Rapamycin Treatment Alleviates Chronic GVHD-Induced Lupus Nephritis in Mice by Recovering IL-2 Production and Regulatory T Cells While Inhibiting Effector T Cells Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Lupus Nephritis in cGVHD Model
2.2. Rapamycin Treatment
2.3. Proteinuria
2.4. ELISA
2.5. Histology
2.6. qPCR
2.7. Immunoblotting
2.8. Flow Cytometry Analysis
2.9. Cell Proliferation by CFSE Analysis
2.10. Statistical Analysis
3. Results
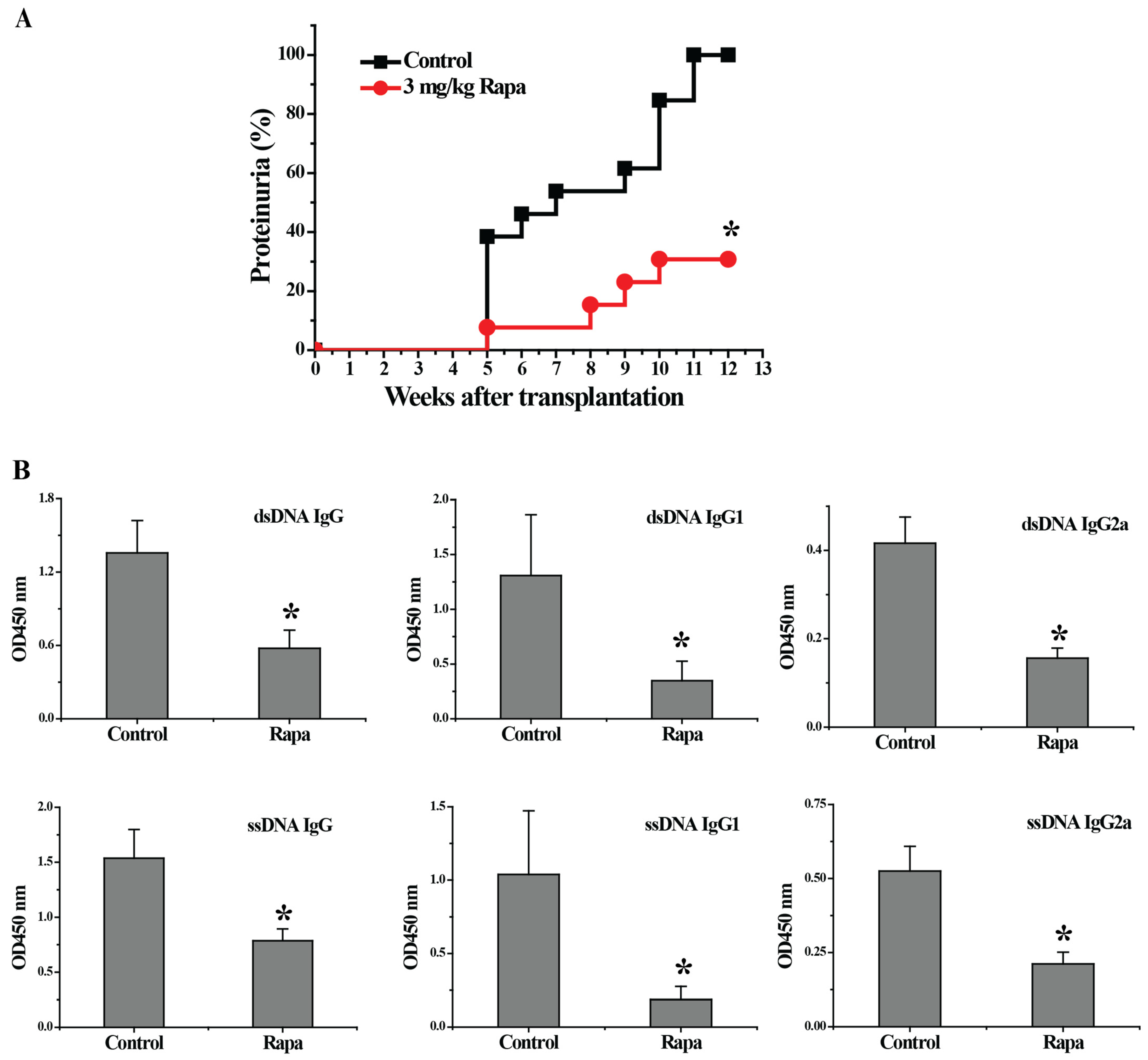
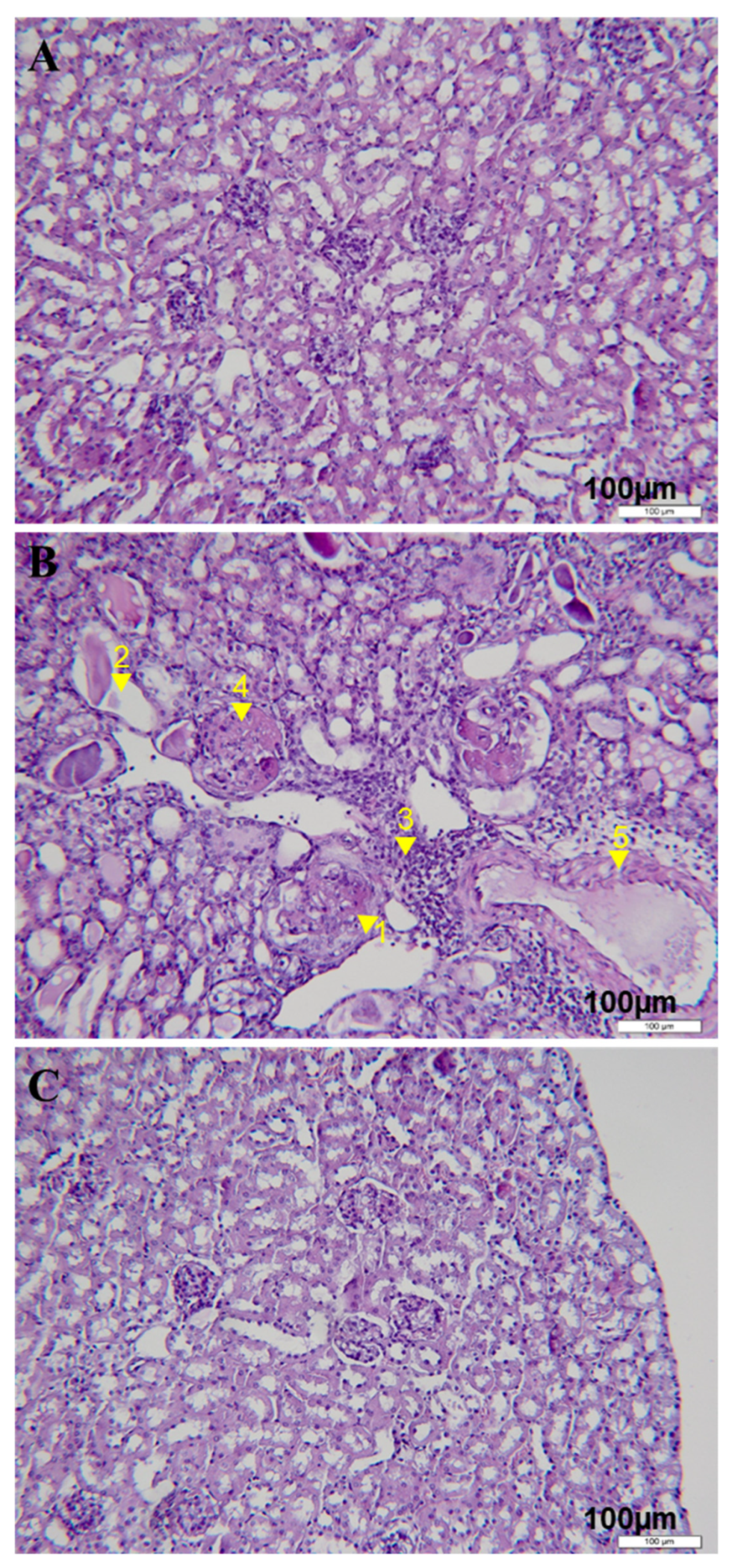
3.1. Rapamycin Treatment Delayed the Onset of Proteinuria, Decreased Serum Autoantibody Levels and Reduced Renal Tissue Damages in Lupus-Nephritis-Bearing Mice
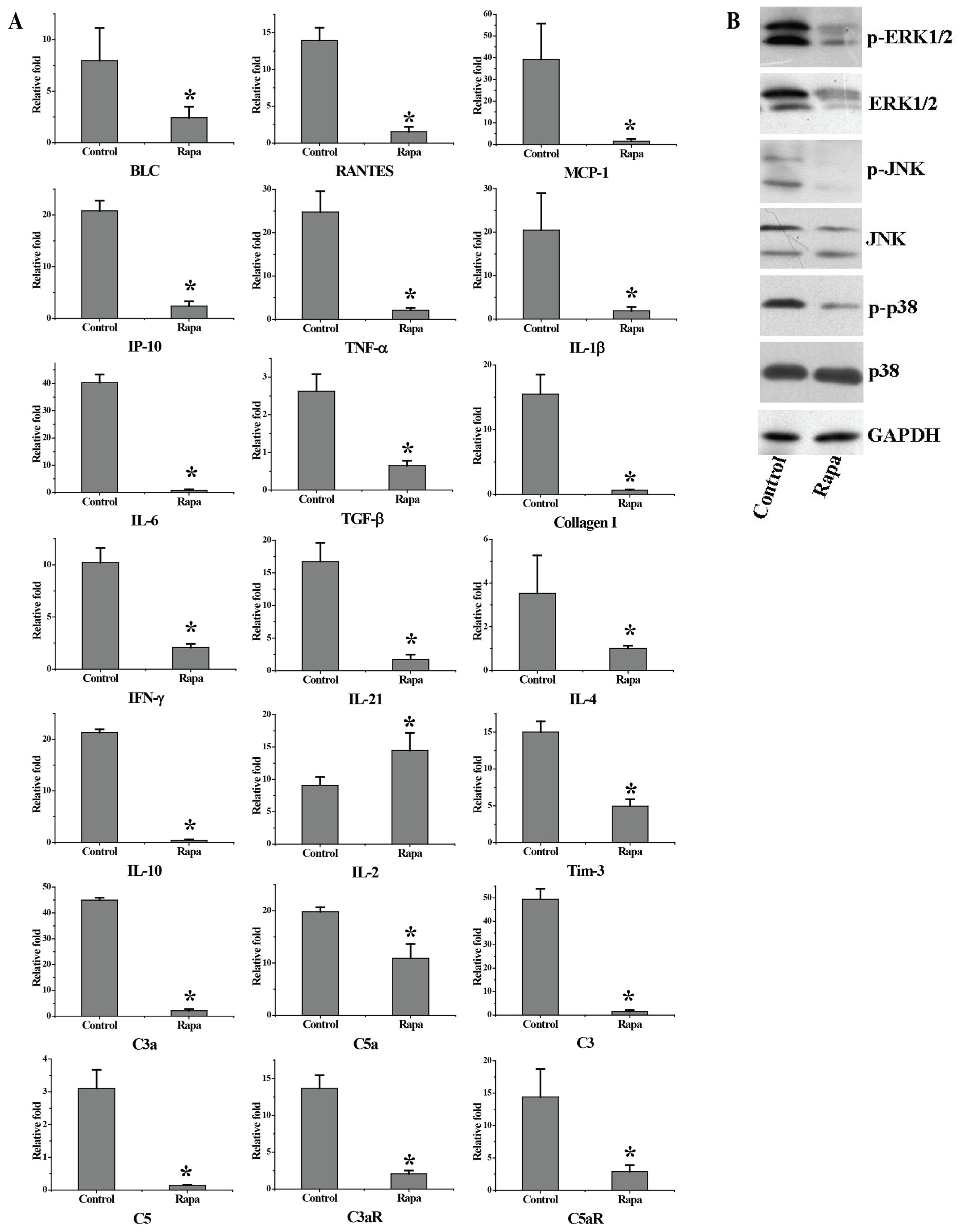
3.2. Rapamycin Treatment Down-Regulated the Expression of Genes Involved in Pathogenesis of Lupus Nephritis and Inflammation-Related Signal Pathways in the Kidney
3.3. Rapamycin Treatment Inhibited Lymphocyte Activation and Proliferation
3.4. Rapamycin Treatment Inhibited Inflammatory Cytokine Production While Enhancing IL-2 Production in Periphery
3.5. Rapamycin Treatment Increased Treg Proportions in Periphery
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.J. New approaches for preventing and treating chronic graft-versus-host disease. Blood 2005, 105, 4200–4206. [Google Scholar] [CrossRef]
- Deeg, H.J.; Storb, R. Graft-versus-host disease: Pathophysiological and clinical aspects. Annu. Rev. Med. 1984, 35, 11–24. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Shulman, H.M.; Storb, R.; Weiden, P.L.; Witherspoon, R.P.; McDonald, G.B.; Schubert, M.M.; Atkinson, K.; Thomas, E.D. Chronic graft-versus-host disease in 52 patients: Adverse natural course and successful treatment with combination immunosuppression. Blood 1981, 57, 267–276. [Google Scholar] [CrossRef]
- Dey, B.; Sykes, M.; Spitzer, T.R. Outcomes of recipients of both bone marrow and solid organ transplants. A review. Medicine 1998, 77, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, S.; Shulman, H.; Ahmed, A.; Shoenfeld, Y.; Peter, J.; McDonald, G.B.; Van de Water, J.; Coppel, R.; Ostlund, C.; Worman, H.J.; et al. Autoantibodies in human chronic graft-versus-host disease after hematopoietic cell transplantation. Clin. Immunol. 1999, 91, 106–116. [Google Scholar] [CrossRef]
