The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Material
2.2. Overall Collagenolytic Activity Evaluation
2.3. Gelatinases’ Content
2.4. Gelatinases’ Western Blot
2.5. Gelatinases’ Activity
2.6. Protein Determination
2.7. Statistical Analysis
3. Results
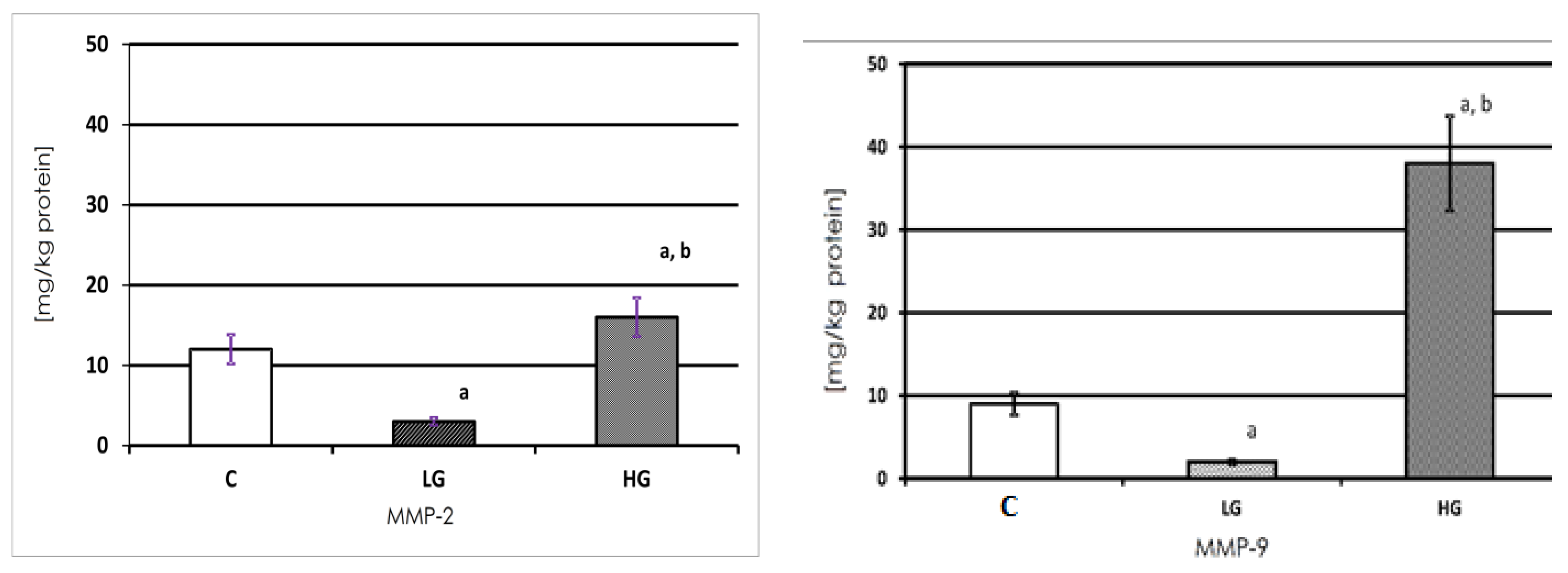
3.1. MMP-2 and MMP-9 Content
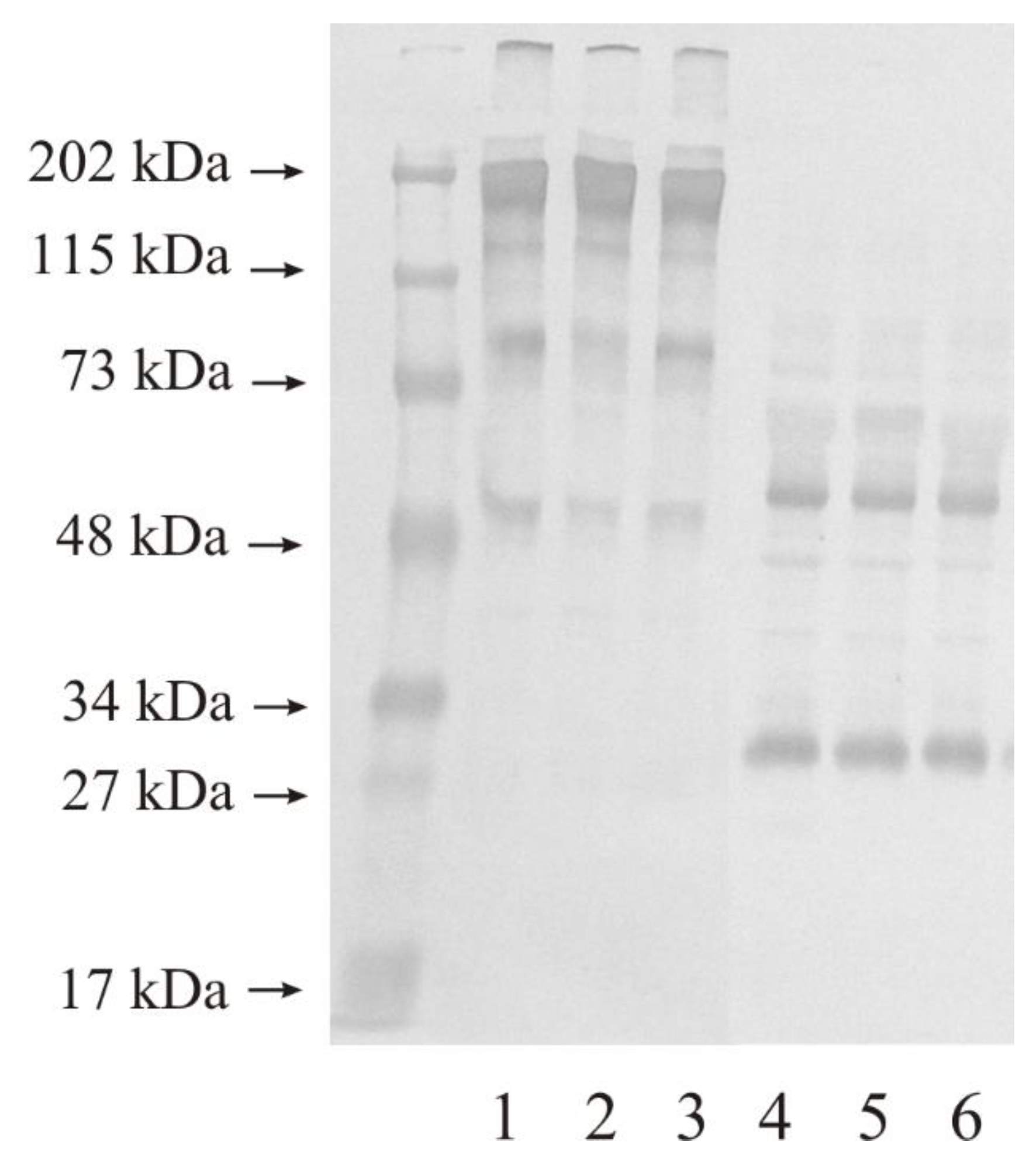
3.2. Western Blot Analysis of Investigated Gelatinases
3.3. Expression of MMP-2 in Human Urinary Bladder
3.4. Expression of MMP-9 in Human Urinary Bladder
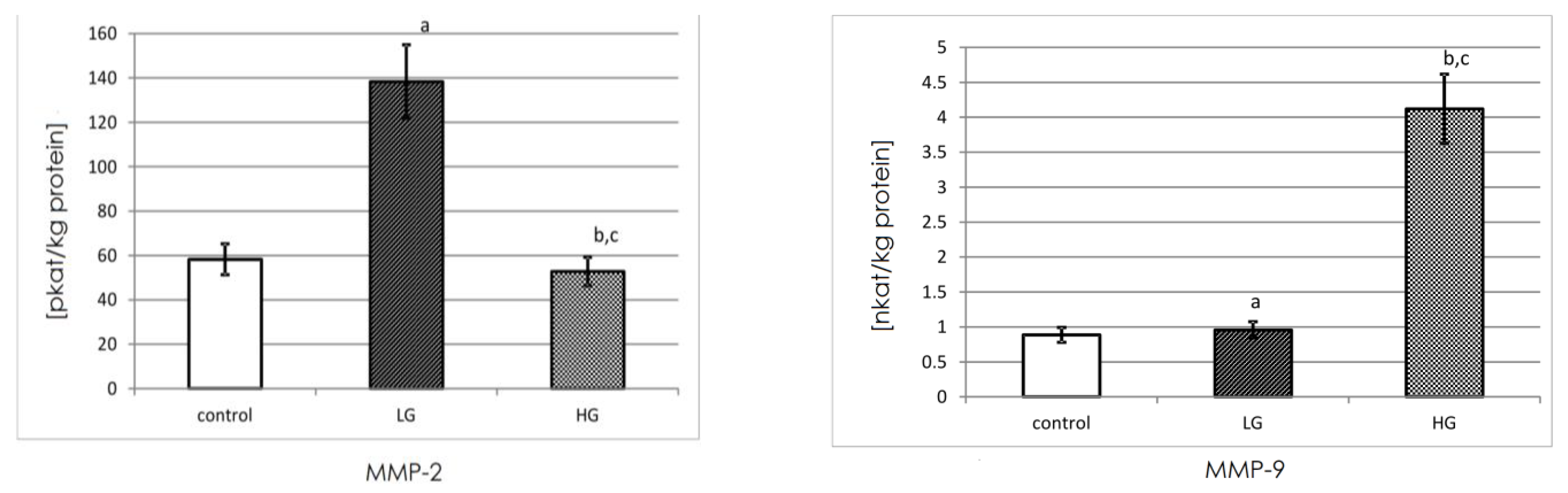
3.5. Actual Activity of MMP-2 and MMP-9
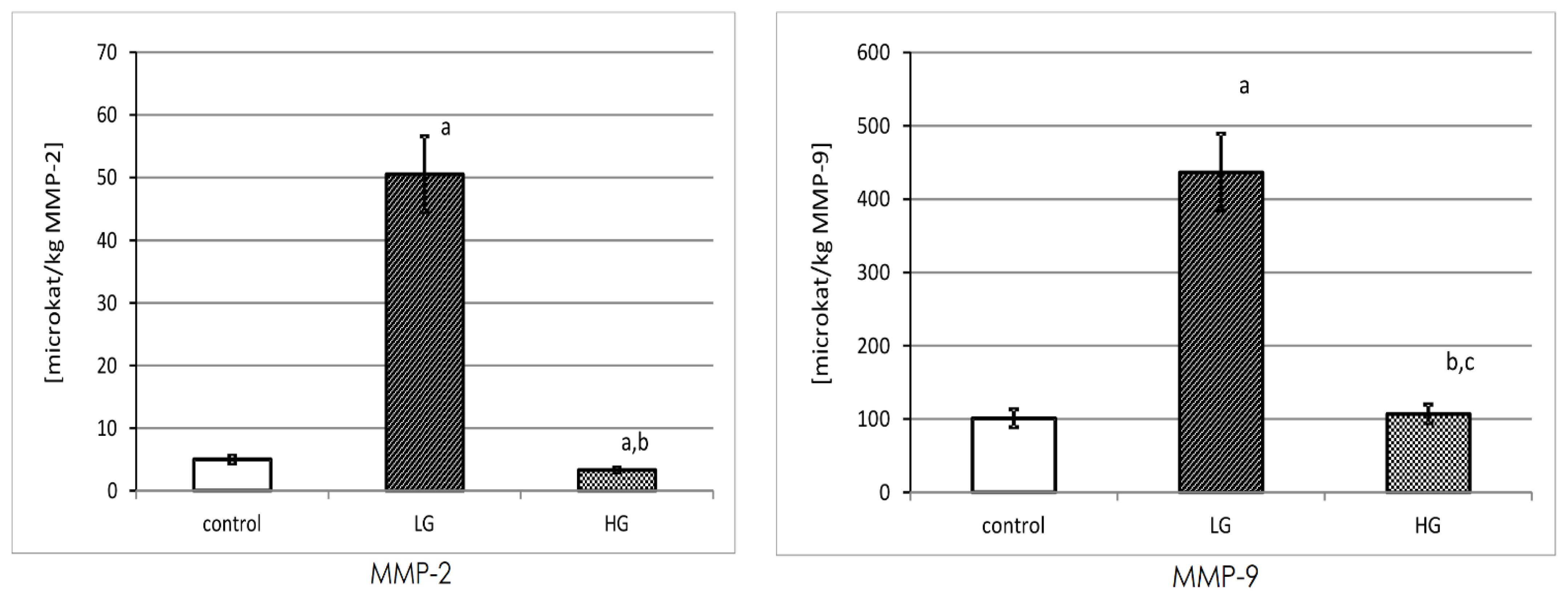
3.6. Specific Activity of MMP-2 and MMP-9
4. Discussion
4.1. Conclusions
4.2. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Wątroba, S.; Wiśniowski, T.; Bryda, J.; Kurzepa, J. The role of matrix metalloproteinases in pathogenesis of human bladder cancer. Acta Biochim. Pol. 2021, 68, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Golabek, T.; Darewicz, B.; Borawska, M.; Socha, K.; Markiewicz, R.; Kudelski, J. Copper, zinc, and Cu/Zn ratio in transitional cell carcinoma of the bladder. Urol. Int. 2012, 89, 342–347. [Google Scholar] [CrossRef]
- Golabek, T.; Darewicz, B.; Kudelski, J.; Socha, K.; Markiewicz-Zukowska, R.; Chlosta, P.; Okon, K.; Borawska, M. Cadmium in urothelial carcinoma of the bladder. Pol. J. Pathol. 2014, 65, 55–59. [Google Scholar] [CrossRef]
