; et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation 2004, 110, II-213–II-218. [Google Scholar] [CrossRef]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.-D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011, 20, 655–667. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Bunnell, B.A. Adipose tissue-derived mesenchymal stem cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Valina, C.; Pinkernell, K.; Song, Y.-H.; Bai, X.; Sadat, S.; Campeau, R.J.; Le Jemtel, T.H.; Alt, E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 2007, 28, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Planat-Bénard, V.; Abizanda, G.; Pelacho, B.; Léobon, B.; Gavira, J.J.; Penuelas, I.; Cemborain, A.; Penicaud, L.; Laharrague, P.; et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 2008, 10, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, T. C-kit-positive cardiac stem cells and myocardial regeneration. Am. J. Cardiovasc. Dis. 2011, 2, 58–67. [Google Scholar] [PubMed]
- Bao, L.; Meng, Q.; Li, Y.; Deng, S.; Yu, Z.; Liu, Z.; Zhang, L.; Fan, H. C-Kit positive cardiac stem cells and bone marrow–derived mesenchymal stem cells synergistically enhance angiogenesis and improve cardiac function after myocardial infarction in a paracrine manner. J. Card. Fail. 2017, 23, 403–415. [Google Scholar] [CrossRef]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Editors. Retraction-cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2019, 393, 1084. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Van Laake, L.W.; Passier, R.; Monshouwer-Kloots, J.; Verkleij, A.J.; Lips, D.J.; Freund, C.; Den Ouden, K.; Ward-van Oostwaard, D.; Korving, J.; Tertoolen, L.G.; et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007, 1, 9–24. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Gaur, M.; Zhang, Y.; Sievers, R.E.; Ritner, C.; Prasad, M.; Boyle, A.; Bernstein, H.S. Myocardial improvement with human embryonic stem cell-derived cardiomyocytes enriched by p38MAPK inhibition. Cytotherapy 2012, 14, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Fernandes, S.; Zhu, W.-Z.; Filice, D.; Muskheli, V.; Kim, J.; Palpant, N.J.; Gantz, J.; Moyes, K.W.; Reinecke, H.; et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012, 489, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, W.; Zhang, H.; Wang, Q.; Yu, J.; Zhang, R.; Chen, Y.; Xia, Y.; Wang, J.; Wang, D. Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Res. Ther. 2020, 11, 73. [Google Scholar] [CrossRef]
- Lancaster, J.J.; Sanchez, P.; Repetti, G.G.; Juneman, E.; Pandey, A.C.; Chinyere, I.R.; Moukabary, T.; LaHood, N.; Daugherty, S.L.; Goldman, S. Human induced pluripotent stem cell–derived cardiomyocyte patch in rats with heart failure. Ann. Thorac. Surg. 2019, 108, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chang, Y.-H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Massé, S.; Magtibay, K.; Kawajiri, H.; et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell–derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef]
- Riegler, J.; Ebert, A.; Qin, X.; Shen, Q.; Wang, M.; Ameen, M.; Kodo, K.; Ong, S.-G.; Lee, W.H.; Lee, G.; et al. Comparison of magnetic resonance imaging and serum biomarkers for detection of human pluripotent stem cell-derived teratomas. Stem Cell Reports 2016, 6, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Lee, A.S.; Volkmer, J.-P.; Sahoo, D.; Nag, D.; Mosley, A.R.; Inlay, M.A.; Ardehali, R.; Chavez, S.L.; Pera, R.R.; et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat. Biotechnol. 2011, 29, 829–834. [Google Scholar] [CrossRef]
- Soma, Y.; Morita, Y.; Kishino, Y.; Kanazawa, H.; Fukuda, K.; Tohyama, S. The present state and future perspectives of cardiac regenerative therapy using human pluripotent stem cells. Front. Cardiovasc. Med. 2021, 8, 774389. [Google Scholar] [CrossRef]
- Lee, M.-O.; Moon, S.H.; Jeong, H.-C.; Yi, J.-Y.; Lee, T.-H.; Shim, S.H.; Rhee, Y.-H.; Lee, S.-H.; Oh, S.-J.; Lee, M.-Y.; et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, E3281–E3290. [Google Scholar] [CrossRef] [PubMed]
- Sougawa, N.; Miyagawa, S.; Fukushima, S.; Kawamura, A.; Yokoyama, J.; Ito, E.; Harada, A.; Okimoto, K.; Mochizuki-Oda, N.; Saito, A.; et al. Immunologic targeting of CD30 eliminates tumourigenic human pluripotent stem cells, allowing safer clinical application of hiPSC-based cell therapy. Sci. Rep. 2018, 8, 3726. [Google Scholar] [CrossRef]
- Tanosaki, S.; Tohyama, S.; Fujita, J.; Someya, S.; Hishiki, T.; Matsuura, T.; Nakanishi, H.; Ohto-Nakanishi, T.; Akiyama, T.; Morita, Y.; et al. Fatty acid synthesis is indispensable for survival of human pluripotent stem cells. iScience 2020, 23, 101535. [Google Scholar] [CrossRef]
