Evaluation of Specific Cellular and Humoral Immune Response to Toxoplasma gondii in Patients with Autoimmune Rheumatic Diseases Immunomodulated Due to the Use of TNF Blockers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Maintenance of T. gondii Cultures and Obtaining Parasites
2.3. Preparation of Soluble Toxoplasma gondii Antigens (STAg) for Immunoenzymatic Assays and for Cell Culture
2.4. ELISA Test for the Detection of Anti-Toxoplasma gondii Total IgG, Its Subclass IgG1, and ELISA-Avidity for Total IgG and IgG1 Anti-T. gondii
2.5. Blood Collection and Isolation and Culture of Peripheral Blood Mononuclear Cells (PBMCs)
2.6. Flow Cytometry
2.7. Determination of Cytokines in the Culture Supernatant by Cytometric Bead Array (CBA)
2.8. Statistical Analysis
3. Results
3.1. General Characteristics of the Studied Individuals
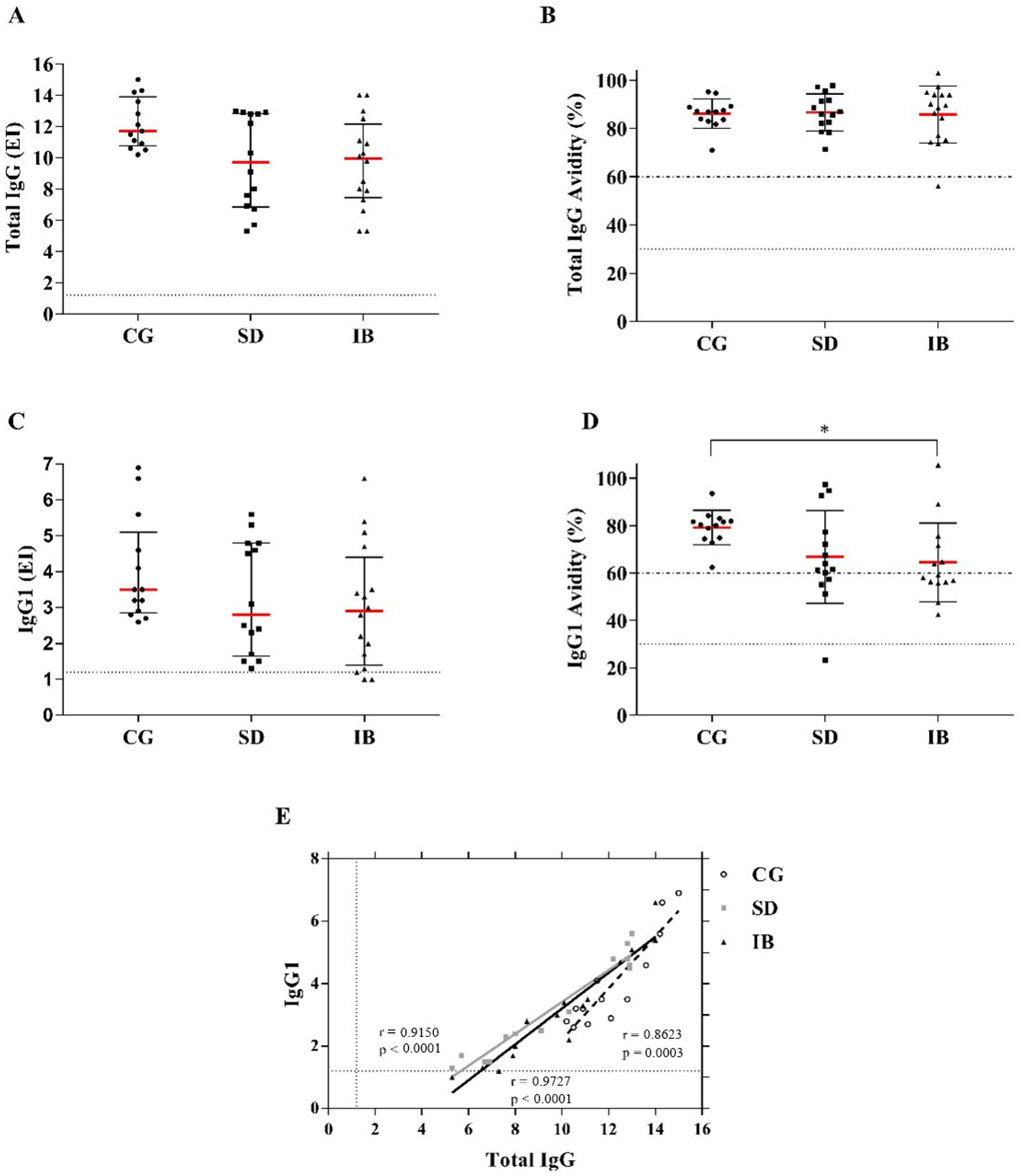
3.2. Detection of Total IgG Antibodies and Subclass IgG1 Anti-Toxoplasma gondii and Avidity of Total IgG and IgG1
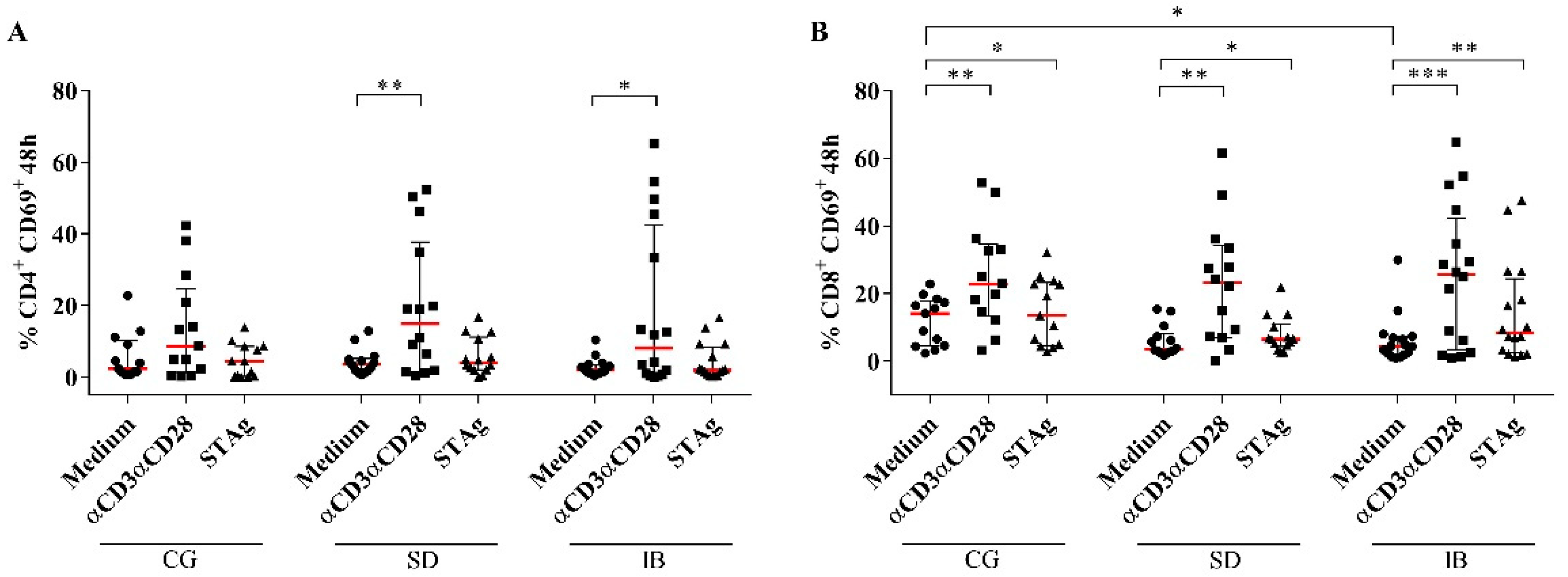
3.3. CD4+ Expressing the Exhaustion Marker PD-1 and Activation Markers CD69+ and CD28+
3.4. Intracellular Expression of IFN-γ, IL-17, and IL-10 Cytokines by CD4+ Cells
3.5. Assessment of Regulatory CD4+ T Populations
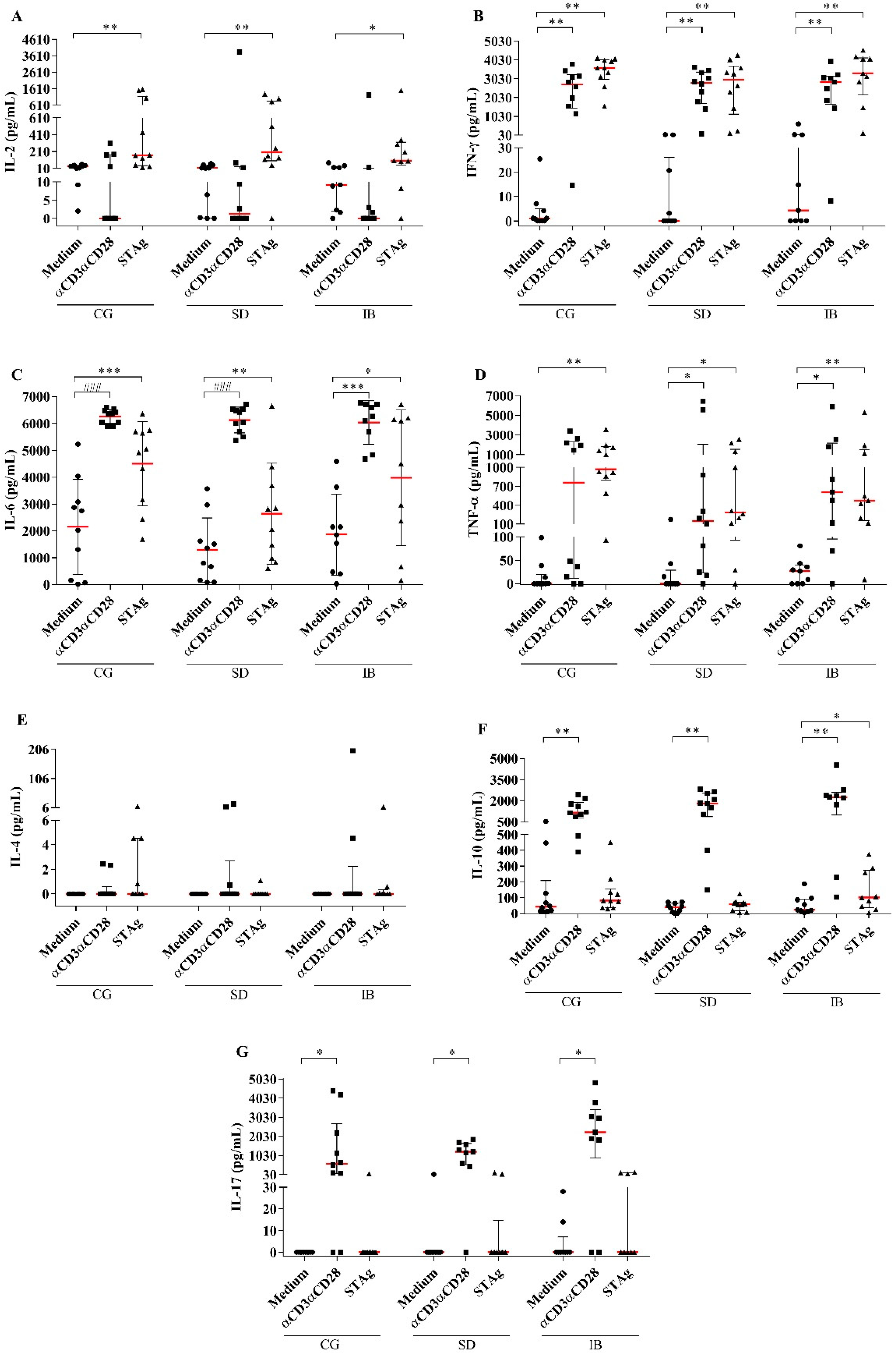
3.6. Production of Cytokines by PBMCs in Patients with Autoimmune Rheumatic Diseases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munoz, M.; Liesenfeld, O.; Heimesaat, M.M. Immunology of Toxoplasma gondii. Immunol. Rev. 2011, 240, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Isa, F.; Saito, K.; Huang, Y.T.; Schuetz, A.; Babady, N.E.; Salvatore, S.; Pessin, M.; Van Besien, K.; Perales, M.A.; Giralt, S.; et al. Implementation of molecular surveillance after a cluster of fatal toxoplasmosis at 2 neighboring transplant centers. Clin. Infect. Dis. 2016, 63, 565–568. [Google Scholar] [CrossRef] [Green Version]
- Passos, L.N.; de Araújo Filho, O.F.; Andrade Junior, H.F. de Toxoplasma Encephalitis in AIDS patients in São Paulo during 1988 and 1991. A comparative retrospective analysis. Rev. Inst. Med. Trop. Sao Paulo 2000, 42, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stray-Pedersen, B. Toxoplasmosis in pregnancy. Baillieres. Clin. Obstet. Gynaecol. 