- Aitken, K.; Bagli, D.R. The bladder extracellular matrix. Part I: Architecture, development and disease. Nat. Rev. Urol. 2009, 6, 596–611. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Kudelski, J.; Młynarczyk, G.; Gudowska-Sawczuk, M.; Mroczko, B.; Darewicz, B.; Bruczko-Goralewska, M.; Sobolewski, K.; Romanowicz, L. Enhanced Expression but Decreased Specific Activity of Matrix Metalloproteinase 10 (MMP-10) in Comparison with Matrix Metalloproteinase 3 (MMP-3) in Human Urinary Bladder Carcinoma. J. Clin. Med. 2021, 10, 3683. [Google Scholar] [CrossRef]
- Zajkowska, M.; Zbucka-Krętowska, M.; Sidorkiewicz, I.; Lubowicka, E.; Będkowska, G.E.; Gacuta, E.; Szmitkowski, M.; Ławicki, S. Human Plasma Levels of Vascular Endothelial Growth Factor, Matrix Metalloproteinase 9, and Tissue Inhibitor of Matrix Metalloproteinase 1 and Their Applicability as Tumor Markers in Diagnoses of Cervical Cancer Based on ROC Analysis. Cancer Control. J. Moffitt Cancer Cent. 2018, 25, 1073274818789357. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim. Biophys. Acta 2012, 1825, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vasala, K.; Paakko, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 immunoreactive protein as a prognostic marker in bladder cancer. Urology 2003, 62, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Gohji, K.; Fujimoto, N.; Komiyama, T.; Fujii, A.; Ohkawa, J.; Kamidono, S.; Nakajima, M. Elevation of serum levels of matrix metalloproteinase-2 and -3 as new predictors of recurrence in patients with urothelial carcinoma. Cancer 1996, 78, 2379–2387. [Google Scholar] [CrossRef]