- Tohyama, S.; Fujita, J.; Hishiki, T.; Matsuura, T.; Hattori, F.; Ohno, R.; Kanazawa, H.; Seki, T.; Nakajima, K.; Kishino, Y.; et al. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 2016, 23, 663–674. [Google Scholar] [CrossRef]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2012, 12, 127–137. [Google Scholar] [CrossRef]
- Nakano, H.; Minami, I.; Braas, D.; Pappoe, H.; Wu, X.; Sagadevan, A.; Vergnes, L.; Fu, K.; Morselli, M.; Dunham, C.; et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife 2017, 6, e29330. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, N.; Shiraki, Y.; Tsuyama, T.; Obata, F.; Miura, M.; Nagae, G.; Aburatani, H.; Kume, K.; Endo, F.; Kume, S. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014, 19, 780–794. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Nakagawa, M.; Garitaonandia, I.; Slavin, I.; Altun, G.; Lacharite, R.M.; Nazor, K.L.; Tran, H.T.; Lynch, C.L.; Leonardo, T.R.; et al. Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis. Cell Res. 2011, 21, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Peh, G.S.L.; Gauthaman, K.; Bongso, A. Separation of SSEA-4 and TRA-1–60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS). Stem Cell Rev. Rep. 2009, 5, 72–80. [Google Scholar] [CrossRef]
- Dubois, N.C.; Craft, A.M.; Sharma, P.; Elliott, D.A.; Stanley, E.G.; Elefanty, A.G.; Gramolini, A.; Keller, G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 2011, 29, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Hattori, F.; Chen, H.; Yamashita, H.; Tohyama, S.; Satoh, Y.-S.; Yuasa, S.; Li, W.; Yamakawa, H.; Tanaka, T.; Onitsuka, T.; et al. Nongenetic method for purifying stem cell–derived cardiomyocytes. Nat. Methods 2009, 7, 61–66. [Google Scholar] [CrossRef]
- Tani, H.; Tohyama, S.; Kishino, Y.; Kanazawa, H.; Fukuda, K. Production of functional cardiomyocytes and cardiac tissue from human induced pluripotent stem cells for regenerative therapy. J. Mol. Cell. Cardiol. 2021, 164, 83–91. [Google Scholar] [CrossRef]
- Ben-David, U.; Gan, Q.-F.; Golan-Lev, T.; Arora, P.; Yanuka, O.; Oren, Y.S.; Leikin-Frenkel, A.; Graf, M.; Garippa, R.; Boehringer, M.; et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 2013, 12, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Miki, K.; Parr, C.J.C.; Hayashi, K.; Takei, I.; Li, J.; Iwasaki, M.; Nakagawa, M.; Yoshida, Y.; Saito, H. Efficient, selective removal of human pluripotent stem cells via ecto-alkaline phosphatase-mediated aggregation of synthetic peptides. Cell Chem. Biol. 2017, 24, 685–694.e4. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Onuma, Y.; Ito, Y.; Minoshima, F.; Saito, S.; Shimizu, M.; Aiki, Y.; Asashima, M.; Hirabayashi, J. Elimination of tumorigenic human pluripotent stem cells by a recombinant lectin-toxin fusion protein. Stem Cell Rep. 2015, 4, 811–820. [Google Scholar] [CrossRef]
- Ben-David, U.; Nudel, N.; Benvenisty, N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat. Commun. 2013, 4, 1992. [Google Scholar] [CrossRef]
- Okada, M.; Tada, Y.; Seki, T.; Tohyama, S.; Fujita, J.; Suzuki, T.; Shimomura, M.; Ofuji, K.; Kishino, Y.; Nakajima, K.; et al. Selective elimination of undifferentiated human pluripotent stem cells using pluripotent state-specific immunogenic antigen Glypican-3. Biochem. Biophys. Res. Commun. 2019, 511, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.J.C.; Katayama, S.; Miki, K.; Kuang, Y.; Yoshida, Y.; Morizane, A.; Takahashi, J.; Yamanaka, S.; Saito, H. MicroRNA-302 switch to identify and eliminate undifferentiated human pluripotent stem cells. Sci. Rep. 2016, 6, 32532. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Soma, Y.; Nakajima, K.; Kanazawa, H.; Tohyama, S.; Tabei, R.; Hirano, A.; Handa, N.; Yamada, Y.; Okuda, S.; et al. Intramyocardial transplantation of human iPS cell–derived cardiac spheroids improves cardiac function in heart failure animals. JACC Basic Transl. Sci. 2021, 6, 239–254. [Google Scholar] [CrossRef]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef]
- Tohyama, S.; Fujita, J.; Fujita, C.; Yamaguchi, M.; Kanaami, S.; Ohno, R.; Sakamoto, K.; Kodama, M.; Kurokawa, J.; Kanazawa, H.; et al. Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes. Stem Cell Rep. 2017, 9, 1406–1414. [Google Scholar] [CrossRef]
- Tabei, R.; Kawaguchi, S.; Kanazawa, H.; Tohyama, S.; Hirano, A.; Handa, N.; Hishikawa, S.; Teratani, T.; Kunita, S.; Fukuda, J.; et al. Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cell–derived cardiomyocytes. J. Heart Lung Transplant. 2019, 38, 203–214. [Google Scholar] [CrossRef]
- Nakamura, K.; Neidig, L.E.; Yang, X.; Weber, G.J.; El-Nachef, D.; Tsuchida, H.; Dupras, S.; Kalucki, F.A.; Jayabalu, A.; Futakuchi-Tsuchida, A.; et al. Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes. Stem Cell Rep. 2021, 16, 2473–2487. [Google Scholar] [CrossRef]