1993, 7, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.G.S.; Sher, A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 1999, 189, 1083–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, M.J.; Maini, R.N.; Feldmann, M.; Kalden, J.R.; Antoni, C.; Smolen, J.S.; Leeb, B.; Breedveld, F.C.; Macfarlane, J.D.; Bijl, J.A.; et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 1994, 344, 1105–1110. [Google Scholar] [CrossRef]
- Baha, A.; Hanazay, C.; Kokturk, N.; Turktas, H. A case of sarcoidosis associated with anti—Tumor necrosis factor treatment. J. Investig. Med. High Impact Case Rep. 2015, 3, 2324709615571366. [Google Scholar] [CrossRef] [Green Version]
- Doran, M.F.; Crowson, C.S.; Pond, G.R.; O’Fallon, W.M.; Gabriel, S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002, 46, 2287–2293. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Muñoz, S.; Soria, N.; Galiana, D.; Bertolaccini, L.; Cuadrado, M.J.; Khamashta, M.A. Autoimmune diseases induced by TNF-targeted therapies: Analysis of 233 cases. Medicine 2007, 86, 242–251. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bononi, I.; Puozzo, A.; Govoni, M.; Foschi, V.; Lanza, G.; Gafa, R.; Gaboriaud, P.; Touzé, F.A.; Selvatici, R.; et al. Merkel cell carcinomas arising in autoimmune disease affected patients treated with biologic drugs including anti-TNF. Clin. Cancer Res. 2017, 23, 3929–3934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, R.S.; Broder, M.S.; Wong, J.Y.; Hanson, M.E.; Beenhouwer, D.O. Granulomatous Infectious Diseases Associated with Tumor Necrosis Factor Antagonists. Clin. Infect. Dis. 2004, 38, 1261–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.J.; Kim, J.K.; Park, M.C.; Park, Y.B.; Lee, S.K. Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-α therapy. J. Rheumatol. 2009, 36, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with Infliximab, a tumor necrosis factor α-neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.; Silva, M.V.; Barros, C.C.O.; Alexandre, P.B.D.; Timóteo, R.P.; Catarino, J.S.; Sales-Campos, H.; Machado, J.R.; Rodrigues, D.B.R.; Oliveira, C.J.; et al. TNF-α blockade impairs in vitro tuberculous granuloma formation and down modulate Th1, Th17 and Treg cytokines. PLoS ONE 2018, 13, e0194430. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, N.M.; Ismail, K.A.; Badawy, A.F.; Elhasanein, K.F. In vivo effect of anti-TNF agent (etanercept) in reactivation of latent toxoplasmosis. J. Parasit. Dis. 2016, 40, 1459–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wang, L.; Xu, D.; Tang, D.; Li, S.; Du, F.; Wang, L.; Zhao, J.; Fang, R. Risk assessment of etanercept in mice chronically infected with Toxoplasma gondii. Front. Microbiol. 2018, 9, 2822. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Zuccoli, G.; Brigo, F.; Trinka, E.; Golaszewski, S. Cerebral toxoplasmosis following adalimumab treatment in rheumatoid arthritis. Rheumatology 2014, 53, 284. [Google Scholar] [CrossRef] [Green Version]
- Pulivarthi, S.; Reshi, R.A.; McGary, C.T.; Krishna Gurram, M. Cerebral Toxoplasmosis in a Patient on Methotrexate and Infliximab for Rheumatoid Arthritis. Intern. Med. 2015, 54, 1433–1436. [Google Scholar] [CrossRef] [Green Version]
- Brasil. Ministério da Saúde Protocolo Clínico e Diretrizes Terapêuticas da Artrite Reumatoide; The Ministry of Health: Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St.Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Eltoum, I.; Wynn, T.A.; Sher, A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 1993, 151, 3672–3681. [Google Scholar] [CrossRef] [PubMed]
- Gumpel, B.Y.J.M. Cyclophosphamide, Gold and Penicillamine—Disease-Modifying Drugs in Rheumatoid Arthritis—Tailored Dosage and Ultimate Success. Rheumatol. Rehabil. 1976, 15, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Furst, D.E.; Bharat, A.; Curtis, J.R.; Kavanaugh, A.F.; Kremer, J.M.; Moreland, L.W.; O’Dell, J.; Winthrop, K.L.; Beukelman, T.; et al. 2012 update of the 2008 American college of rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012, 64, 625–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, D.P.; Freitas, M.M.P.; Cardoso, M.R.D.; Pajuaba, A.C.A.M.; Silva, N.M.; Mineo, T.W.P.; Silva, J.S.; Mineo, J.R.; Silva, D.A.O. CpG-ODN combined with Neospora caninum lysate, but not with excreted-secreted antigen, enhances protection against infection in mice. Vaccine 2009, 27, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Hedman, K.; Lappalainen, M.; Soderlund, M.; Hedman, L. Avidity of IgG in serodiagnosis of infectious diseases. Rev. Med. Microbiol. 1993, 4, 123–129. [Google Scholar] [CrossRef]