- Vasala, L.; Kuvaja, P.; Turpeenniemi-Hujanen, T. Low circulating levels of Pro-MMP-2 are associated with adverse prognosis in bladder cancer. Tumour Biol. 2008, 29, 279–286. [Google Scholar] [CrossRef]
- Brooks, M.; Mo, Q.; Krasnow, R.; Levy, P.; Lee, Y.; Xiao, J.; Kurtova, A.; Lerner, S.; Godoy, G.; Jian, W.; et al. Positive association of collagen type I with non-muscle invasive bladder cancer progression. Oncotarget 2016, 7, 82609–82619. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Ismael, N.R.; Shaarawy, S.M.; El-Merzabani, M.M. Prognostic value of membrane type 1 and 2 matrix metalloproteinase expression and gelatinase A activity in bladder cancer. Int. J. Biol. Markers 2010, 25, 69–74. [Google Scholar] [CrossRef]
- Ozdemir, E.; Kakehi, Y.; Okuno, H.; Yoshida, O. Role of matrix metalloproteinase-9 in the basement membrane destruction of superficial urothelial carcinomas. J. Urol. 1999, 161, 1359–1363. [Google Scholar] [CrossRef]
- Sier, C.F.; Casetta, G.; Verheijen, J.H.; Tizzani, A.; Agape, V.; Kos, J.; Blasi, F.; Hanemaaijer, R. Enhanced urinary gelatinase activities (matrix metalloproteinases 2 and 9) are associated with early-stage bladder carcinoma: A comparison with clinically used tumor markers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 2333–2340. [Google Scholar]
- Vasala, K.; Paakko, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-9 immunoreactive protein in urinary bladder cancer: A marker of favorable prognosis. Anticancer Res. 2008, 28, 1757–1761. [Google Scholar] [PubMed]