- Hou, D.; Youssef, E.A.S.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112 (Suppl. S9), I150–I156. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 2005, 111, 2198–2202. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tohyama, S.; Kanazawa, H.; Ichimura, H.; Chino, S.; Tanaka, Y.; Suzuki, Y.; Zhao, J.; Shiba, N.; Kadota, S.; et al. Intracoronary transplantation of pluripotent stem cell-derived cardiomyocytes: Inefficient procedure for cardiac regeneration. J. Mol. Cell. Cardiol. 2022, 174, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C. Intravenous, intracoronary, transendocardial, and advential delivery. In Stem Cell and Gene Therapy for Cardiovascular Disease, 1st ed; Perin, E.C., Miller, L.W., Taylor, D.A., Willerson, J.T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 279–287. [Google Scholar] [CrossRef]
- Bellamy, V.; Vanneaux, V.; Bel, A.; Nemetalla, H.; Boitard, S.E.; Farouz, Y.; Joanne, P.; Perier, M.-C.; Robidel, E.; Mandet, C.; et al. Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J. Heart Lung Transplant. 2015, 34, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wu, Q.; Ni, C.; Zhang, P.; Zhong, Z.; Wu, Y.; Wang, Y.; Xu, Y.; Kong, M.; Cheng, H.; et al. Lack of remuscularization following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ. Res. 2018, 122, 958–969. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhang, J.; Qu, X.; Kawamura, T.; Miyagawa, S.; Sawa, Y. Engineered tissue for cardiac regeneration: Current status and future perspectives. Bioengineering 2022, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Kahn-Krell, A.; Pretorius, D.; Guragain, B.; Lou, X.; Wei, Y.; Zhang, J.; Qiao, A.; Nakada, Y.; Kamp, T.J.; Ye, L.; et al. A three-dimensional culture system for generating cardiac spheroids composed of cardiomyocytes, endothelial cells, smooth-muscle cells, and cardiac fibroblasts derived from human induced-pluripotent stem cells. Front. Bioeng. Biotechnol. 2022, 10, 908848. [Google Scholar] [CrossRef] [PubMed]
- Gerbin, K.A.; Yang, X.; Murry, C.E.; Coulombe, K.L.K. Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PLoS ONE 2015, 10, e0131446. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyagawa, S.; Liu, L.; Kawamura, T.; Li, J.; Qu, X.; Harada, A.; Toda, K.; Yoshioka, D.; Kainuma, S.; et al. Therapeutic efficacy of large aligned cardiac tissue derived from induced pluripotent stem cell in a porcine ischemic cardiomyopathy model. J. Heart Lung Transplant. 2021, 40, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Querdel, E.; Reinsch, M.; Castro, L.; Köse, D.; Bähr, A.; Reich, S.; Geertz, B.; Ulmer, B.; Schulze, M.; Lemoine, M.D.; et al. Human engineered heart tissue patches remuscularize the injured heart in a dose-dependent manner. Circulation 2021, 143, 1991–2006. [Google Scholar] [CrossRef]
- Hirata, Y.; Yamada, H.; Sata, M. Epicardial fat and pericardial fat surrounding the heart have different characteristics. Circ. J. 2018, 82, 2475–2476. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Miyagawa, S.; Pearson, J.T.; Fukushima, S.; Saito, A.; Tsuchimochi, H.; Sonobe, T.; Fujii, Y.; Yagi, N.; Astolfo, A.; et al. Functional and electrical integration of induced pluripotent stem cell-derived cardiomyocytes in a myocardial infarction rat heart. Cell Transplant. 2015, 24, 2479–2489. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, R.Y.; Park, B.-W.; Lee, S.; Choi, S.W.; Park, J.-H.; Choi, J.J.; Kim, S.-W.; Jang, J.; Cho, D.-W.; et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019, 10, 3123. [Google Scholar] [CrossRef] [PubMed]
- Kahn-Krell, A.; Pretorius, D.; Ou, J.; Fast, V.G.; Litovsky, S.; Berry, J.; Liu, X.M.; Zhang, J. Bioreactor suspension culture: Differentiation and production of cardiomyocyte spheroids from human induced pluripotent stem cells. Front. Bioeng. Biotechnol. 2021, 9, 674260. [Google Scholar] [CrossRef]
- Souidi, M.; Sleiman, Y.; Acimovic, I.; Pribyl, J.; Charrabi, A.; Baecker, V.; Scheuermann, V.; Pesl, M.; Jelinkova, S.; Skladal, P.; et al. Oxygen is an ambivalent factor for the differentiation of human pluripotent stem cells in cardiac 2D monolayer and 3D cardiac spheroids. Int. J. Mol. Sci. 2021, 22, 662. [Google Scholar] [CrossRef]
- Hemmi, N.; Tohyama, S.; Nakajima, K.; Kanazawa, H.; Suzuki, T.; Hattori, F.; Seki, T.; Kishino, Y.; Hirano, A.; Okada, M.; et al. A massive suspension culture system with metabolic purification for human pluripotent stem cell-derived cardiomyocytes. Stem Cells Transl. Med. 2014, 3, 1473–1483. [Google Scholar] [CrossRef]
- Clinicaltrials.gov (NCT04945018). A Study of iPS Cell-Derived Cardiomyocyte Spheroids (HS-001) in Patients with Heart Failure (LAPiS Study) (LAPiS). Available online: https://clinicaltrials.gov/ct2/show/NCT04945018?term=NCT04945018 (accessed on 22 November 2022).