- Da Silva, M.V.; Massaro Junior, V.J.; Machado, J.R.; Silva, D.A.A.; Castellano, L.R.; Alexandre, P.B.D.; Rodrigues, D.B.R.; Rodrigues, V.; Di Carlo, P. Expression pattern of transcription factors and intracellular cytokines reveals that clinically cured tuberculosis is accompanied by an increase in mycobacterium-specific Th1, Th2, and Th17 cells. Biomed Res. Int. 2015, 2015, 591237. [Google Scholar] [CrossRef]
- Miot, H.A. Avaliação da normalidade dos dados em estudos clínicos e experimentais [Assessing normality of data in clinical and experimental trials]. J. Vasc. Bras. 2017, 16, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Torman, V.B.; Coster, R.; Riboldi, J. Normality of variables: Diagnosis methods and comparison of some nonparametric tests by simulation. Clin. Biomed. Res. 2012, 32, 227–234. [Google Scholar]
- Huskinson, J.; Stepick-Biek, P.N.; Araujo, F.G.; Thulliez, P.; Suzuki, Y.; Remington, J.S. Toxoplasma antigens recognized by immunoglobulin G subclasses during acute and chronic infection. J. Clin. Microbiol. 1989, 27, 2031–2038. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, A.H.; Freeman, G.J. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002, 2, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli, R.T.; Wysocka, M.; Hayashi, S.; Denkers, E.Y.; Hieny, S.; Caspar, P.; Trinchieri, G.; Sher, A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994, 153, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Mota, L.M.H.; Cruz, B.A.; Brenol, C.V.; Pereira, I.A.; Rezende-Fronza, L.S.; Bertolo, M.B.; Freitas, M.V.C.; da Silva, N.A.; Louzada-Júnior, P.; Giorgi, R.D.N.; et al. Consenso 2012 da Sociedade Brasileira de Reumatologia para o tratamento da artrite reumatoide. Rev. Bras. Reumatol. 2012, 52, 135–174. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.; Bezerra, M.; Ribeiro, A.; Medeiros, M. Perfil do uso de agentes biológicos no tratamento da artrite reumatoide: Experiência do Hospital Universitário Walter Cantídio. Rev. Med. UFC 2015, 55, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Hosseininejad, Z.; Sharif, M.; Sarvi, S.; Amouei, A.; Hosseini, S.A.; Chegeni, T.N.; Anvari, D.; Saberi, R.; Gohardehi, S.; Mizani, A.; et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006545. [Google Scholar] [CrossRef]