- Kader, A.K.; Shao, L.; Dinney, C.P.; Schabath, M.B.; Wang, Y.; Liu, J.; Gu, J.; Grossman, H.B.; Wu, X. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006, 66, 11644–11648. [Google Scholar] [CrossRef]
- Guan, K.P.; Ye, H.Y.; Yan, Z.; Wang, Y.; Hou, S.K. Serum levels of endostatin and matix metalloproteinase-9 associated with high stage and grade primary transitional cell carcinoma of the bladder. Urology 2003, 61, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Gerhards, S.; Jung, K.; Koenig, F.; Daniltchenko, D.; Hauptmann, S.; Schnorr, D.; Loening, S.A. Excretion of matrix metalloproteinase 2 and 9 are associated with a high stage and grade of bladder carcinoma. Urology 2001, 57, 675–679. [Google Scholar] [CrossRef]
- Eissa, S.; Ali-Labib, R.; Swellam, M.; Bassiony, M.; Tash, F.; El-Zayat, T.M. Noninvasive diagnosis of bladder cancer by detection of matrix metalloproteinases (MMP-2 and MMP-9) and their inhibitor (TIMP-2) in urine. Eur. Urol. 2007, 52, 1388–1396. [Google Scholar] [CrossRef]
- Bruczko, M.; Gogiel, T.; Wolańska, M.; Kowalewski, R.; Sobolewski, K.; Romanowicz, L. MT1-MMP evaluation in neointimal hyperplasia in the late follow-up after prosthesis implantation. Int. J. Exp. Pathol. 2019, 100, 94–101. [Google Scholar] [CrossRef]
- Unemori, E.N.; Werb, Z. Reorganization of polymerized actin: A possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J. Cell Biol. 1989, 103, 1021–1031. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Watkins, G.A.; Fung Jones, E.; Scott Shell, M.; VanBrocklin, H.F.; Pan, M.