- Slayback, D.L.; Dobkins, J.A.; Harper, J.M.; Allen, R.D. Genetic factors influencing the development of chronic graft-versus-host disease in a murine model. Bone Marrow Transpl. 2000, 26, 931–938. [Google Scholar] [CrossRef]
- Kuppers, R.C.; Suiter, T.; Gleichmann, E.; Rose, N.R. The induction of organ-specific antibodies during the graft-vs.-host reaction. Eur. J. Immunol. 1988, 18, 161–166. [Google Scholar] [CrossRef]
- Rus, V.; Svetic, A.; Nguyen, P.; Gause, W.C.; Via, C.S. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells. J. Immunol. 1995, 155, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Gleichmann, E.; Pals, S.T.; Rolink, A.G.; Radaszkiewicz, T.; Gleichmann, H. Graft-versus-host reactions: Clues to the etiopathology of a spectrum of immunological diseases. Immunol. Today 1984, 5, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Rolink, A.G.; Gleichmann, E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J. Exp. Med. 1983, 158, 546–558. [Google Scholar] [CrossRef]
- Sasaki, M.; Hasegawa, H.; Kohno, M.; Inoue, A.; Ito, M.R.; Fujita, S. Antagonist of secondary lymphoid-tissue chemokine (CCR ligand 21) prevents the development of chronic graft-versus-host disease in mice. J. Immunol. 2003, 170, 588–596. [Google Scholar] [CrossRef]
- Kahan, B.D.; Camardo, J.S. Rapamycin: Clinical results and future opportunities. Transplantation 2001, 72, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Berney, J.; Koechlin, C.; Soldati, G.; Cuendet, A.; Younessian, S. Surgical correction of a frontonasal encephalocele. Rev. Otoneuroophtalmol. 1968, 40, 344–350. [Google Scholar] [PubMed]
- Blazar, B.R.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Vallera, D.A. Rapamycin inhibits the generation of graft-versus-host disease- and graft-versus-leukemia-causing T cells by interfering with the production of Th1 or Th1 cytotoxic cytokines. J. Immunol. 1998, 160, 5355–5365. [Google Scholar] [CrossRef]