- Clinicaltrials.gov (NCT04982081). Treating Congestive HF with hiPSC-CMs through Endocardial Injection. Available online: https://clinicaltrials.gov/ct2/show/NCT04982081?term=NCT04982081&draw=2&rank=1 (accessed on 22 November 2022).
- Clinicaltrials.gov (NCT05566600). Allogeneic iPSC-Derived Cardiomyocyte Therapy in Patients with Worsening Ischemic Heart Failure. Available online: https://clinicaltrials.gov/ct2/show/NCT05566600?term=NCT05566600 (accessed on 22 November 2022).
- Zhang, H.; Xue, Y.; Pan, T.; Zhu, X.; Chong, H.; Xu, C.; Fan, F.; Cao, H.; Zhang, B.; Pan, J.; et al. Epicardial injection of allogeneic human-induced-pluripotent stem cell-derived cardiomyocytes in patients with advanced heart failure: Protocol for a phase I/IIa dose-escalation clinical trial. BMJ Open 2022, 12, e056264. [Google Scholar] [CrossRef]
- Clinicaltrials.gov (NCT04696328). Clinical Trial Of Human (allogeneic) iPS Cell-Derived Cardiomyocytes Sheet for Ischemic Cardiomyopathy. Available online: https://clinicaltrials.gov/ct2/show/NCT04696328?term=NCT04696328&draw=2&rank=1 (accessed on 22 November 2022).
- Clinicaltrials.gov (NCT04396899). Safety and Efficacy of Induced Pluripotent Stem Cell-Derived Engineered Human Myocardium as Biological Ventricular Assist Tissue in Terminal Heart Failure (BioVAT-HF). Available online: https://clinicaltrials.gov/ct2/show/NCT04396899?term=NCT04396899 (accessed on 22 November 2022).
- Clinicaltrials.gov (NCT05068674). Human Embryonic Stem Cell-Derived Cardiomyocyte Therapy for Chronic Ischemic Left Ventricular Dysfunction (HECTOR). Available online: https://clinicaltrials.gov/ct2/show/NCT05068674?term=NCT05068674&draw=2&rank=1 (accessed on 22 November 2022).
| Key Findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Patient Population | Cell Type (Number) | Auto/ Allo | Phase | n | Follow-Up | Delivery Route | LVEF LV Volumes | Infarct/ Scar Size | QoL | Other |
| BM-MNCs | ||||||||||
| Ruan 2005 [12] MI and LAD occlusion | BM-MNCs (not specified) | Auto | ? | 20 | 6 months | IC | Improved (BM-MNCs, 53.37–59.33%; control, 53.51–50.30%) Improved | – | – | – |
| Janssens 2006 [13] NCT00264316 STEMI and PCI | BM-MNCs (304 × 106 nucleated cells, 172 × 106 MNCs) | Auto | ? | 77 | 4 months | IC | ns (BM-MNCs, 48.5–51.8%; placebo, 46.9–49.1%) | – | – | – |
| Assmus 2009 [14] NCT00279175 STEMI with successful stent and LVEF ≤ 45% | BM-PCs 1 | Auto | ? | 204 | 2 years | IC | ns (BM-MNCs, 46.5–53.7%; placebo, 40.4–46.8% at 2 years) ns | – | – | Improvement in composite primary endpoint vs. placebo (death, MI, or need for revascularization) |
| Traverse 2010 [15] STEMI with successful stent/angioplasty and LVEF ≤ 50% | BM-MNCs (100 × 106 cells) | Auto | 1 | 40 | 1 year | IC | ns (BM-MNCs, 49.0–55.2%; placebo, 48.6–57.0% at 6 months) ns | – | – | – |
| Hu 2011 [16] CHF due to severe ischemic cardiomyopathy (LVEF < 30%) | BM-MNCs (100 × 106 cells) | Auto | ? | 60 | 6 months | IC | Improved (BM-MNCs, 22.78–33.80%; placebo, 24.95–31.82%) Improved | – | – | 6MWT improved Reduction in BNP |
| ASTAMI Beitnes 2011 [17] Anterior STEMI and PCI | BM-MNCs (median: 68 × 106 cells) | Auto | ? | 100 | 3 years | IC | ns (BM-MNCs, 45.7–47.5%; placebo, 46.9–46.8%) ns | – | – | – |
| FOCUS-CCTRN Perin 2012 [18] NCT00824005 HF (NYHA class II–III or CCS class II–IV) and LVEF ≤ 45% | BM-MNCs (100 × 106 cells) | Auto | 2 | 92 | 6 months | TE | ns (BM-MNCs, +1.4% from baseline; placebo, −1.3% from baseline) ns | ns | – | Maximum O2 consumption ns NT-proBNP ns |
| SCAMI Wohrle 2013 [19] Wohrle 2010 [20] MI and PCI conducted 6–48 h after symptoms | BM-MNCs (median: 324 × 106 cells) | Auto | ? | 42 | 3 years 6 months | IC | ns (BM-MNCs, 53.5–54.0%; placebo, 55.7–59.4% at 3 years) ns | ns | – | – |
| Lu 2013 [21] Chronic MI (≥3 months), LVEF ≤ 35%, admitted for elective CABG | BM-MNCs (‘average’: 133.8 × 106 cells) | Auto | ? | 50 | 12 months | IC | Improved (BM-MNCs, +13.5%; control, +8.0%) – | ns | – | – |