-H.; Hanrahan, S.M.; Feng, J.J.; He, J.; Sounni, N.E.; Dill, K.A.; et al. Development of an optimized activatable MMP-14 targeted SPECT imaging probe. Bioorg. Med. Chem. 2009, 17, 653–659. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.; Salem, H.; Ellakwa, D.E.; Abdel-Hamid, M. MMP-2 and MMP-9 as prognostic markers for the early detection of urinary bladder cancer. J. Biochem. Mol. Toxicol. 2019, 33, e22275. [Google Scholar] [CrossRef] [PubMed]
- Ramón de Fata, F.; Ferruelo, A.; Andrés, G.; Gimbernat, H.; Sánchez-Chapado, M.; Angulo, J.C. The role of matrix metalloproteinase MMP-9 and TIMP-2 tissue inhibitor of metalloproteinases as serum markers of bladder cancer. Actas Urol. Esp. 2013, 37, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Monier, F.; Surla, A.; Guillot, M.; Morel, F. Gelatinase isoforms in urine from bladder cancer patients. Clin. Chim. Acta Int. J. Clin. Chem. 2000, 299, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.; Tzankov, A. The role of structural extracellular matrix proteins in urothelial bladder cancer (review). Biomark. Insights 2007, 2, 418–427. [Google Scholar] [CrossRef]
- Kanda, K.; Takahashi, M.; Murakami, Y.; Kanayama, H.; Kagawa, S. The role of the activated form of matrix metalloproteinase-2 in urothelial cancer. BJU Int. 2000, 86, 553–557. [Google Scholar] [CrossRef]
- Wu, G.J.; Bao, J.S.; Yue, Z.J.; Zeng, F.C.; Cen, S.; Tang, Z.Y.; Kang, X.L. Elevated expression of matrix metalloproteinase-9 is associated with bladder cancer pathogenesis. J. Can. Res. Ther. 2018, 14, S54–S59. [Google Scholar]