- Cutler, C.; Antin, J.H. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transpl. 2004, 34, 471–476. [Google Scholar] [CrossRef]
- Chen, B.J.; Morris, R.E.; Chao, N.J. Graft-versus-host disease prevention by rapamycin: Cellular mechanisms. Biol Blood Marrow Transpl. 2000, 6, 529–536. [Google Scholar] [CrossRef]
- Schmucki, K.; Hofmann, P.; Fehr, T.; Inci, I.; Kohler, M.; Schuurmans, M.M. Mammalian Target of Rapamycin Inhibitors and Kidney Function After Thoracic Transplantation: A Systematic Review and Recommendations for Management of Lung Transplant Recipients. Transplantation 2023, 107, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N. Rapamune (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem. 1998, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.T.; Wiederrecht, G.J. Immunopharmacology of rapamycin. Annu. Rev. Immunol. 1996, 14, 483–510. [Google Scholar] [CrossRef]
- Bjornsti, M.A.; Houghton, P.J. The TOR pathway: A target for cancer therapy. Nat. Rev. Cancer 2004, 4, 335–348. [Google Scholar] [CrossRef]
- Wiederrecht, G.J.; Sabers, C.J.; Brunn, G.J.; Martin, M.M.; Dumont, F.J.; Abraham, R.T. Mechanism of action of rapamycin: New insights into the regulation of G1-phase progression in eukaryotic cells. Prog. Cell Cycle Res. 1995, 1, 53–71. [Google Scholar] [CrossRef]
- Suzuki, O.; Koura, M.; Uchio-Yamada, K.; Sasaki, M. Urinary protein analysis in mice lacking major urinary proteins. Exp. Anim. 2021, 70, 406–411. [Google Scholar] [CrossRef]
- Hurst, J.L.; Beynon, R.J.; Armstrong, S.D.; Davidson, A.J.; Roberts, S.A.; Gomez-Baena, G.; Smadja, C.M.; Ganem, G. Molecular heterogeneity in major urinary proteins of Mus musculus subspecies: Potential candidates involved in speciation. Sci. Rep. 2017, 7, 44992. [Google Scholar] [CrossRef]
- Hill, G.R.; Betts, B.C.; Tkachev, V.; Kean, L.S.; Blazar, B.R. Current Concepts and Advances in Graft-Versus-Host Disease Immunology. Annu. Rev. Immunol. 2021, 39, 19–49. [Google Scholar] [CrossRef]
- Hill, G.R.; Koyama, M. Cytokines and costimulation in acute graft-versus-host disease. Blood 2020, 136, 418–428. [Google Scholar] [CrossRef]