- Oliveira-Scussel, A.C.d.M.; Ferreira, P.T.M.; Resende, R.d.S.; Ratkevicius-Andrade, C.M.; Gomes, A.d.O.; Paschoini, M.C.; De Vito, F.B.; Farnesi-de-Assunção, T.S.; da Silva, M.V.; Mineo, J.R.; et al. Association of gestational diabetes mellitus and negative modulation of the specific humoral and cellular immune response against Toxoplasma gondii. Front. Immunol. 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [Green Version]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, R.; Gigley, J.P.; Khan, I.A. PD-1-Mediated Attrition of Polyfunctional Memory CD8+ T Cells in Chronic Toxoplasma Infection. J. Infect. Dis. 2012, 206, 125–134. [Google Scholar] [CrossRef]
- Bhadra, R.; Gigley, J.P.; Weiss, L.M.; Khan, I.A. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc. Natl. Acad. Sci. USA 2011, 108, 9196–9201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Li, Y.; Yolken, R.H.; Viscidi, R.P. PD-1 immune checkpoint blockade promotes brain leukocyte infiltration and diminishes cyst burden in a mouse model of Toxoplasma infection. J. Neuroimmunol. 2018, 319, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Zheng, Y.; Li, R.; Guo, T.B.; He, D.; Fang, L.; Liu, X.; Xiao, L.; Chen, X.; Wan, B.; et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 2013, 19, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lv, Q.; Wei, Q.; Jiang, Y.; Qi, J.; Xiao, M.; Fang, L.; Xie, Y.; Cao, S.; Lin, Z.; et al. TNF-α inhibitor therapy can improve the immune imbalance of CD4+ T cells and negative regulatory cells but not CD8+ T cells in ankylosing spondylitis. Arthritis Res. Ther. 2020, 22, 149. [Google Scholar] [CrossRef]
- Biton, J.; Semerano, L.; Delavallée, L.; Lemeiter, D.; Laborie, M.; Grouard-Vogel, G.; Boissier, M.-C.; Bessis, N. Interplay between TNF and Regulatory T Cells in a TNF-Driven Murine Model of Arthritis. J. Immunol. 2011, 186, 3899–3910. [Google Scholar] [CrossRef] [Green Version]
- Oldenhove, G.; Bouladoux, N.; Wohlfert, E.A.; Hall, J.A.; Chou, D.; Dos Santos, L.; O’brien, S.; Blank, R.; Lamb, E.; Natarajan, S.; et al. Decrease of Foxp3+ Treg Cell Number and Acquisition of Effector Cell Phenotype during Lethal Infection. Immunity 2009, 31, 772–786. [Google Scholar] [CrossRef] [Green Version]