| TAC-HFT Heldman 2014 [22] NCT00768066 Ischemic cardiomyopathy and LVEF < 50% | BM-MNCs (CardiAMP®) | Auto | 1/2 | 65 | 12 months | TE | ns (no change in LVEF) ns | ns | Improved | Functional capacity ns |
| Patila 2014 [23] NCT00418418 HF (NYHA class II–IV; LVEF 15–45%) and scheduled for CABG | BM-MNCs (median: 840 × 106 cells) | Auto | ? | 104 | 12 months | IMI | ns (BM-MNCs, +4.8%; control, +5.6%) ns | – | – | NT-proBNP ns Myocardial viability ns |
| Hu 2015 [24] NCT01234181 STEMI and PCI and LV wall motion abnormality | Hypoxia pre-conditioned BM-MNCs (100 × 106 cells) | Auto | 1 | 36 | 12 months | IC | ns (normoxia BM-MNCs, 56.9–56.8%; hypoxia BM-MNCs, 50.9–56.1%; control, 57.1–59.6%) Improved | – | – | Pre-conditioned cells superior to non-pre-conditioned |
| REGENERATE-AMI Choudry 2016 [25] NCT00765453 STEMI and regional wall motion abnormality | BM-MNCs (mean: 59.8 × 106 cells) | Auto | 2 | 100 | 12 months | IC | ns (BM-MNCs, +5.1%; placebo, +2.8%) – | ns | ns | NYHA class ns Myocardial salvage index improved NT-proBNP decreased in both groups |
| Mi-Heart Martino 2015 [26] NCT00333827 Non-ischemic dilated cardiomyopathy (LVEF < 35%) | BM-MNCs (mean: 236 × 106 cells) | Auto | ? | 160 | 12 months | IC | ns (BM-MNCs, 24.0–19.9%; placebo, 24.3–22.1%) ns | – | ns | – BNP ns |
| BOOST-2 Wollert 2017 [27] STEMI and reduced LVEF Subgroup analysis of patients with S-CMR Seitz 2020 [28] ISRCTN17457407 | BM-MNCs (mean: high 2060 × 106 cells; low 700 × 106 cells) | Auto | ? | 153 51 | 6 months | IC | ns (high BM-MNCs, +4.3%; low BM-MNCs, +3.8%; control, +3.3%) ns | – | – | – BM-MNCs did not enhance infarct perfusion |
| TIME Traverse 2012 [29] STEMI and PCI (LVEF ≤45%) Follow-up analysis Traverse 2018 [30] NCT00684021 | BM-MNCs (150 × 106 cells) | Auto | ? | 120 | 6 months 2 years | IC | ns (BM-MNCs, 45.2–48.3%; placebo, 44.5–47.8%) ns ns (BM-MNCs, +2.8%; placebo, +4.7%) Increase in LVEDVI with BM-MNCs | – – | – – | – – |
| Nicolau 2018 [31] STEMI and angioplasty (LVEF ≤ 50%) | BM-MNCs (100 × 106 cells) | Auto | ? | 121 | 6 months | IC | ns (BM-MNCs, 44.63–44.74%; placebo, 42.23–43.50%) ns | ns | – | – |
| COMPARE-CPM-RMI Naseri 2018 [32] NCT01167751 STEMI (LVEF 20–45%) | BM-MNCs (mean: 564.63 × 106 cells) | Auto | 2/3 | 77 | 6 months 18 months | IMI | Improved (BM-MNCs, +7% vs. placebo; CD133+ cells, +9% vs. placebo) – | – | – | BM-MNCs were inferior to CD133+ cells |
| BM-MSCs | ||||||||||
| Hare 2009 [33] MI and LVEF 30–60% | BM-MSCs (0.5, 1.6, 6 × 106 cells/kg) | Allo | ? | 53 | 6 months | i.v. | ns (BM-MNCs, 50.4–56.9%; placebo, 48.7–56.1%) ns | – | – | 6MWT ns Global symptom score improved |
| TAC-HFT Heldman 2014 [22] NCT00768066 Ischemic cardiomyopathy (LVEF < 50%) | BM-MSCs (not specified) | Auto | 1/2 | 65 | 12 months | TE | ns (no change in LVEF) ns | Reduced | Improved | 6MWT improved Regional myocardial function improved |
| MSC-HF Mathiasen 2015 [34] Mathiasen 2020 [35] NCT00644410 Severe ischemic HF (NYHA class II–III; LVEF < 45%) | BM-MSCs (mean: 77.5 × 106 cells) | Auto | 2 | 60 | 6 months 12 months 4 years | IMI | Improved (+6.2% vs. placebo at 6 and 12 months) LVESV reduced by 13 mL (6 months) and 17 mL (12 months) vs. placebo | ns | – | 6MWT ns NYHA class ns 4 years: hospitalizations for angina reduced |
| Chullikana 2015 [36] AMI and PCI NCT00883727 | BM-MSCs (4.0 × 106 cells) | Allo | 1/2 | 20 | 2 years | i.v. | ns (BM-MSCs 43.06–47.80%; placebo, 43.44–45.33%) – | ns | – | – |
| TRIDENT Florea 2017 [37] NCT02013674 Ischemic cardiomyopathy secondary to MI (LVEF ≤ 50%) | BM-MSCs (low [20 × 106 cells] vs. high dose [100 × 106 cells]) | Allo | 2 | 30 | 12 months | TE | Improved with high dose by 3.7 units – | Reduced | – | NYHA class improved NT-proBNP increased with low dose |