- Holtzman, N.G.; Pavletic, S.Z. The clinical landscape of chronic graft-versus-host disease management in 2021. Br. J. Haematol. 2022, 196, 830–848. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef]
- Martinez-Cibrian, N.; Zeiser, R.; Perez-Simon, J.A. Graft-versus-host disease prophylaxis: Pathophysiology-based review on current approaches and future directions. Blood Rev. 2021, 48, 100792. [Google Scholar] [CrossRef]
- Martini, D.J.; Chen, Y.B.; DeFilipp, Z. Recent FDA Approvals in the Treatment of Graft-Versus-Host Disease. Oncologist 2022, 27, 685–693. [Google Scholar] [CrossRef]
- Braun, L.M.; Zeiser, R. Kinase Inhibition as Treatment for Acute and Chronic Graft-Versus-Host Disease. Front. Immunol. 2021, 12, 760199. [Google Scholar] [CrossRef]
- Jeon, H.J.; Lee, H.E.; Yang, J. Safety and efficacy of Rapamune(R) (Sirolimus) in kidney transplant recipients: Results of a prospective post-marketing surveillance study in Korea. BMC Nephrol. 2018, 19, 201. [Google Scholar] [CrossRef]
- Pidala, J.; Hamadani, M.; Dawson, P.; Martens, M.; Alousi, A.M.; Jagasia, M.; Efebera, Y.A.; Chhabra, S.; Pusic, I.; Holtan, S.G.; et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: The BMT CTN 1501 trial. Blood 2020, 135, 97–107. [Google Scholar] [CrossRef]
- Bejanyan, N.; Pidala, J.A.; Wang, X.; Thapa, R.; Nishihori, T.; Elmariah, H.; Lazaryan, A.; Khimani, F.; Davila, M.L.; Mishra, A.; et al. A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv. 2021, 5, 1154–1163. [Google Scholar] [CrossRef]
- Zeng, J.; Zhong, Q.; Feng, X.; Li, L.; Feng, S.; Fan, Y.; Song, T.; Huang, Z.; Wang, X.; Lin, T. Conversion From Calcineurin Inhibitors to Mammalian Target of Rapamycin Inhibitors in Kidney Transplant Recipients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Immunol. 2021, 12, 663602. [Google Scholar] [CrossRef]