- McGovern, J.L.; Nguyen, D.X.; Notley, C.A.; Mauri, C.; Isenberg, D.A.; Ehrenstein, M.R. Th17 cells are restrained by treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012, 64, 3129–3138. [Google Scholar] [CrossRef] [Green Version]
- Berner, B.; Akça, D.; Jung, T.; Muller, G.A.; Reuss-Borst, M.A. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rlaeumatoid arthritis by flow cytometry. J. Rheumatol. 2000, 27, 1128–1135. [Google Scholar]
- Sauzullo, I.; Scrivo, R.; Sessa, P.; Mengoni, F.; Vullo, V.; Valesini, G.; Mastroianni, C.M. Changes in T cell effector functions over an 8-year period with TNF antagonists in patients with chronic inflammatory rheumatic diseases. Sci. Rep. 2018, 8, 7881. [Google Scholar] [CrossRef] [Green Version]
- Furiati, S.C.; Catarino, J.S.; Silva, M.V.; Silva, R.F.; Estevam, R.B.; Teodoro, R.B.; Pereira, S.L.; Ataide, M.; Rodrigues, V.; Rodrigues, D.B.R. Th1, Th17, and Treg Responses are Differently Modulated by TNF-α Inhibitors and Methotrexate in Psoriasis Patients. Sci. Rep. 2019, 9, 7526. [Google Scholar] [CrossRef] [Green Version]
- Seong, S.-S.; Choi, C.-B.; Woo, J.-H.; Bae, K.W.; Joung, C.-L.; Uhm, W.-S.; Kim, T.-H.; Jun, J.-B.; Yoo, D.-H.; Lee, J.-T.; et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): Effects of RA itself and of tumor necrosis factor blockers. J. Rheumatol. 2007, 34, 706–711. [Google Scholar] [PubMed]
- Bowen, L.N.; Smith, B.; Reich, D.; Quezado, M.; Nath, A. HIV-associated opportunistic CNS infections: Pathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2016, 12, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Wangsiricharoen, S.; Ligon, C.; Gedmintas, L.; Dehrab, A.; Tungsiripat, M.; Bingham III, C.; Lozada, C.; Calabrese, L. Rates of Serious Infections in HIV-Infected Patients Receiving Tumor. Arthritis Care Res. 2016, 69, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallitano, S.M.; McDermott, L.; Brar, K.; Lowenstein, E. Use of tumor necrosis factor (TNF) inhibitors in patients with HIV/AIDS. J. Am. Acad. Dermatol. 2016, 74, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Naovarat, B.S.; Salazar, G.; Ishimori, M.; Williams, F.M.; Reveille, J.D. Biological treatment usage in patients with HIV and rheumatic disease, 2003–2021: Long-term safety and follow-up. RMD Open 2022, 8, e002282. [Google Scholar] [CrossRef] [PubMed]