| CHART-1 Bartunek 2017 [38] Follow-up: Bartunek 2020 [39] NCT01768702 Symptomatic ischemic HF (LVEF ≤ 35%) | Cardiopoietic BM-MSCs (24 × 106 cells) | Auto | 3 | 315 | 39 weeks 104 weeks | TE | – – | – | – | ns for composite primary endpoint Subgroup analysis suggests a beneficial effect in patients with low LVEDV 2-year follow-up confirmed benefits in patients with LV enlargement |
| DREAM-HF Borow 2019 [40] Perin 2023 [41] NCT02032004 Advanced stable chronic HFrEF | BM-MSCs (not specified) | Allo | 3 | 565 (537 treated) | Median ~30 months | TE | ? | ? | ? | Did not meet primary endpoint 58% reduction in MI or stroke 28% reduction in 3-point MACE |
| COMPARE-AMI Haddad 2020 [42] STEMI and LV dysfunction after PCI | CD133+ enriched BM-MSCs 10 × 106 cells (one patient was injected with 5.2 × 106 cells) | Allo | 2 | 38 | 10 years | IC | ? | – | – | 10-year event-free survival ns |
| CONCERT_CCRTN Bolli 2021 [43] HF caused by ischemic cardiomyopathy (NYHA class I–III; LVEF ≤ 40%; scar ≥ 5% LV volume) | BM-MSCs ± CPCs (BM-MSCs, 150 × 106 cells; CPCs, 5 × 106 cells) | Auto | 2 | 125 | 12 months | TE | ns ns (BM-MSCs + CPCs, 29.21–29.91%; CPCs 26.31–26.96%; BM-MSCs, 29.26–31.12%; placebo, 29.66–29.35%) | – | Improved with MSCs + CPCs and with MSCs alone | 6MWT ns Peak O2 consumption ns MACE decreased with CPCs NT-proBNP ns |
| UC-MSCs | ||||||||||
| Gao 2015 [44] UC-MSCs STEMI and successful stent | UC-MSCs (6 × 106 cells) | Allo | ? | 116 | 18 months | IC | Improved (UC-MSCs, +7.8%, placebo, 2.8%) Improved | – | – | Increase in myocardial viability with UC-MSCs |
| RIMECARD Bartolucci 2017 [45] NCT01739777 HFrEF (NYHA class I–III; LVEF ≤ 40%) | UC-MSCs (1 × 106 cells/kg) | Allo | 1/2 | 30 | 12 months | i.v. | Improved (TTE LVEF: UC-MSCs, 33.50–40.57%; placebo, 31.53–33.39%; CMR LVEF: UC-MSCs, 32.64–37.43%; placebo, 29.62–31.31%) ns | – | Improved | NYHA class improved Decreased BNP |
| He 2020 [46] NCT02635464 Chronic ischemic heart disease (LVEF ≤ 45%) requiring CABG | UC-MSCs in collagen hydrogel (100 × 106 cells) | Allo | 1 | 50 | 12 months | IMI | – – | Reduced | – | – |
| ADRCs | ||||||||||
| PRECISE Perin 2014 [47] NCT00426868 Ischemic cardiomyopathy (NYHA class II–III or CCS class II–IV; LVEF ≤ 35%) not amenable to revascularization | ADRCs (0.4, 0.8, 1.2 × 106 cells/kg) (mean: 42 × 106 cells) | Auto | 1 | 27 | 36 months | TE | ns ns | – | – | VO2 max ns |
| ATHENA I and II Henry 2017 [48] Multivessel CAD (NYHA class II–III or CCS class II–IV; LVEF 20–45%) not amenable to revascularization DISCONTINUED | ADRCs (ATHENA I, 40 × 106 cells; ATHENA II, 80 × 106 cells) | Auto | ? | 28 3 | 12 months | IMI | – – | – | Enrolment terminated prematurely due to non-ADRC-related AEs | |
| Myoblasts | ||||||||||
| MAGIC Menasche 2008 [49] Ischemic cardiomyopathy (LVEF 15–35%) and indication for CABG | Myoblasts (low dose, 400 × 106 cells; high dose, 800 × 106 cells) | Auto | 1 | 97 | 6 months | IMI | ns (low dose, +3.4%; high dose, +5.2%; placebo, +4.4%) Improved | ns | – | – |
| MARVEL Povsic 2011 [50] HF (NYHA class II–IV; LVEF < 35%) DISCONTINUED | Skeletal myoblasts (400 × 106 cells or 800 × 106 cells) | Auto | 2b/3 | 23 | 6 months | IMI | – – | – | Discontinued for financial reasons following enrolment of 23 out of 330 planned patients Larger BNP increases with placebo vs. myoblast treatment | |
| ALLSTAR Makkar 2020 [51] Post-MI LV dysfunction (NYHA class II–IV; LVEF ≤ 45%; LV scar ≥15% LV mass) DISCONTINUED | CDCs (25 × 106 cells) | Allo | ? | 142 | Interim analysis at 6 months | IC | – Improved | ns | NT-proBNP reduced Discontinued based on prespecified interim analysis at 6 months that indicated futility with respect to primary endpoint | |
| CAREMI Fernandez-Aviles 2018 [52] STEMI and LVEF ≤ 45% and infarct > 25% LV mass | CSCs (35 × 106 cells) | Allo | 1/2 | 49 | 12 months | IC | ns (CSCs, +7.7%; placebo, +8.6%) ns | ns | – | NT-proBNP changes ns |