- Carpenter, P.A.; Logan, B.R.; Lee, S.J.; Weisdorf, D.J.; Johnston, L.; Costa, L.J.; Kitko, C.L.; Bolanos-Meade, J.; Sarantopoulos, S.; Alousi, A.M.; et al. A phase II/III randomized, multicenter trial of prednisone/sirolimus versus prednisone/ sirolimus/calcineurin inhibitor for the treatment of chronic graft-versus-host disease: BMT CTN 0801. Haematologica 2018, 103, 1915–1924. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.C.; Zheng, X.X.; Wells, A.D.; Turka, L.A.; Strom, T.B. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat. Med. 1999, 5, 1298–1302. [Google Scholar] [CrossRef]
- Blaha, P.; Bigenzahn, S.; Koporc, Z.; Schmid, M.; Langer, F.; Selzer, E.; Bergmeister, H.; Wrba, F.; Kurtz, J.; Kiss, C.; et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood 2003, 101, 2886–2893. [Google Scholar] [CrossRef]
- Turnquist, H.R.; Raimondi, G.; Zahorchak, A.F.; Fischer, R.T.; Wang, Z.; Thomson, A.W. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 2007, 178, 7018–7031. [Google Scholar] [CrossRef]
- Battaglia, M.; Stabilini, A.; Roncarolo, M.G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005, 105, 4743–4748. [Google Scholar] [CrossRef]
- Coenen, J.J.; Koenen, H.J.; van Rijssen, E.; Hilbrands, L.B.; Joosten, I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 2006, 107, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Game, D.S.; Hernandez-Fuentes, M.P.; Lechler, R.I. Everolimus and basiliximab permit suppression by human CD4+CD25+ cells in vitro. Am. J. Transplant. 2005, 5, 454–464. [Google Scholar] [CrossRef]
- Hippen, K.L.; Merkel, S.C.; Schirm, D.K.; Nelson, C.; Tennis, N.C.; Riley, J.L.; June, C.H.; Miller, J.S.; Wagner, J.E.; Blazar, B.R. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am. J. Transplant. 2011, 11, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Stabilini, A.; Migliavacca, B.; Horejs-Hoeck, J.; Kaupper, T.; Roncarolo, M.G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 2006, 177, 8338–8347. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Leveson-Gower, D.B.; Zambricki, E.A.; Kambham, N.; Beilhack, A.; Loh, J.; Hou, J.Z.; Negrin, R.S. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 2008, 111, 453–462. [Google Scholar] [CrossRef]