- Papathoma, A.S.; Petraki, C.; Grigorakis, A.; Papakonstantinou, H.; Karavana, V.; Stefanakis, S.; Sotsiou, F.; Pintzas, A. Prognostic significance of matrix metalloproteinases 2 and 9 in bladder cancer. Anticancer. Res. B 2000, 20, 2009–2013. [Google Scholar]
- Davies, B.; Waxman, J.; Wasan, H.; Abel, P.; Williams, G.; Krausz, T.; Neal, D.; Thomas, D.; Hanby, A.; Balkwill, F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993, 53, 5365–5369. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudelski, J.; Tokarzewicz, A.; Gudowska-Sawczuk, M.; Mroczko, B.; Chłosta, P.; Bruczko-Goralewska, M.; Mitura, P.; Młynarczyk, G. The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer. Biomedicines 2023, 11, 956. https://doi.org/10.3390/biomedicines11030956
Kudelski J, Tokarzewicz A, Gudowska-Sawczuk M, Mroczko B, Chłosta P, Bruczko-Goralewska M, Mitura P, Młynarczyk G. The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer. Biomedicines. 2023; 11(3):956. https://doi.org/10.3390/biomedicines11030956
Chicago/Turabian StyleKudelski, Jacek, Anna Tokarzewicz, Monika Gudowska-Sawczuk, Barbara Mroczko, Piotr Chłosta, Marta Bruczko-Goralewska, Przemysław Mitura, and Grzegorz Młynarczyk. 2023. "The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer" Biomedicines 11, no. 3: 956. https://doi.org/10.3390/biomedicines11030956
APA StyleKudelski, J., Tokarzewicz, A., Gudowska-Sawczuk, M., Mroczko, B., Chłosta, P., Bruczko-Goralewska, M., Mitura, P., & Młynarczyk, G. (2023). The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer. Biomedicines, 11(3), 956. https://doi.org/10.3390/biomedicines11030956