| Ongoing trials/trials with results awaited | ||||||||||
| CardiAMP® Biocardia [53] Raval 2021 [54] Johnston 2022 [55] NCT02438306 Chronic LV dysfunction (NYHA class II–III; LVEF 20–40%) secondary to MI | BM-MNCs (not specified) | Auto | 3 | 250 | 2 years | CardiAMP® cell therapy system | 1o: composite 2 2o: survival, MACE, QoL | Estimated completion December 2024 Open-label, roll-in cohort (n = 10): 12 months: trend improvement in LVEF, 6MWT, QoL, NYHA 2 years: 100% survival; improved 6MWT and LVEF vs. baseline | ||
| SCIENCE Paitazoglou 2019 [56] NCT02673164 Chronic ischemic HF (NYHA class II–III; LVEF < 45%) | ADRCs 3 (100 × 106 cells) | Allo | 2 | 133 | 12 months | TE | 1o: LVESV 2o: SAEs | Completed December 2020 | ||
| CSCC_ASCII [57] NCT03092284 Chronic stable ischemic heart disease (NYHA class II–III; LVEF ≤ 45%) | AD-MSCs (100 × 106 cells) | Allo | 2 | 81 | 12 months | TE | 1o: LVESV 2o: TEAEs, LVEF, KCCQ, Seattle Angina Questionnaire; 6MWT | Completed July 2022 | ||
| Approach | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Cell sorting using MACS or FACS | |||
| Lectins [96] | hPSC-specific biomarker (lectin) mediated cell separation by MACS |
|
|
| SSEA-5 [87] | Antibody targeting hPSC-specific cell surface H type-1 glycan and cells separated by FACS |
|
|
| TRA-1 60, SSEA-4 [97] | Antibody targeting hESC-specific cell surface H type-1 glycan and cells separated by MACS and FACS |
|
|
| SIRPA [98] | hPSC-CM-specific markers |
| |
| Mitochondria [99] | Differences in mitochondrial number identified by accumulation of fluorescent mitochondrion-specific dye in CMs |
| |
| Metabolic selection | |||
| Glucose/glutamine depletion [92,100] | CMs, but not undifferentiated hPSCs, can utilize lactate to generate energy in the absence of glucose and glutamine. Incubation of cells in glucose- and glutamine-free media supplemented with lactate results in elimination of undifferentiated cells |
|
|
| Methionine depletion [95] | hPSCs require high amounts of methionine. Prolonged methionine depletion induced apoptosis of hPSCs |
|
|
| PluriSIns [101] | Pluripotent cell-specific inhibitor of stearoyl-coA desaturase, a key enzyme in oleate synthesis, which induces apoptosis of hPSCs |
| |
| Fatty acid synthase inhibition [91] | Undifferentiated hPSCs express different fatty acid biosynthesis enzymes to differentiated cells Inhibition of fatty acid synthase reduces phosphatidylcholine, a key metabolite for survival, inducing apoptosis of hPSCs, but not hPSC-derived cells, including CMs |
| |
| Addition of compounds | |||
| Inhibitors of survivin [89] | Inhibition of hPSC-specific antiapoptotic factor |
| |
| D-3 [102] | A phospho-D peptide that causes cell death when dephosphorylated by alkaline phosphatases, which are overexpressed on hPSCs, but not hPSC-CMs |
|
|
| Lectin-toxin fusion protein [103] | Binds to hPSCs only and delivers cytotoxic protein when internalized, eliminating hPSCs | ||
| Clostridium perfringens enterotoxin [104] | Toxic that binds to Claudin-6, a tight-junction protein specific to hPSCs, and kills undifferentiated cells | ||
| Other | |||
| Glypican-3 [105] | Pluripotent-state specific immunogenic antigen targeted by glypican-3-reactive cytotoxic T lymphocytes |
|
|
| Brentuximab vedotin [90] | Antibody-drug conjugate targeting CD30, a cell surface antigen expressed specifically on hiPSCs | ||
| MicroRNA-302a-5p [106] | MicroRNA-302a-5p is highly expressed in hPSCs, but not differentiated cells microRNA switch hPSC elimination system using miR-302a switch for controlling puromycin resistance before adding puromycin to kill undifferentiated cells |
|
|
| ClinicalTrials.gov ID Location Phase | Participants | Cells | Duration | Doses | Delivery | Endpoints | Estimated Study Completion | Status |
|---|---|---|---|---|---|---|---|---|