- Strauss, L.; Whiteside, T.L.; Knights, A.; Bergmann, C.; Knuth, A.; Zippelius, A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J. Immunol. 2007, 178, 320–329. [Google Scholar] [CrossRef]
- Zorn, E.; Nelson, E.A.; Mohseni, M.; Porcheray, F.; Kim, H.; Litsa, D.; Bellucci, R.; Raderschall, E.; Canning, C.; Soiffer, R.J.; et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006, 108, 1571–1579. [Google Scholar] [CrossRef]
- Long, S.A.; Buckner, J.H. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J. Autoimmun. 2008, 30, 293–302. [Google Scholar] [CrossRef]
- Torng, S.; Rigatto, C.; Rush, D.N.; Nickerson, P.; Jeffery, J.R. The urine protein to creatinine ratio (P/C) as a predictor of 24-hour urine protein excretion in renal transplant patients. Transplantation 2001, 72, 1453–1456. [Google Scholar] [CrossRef]
- Douglas, C.E.; Roem, J.; Flynn, J.T.; Furth, S.L.; Warady, B.A.; Halbach, S.M.; Chronic Kidney Disease in Children Study Investigators. Effect of Age on Hypertension Recognition in Children with Chronic Kidney Disease: A Report from the Chronic Kidney Disease in Children Study. Hypertension 2023, 80. [Google Scholar] [CrossRef]
- Korbut, A.I.; Romanov, V.V.; Klimontov, V.V. Urinary Markers of Tubular Injury and Renal Fibrosis in Patients with Type 2 Diabetes and Different Phenotypes of Chronic Kidney Disease. Life 2023, 13, 343. [Google Scholar] [CrossRef] [PubMed]


| Genes | Primer 1 (Forward, 5′→3′) | Primer 2 (Reverse, 5′→3′) |
|---|---|---|
| BLC | atgaggctcagcacagcaacgctgcttc | tccgatctatgatgtttagaccgacaacag |
| RANTES | atgaagatctctgcagctgccctc | tgctggtgtagaaatactccttgac |
| MCP-1 | atgcaggtccctgtcatgcttctg | ggtgatcctcttgtagctctccag |
| IP-10 | atccctctcgcaaggacggtccgctg | tcttagattccggattcagacatc |
| TNF-α | attcgagtgacaagcctgtagcccac | ctgggagtagacaaggtacaacccat |
| IL-1β | gcccatcctctgtgactcat | aggccacaggtattttgtcg |
| IL-6 | ttccctacttcacaagtccggagaggag | ctgcaagtgcatcatcgttgttc |
| TGF-β1 | ataccaactattgcttcagctccacag | gtactgtgtgtccaggctccaaatat |
| pro (α1) I collagen | aggcaacagtcgcttcacctacagc | caatgtctagtccgaattcctggtct |
| IFN-γ | gcacagtcattgaaagcctagaaagtc | ggtagaaagagataatctggctctg |