- Saliu, O.Y.; Sofer, C.; Stein, D.S.; Schwander, S.K.; Wallis, R.S. Tumor-Necrosis-Factor Blockers: Differential Effects on Mycobacterial Immunity. J. Infect. Dis. 2006, 194, 486–492. [Google Scholar] [CrossRef] [Green Version]




| Wells/Tubes | Culture Time | Extracellular Labeling * | Intracellular Labeling * |
|---|---|---|---|
| B | 48 h | ----- | ----- |
| 1 | 48 h | CD8 BB515 | ----- |
| CD69 PE | |||
| CD4 PE-Cy7 | |||
| B | 96 h | ----- | ----- |
| 2 | 96 h | CD8 BB515 | ----- |
| PD1 PE (CD279) | |||
| CD4 PE-Cy7 | |||
| CD28 APC | |||
| 3 | 96 h | CD25 FITC (IL-2RA) | ----- |
| ----- | FOXP3 PE | ||
| CD4 PE-Cy7 | ----- | ||
| LAP Alexa 647 | ----- | ||
| 4 | 96 h | ----- | IL-17 Alexa 488 |
| ----- | IL-10 PE | ||
| CD4 PE-Cy7 | ----- | ||
| ----- | IFN Alexa 647 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, C.M.R.; de Lima Marques, A.C.; Timóteo, R.P.; de Morais Oliveira-Scussel, A.C.; De Vito, F.B.; da Silva, M.V.; Mineo, J.R.; Teodoro, R.B.; Rodrigues, D.B.R.; Júnior, V.R. Evaluation of Specific Cellular and Humoral Immune Response to Toxoplasma gondii in Patients with Autoimmune Rheumatic Diseases Immunomodulated Due to the Use of TNF Blockers. Biomedicines 2023, 11, 930. https://doi.org/10.3390/biomedicines11030930
Andrade CMR, de Lima Marques AC, Timóteo RP, de Morais Oliveira-Scussel AC, De Vito FB, da Silva MV, Mineo JR, Teodoro RB, Rodrigues DBR, Júnior VR. Evaluation of Specific Cellular and Humoral Immune Response to Toxoplasma gondii in Patients with Autoimmune Rheumatic Diseases Immunomodulated Due to the Use of TNF Blockers. Biomedicines. 2023; 11(3):930. https://doi.org/10.3390/biomedicines11030930
Chicago/Turabian StyleAndrade, Cristhianne Molinero Ratkevicius, Aline Caroline de Lima Marques, Rodolfo Pessato Timóteo, Ana Carolina de Morais Oliveira-Scussel, Fernanda Bernadelli De Vito, Marcos Vinícius da Silva, José Roberto Mineo, Reginaldo Botelho Teodoro, Denise Bertulucci Rocha Rodrigues, and Virmondes Rodrigues Júnior. 2023. "Evaluation of Specific Cellular and Humoral Immune Response to Toxoplasma gondii in Patients with Autoimmune Rheumatic Diseases Immunomodulated Due to the Use of TNF Blockers" Biomedicines 11, no. 3: 930. https://doi.org/10.3390/biomedicines11030930