| NCT04945018 LAPiS [130] Japan Phase 1/2 Open-label | 10 patients with severe ischemic HFrEF (LVEF ≤ 40%) secondary to IHD | Allogeneic hiPSC-CM spheroids (HS-001) | 12 months | ‘Low dose (50 million)’ vs. ‘high dose (150 million)’ | Injection using needle ‘SEEDPLANTER®’ | 1o: safety and tolerability (26 weeks) 2o: LVEF (Echo/MRI); myocardial wall motion; myocardial blood flow and viability (SPECT); 6MWT; KCCQ; EQ-5D-5L; NT-proBNP | March 2024 | Recruiting |
| NCT04982081 [131] China Phase 1 Randomized double-blind parallel group | 20 patients with severe congestive HFrEF (LVEF < 40%, both ischemic and non-ischemic) | Allogeneic hiPSC-CMs (HiCM-188) | 12 months | 100 × 106 (n = 10) or 400 × 106 (n = 10) cells | Catheter-based EC injection | 1o: major SAEs 1 2o: arrhythmias; tumors; immunogenicity; LV systolic function (Echo/MRI); 6MWT; NYHA; MLHFQ | July 2023 | Recruiting |
| NCT05566600 [132] China Phase 1 Open-label | 32 patients with worsening chronic ischemic HFrEF (LVEF < 40%, ischemic) | Allogeneic hiPSC-CMs in patients undergoing CABG | 12 months | 100, 200, or 400 × 106 cells with CABG, or CABG only | Epicardial injection during CABG | 1o: safety 2o: AEs; Holter monitoring; tumors; immunogenicity; LV systolic function (Echo/MRI); 6MWT; NYHA; MLHFQ; hospitalization for HF | July 2025 | Not yet recruiting |
| NCT03763136 HEAL-CHF [133] China Phase 1/2 Randomized double-blind | 20 patients with chronic LV dysfunction (LVEF ≥ 20% and ≤ 45%) | Allogeneic hPSC-CM | 12 months | 200 × 106 cells in 2.5–5 mL medium suspension with CABG, or CABG only | Injection during CABG | 1o: sustained ventricular arrhythmias; tumors 2o: overall left ventricular systolic performance; 6MWT; NYHA; MLHFQ; MACE; SAEs; penal reactive antibodies; donor-specific antibodies; severe arrhythmia; NT-proBNP | July 2023 | Recruiting |
| NCT04696328 [134] Japan Phase 1 Open-label | 10 patients with ischemic cardiomyopathy (LVEF ≤ 35%) | Allogeneic hiPSC-CM sheet | 12 months | NR | 1o: LVEF (Echo); safety 2o: number of responders; LV contraction; LV remodeling; NYHA; SAS; MLHFQ; SF-36; 6MWT; BNP; exercise tolerance; rejections | May 2023 | Recruiting | |
| NCT04396899 BioVAT-HF [135] Germany Phase 1/2 Open-label | 53 patients with HFrEF (EF ≤ 35%, both ischemic and non-ischemic) with no realistic chance of a HT | BioVAT tissue: defined mixtures of hiPSC-CMs and stromal cells in a bovine collagen type 1 hydrogel | 12 months | NA | Implantation on myocardium | 1o: target heart wall thickness (Echo/MRI) and heart wall thickening fraction | October 2024 | Recruiting |
| NCT05068674 HECTOR [136] USA Phase 1 Open-label | 18 patients with chronic ischemic LV dysfunction (LVEF < 40%) secondary to MI treated with appropriate maximal medical therapy and a candidate for cardiac catheterization | Allogeneic hESC-CMs | 36 months | 50, 150, or 300 million cells spread over 10 injections | NR | 1o: safety | October 2025 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishino, Y.; Fukuda, K. Unlocking the Pragmatic Potential of Regenerative Therapies in Heart Failure with Next-Generation Treatments. Biomedicines 2023, 11, 915. https://doi.org/10.3390/biomedicines11030915
Kishino Y, Fukuda K. Unlocking the Pragmatic Potential of Regenerative Therapies in Heart Failure with Next-Generation Treatments. Biomedicines. 2023; 11(3):915. https://doi.org/10.3390/biomedicines11030915
Chicago/Turabian StyleKishino, Yoshikazu, and Keiichi Fukuda. 2023. "Unlocking the Pragmatic Potential of Regenerative Therapies in Heart Failure with Next-Generation Treatments" Biomedicines 11, no. 3: 915. https://doi.org/10.3390/biomedicines11030915
APA StyleKishino, Y., & Fukuda, K. (2023). Unlocking the Pragmatic Potential of Regenerative Therapies in Heart Failure with Next-Generation Treatments. Biomedicines, 11(3), 915. https://doi.org/10.3390/biomedicines11030915