| IL-21 | agacattcatcattgacctcgtg | gaatcatcttttgaaggagcca |
| IL-4 | ctccatgcttgaagaagaactctag | atttgcatgatgctctttaggct |
| IL-10 | actgctaaccgactccttaatg | catcctgagggtcttcagcttct |
| IL-2 [21] | cccacttcaagctccacttc | atcctggggagtttcaggtt |
| Tim-3 | cacttgtgcctatgtgctg | ttgttgagatcgcccttta |
| C3a | tcggaagtgttgtgaggat | gttgcagcagtctatgaagg |
| C5a | agaaatgctgctatgacgga | ttcttttcggatcttgttcg |
| C3 | gggagaagttcggcatag | gttgttgaaggcagcatag |
| C5 | gctgcctgactcactaac | tcctcgcacaacagaata |
| C3aR [21] | taaccagatgagcaccacca | tgtgaatgttgtgtgcatgg |
| C5aR [22] | gatgccaccgcctgtatagt | acgaaggatggaatggtgag |
| GAPDH | tgaaggtcggtgtgaacggatttg | gttgaatttgccgtgagtggagtc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, X.; Wang, R.; Chen, G.; Wang, J.; Feng, J.; Li, Y.; Yu, Z.; Xiao, H. Rapamycin Treatment Alleviates Chronic GVHD-Induced Lupus Nephritis in Mice by Recovering IL-2 Production and Regulatory T Cells While Inhibiting Effector T Cells Activation. Biomedicines 2023, 11, 949. https://doi.org/10.3390/biomedicines11030949
Zhang J, Wang X, Wang R, Chen G, Wang J, Feng J, Li Y, Yu Z, Xiao H. Rapamycin Treatment Alleviates Chronic GVHD-Induced Lupus Nephritis in Mice by Recovering IL-2 Production and Regulatory T Cells While Inhibiting Effector T Cells Activation. Biomedicines. 2023; 11(3):949. https://doi.org/10.3390/biomedicines11030949
Chicago/Turabian StyleZhang, Jilu, Xun Wang, Renxi Wang, Guojiang Chen, Jing Wang, Jiannan Feng, Yan Li, Zuyin Yu, and He Xiao. 2023. "Rapamycin Treatment Alleviates Chronic GVHD-Induced Lupus Nephritis in Mice by Recovering IL-2 Production and Regulatory T Cells While Inhibiting Effector T Cells Activation" Biomedicines 11, no. 3: 949. https://doi.org/10.3390/biomedicines11030949
APA StyleZhang, J., Wang, X., Wang, R., Chen, G., Wang, J., Feng, J., Li, Y., Yu, Z., & Xiao, H. (2023). Rapamycin Treatment Alleviates Chronic GVHD-Induced Lupus Nephritis in Mice by Recovering IL-2 Production and Regulatory T Cells While Inhibiting Effector T Cells Activation. Biomedicines, 11(3), 949. https://doi.org/10.3390/biomedicines11030949






