Updates on Lymphovascular Invasion in Breast Cancer
Abstract
:1. Introduction
2. Histological Characteristics of Lymphovascular Invasion
2.1. Histological Diagnosis
Lymphovascular Invasion Quantification
2.2. Pitfalls
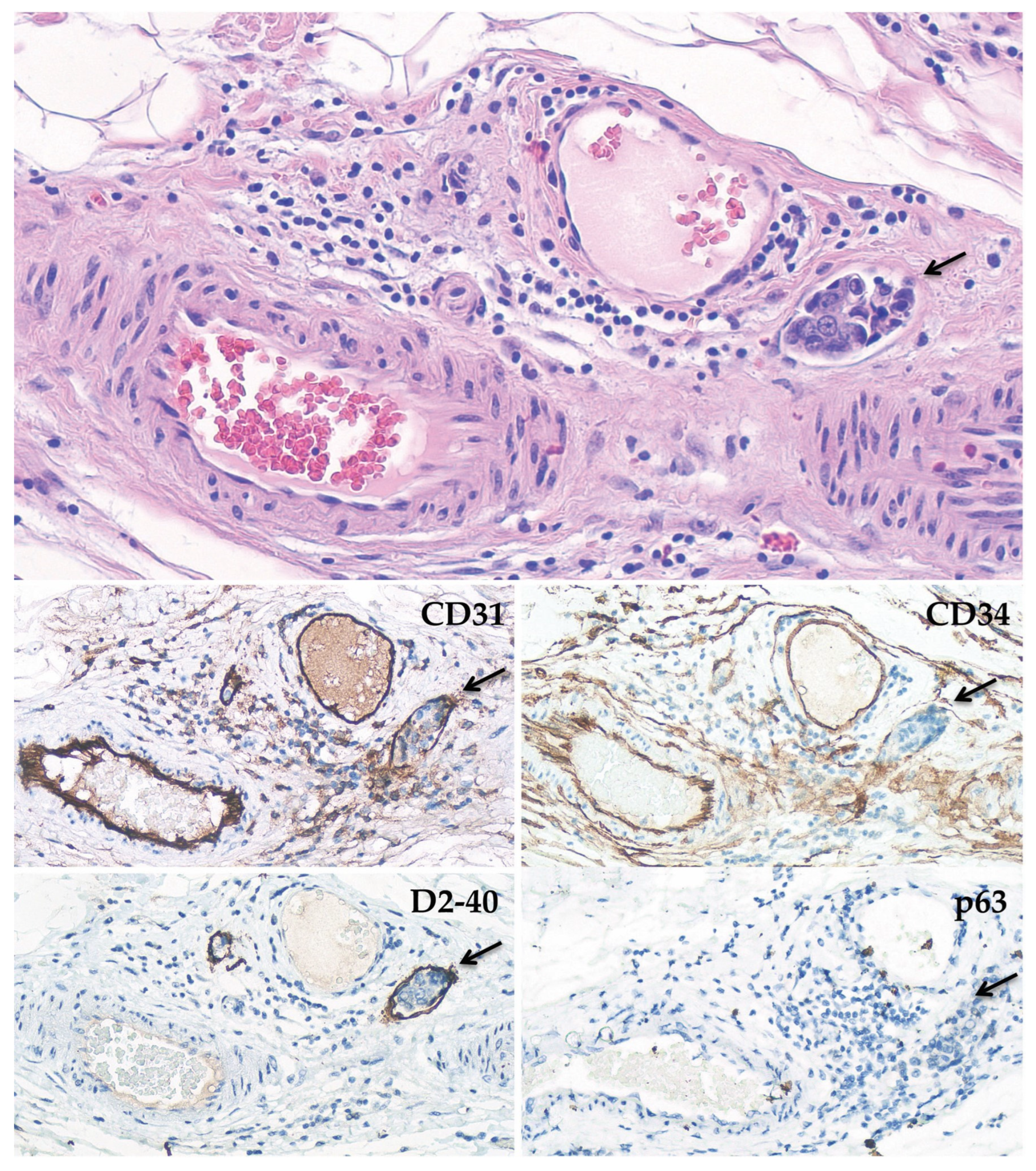
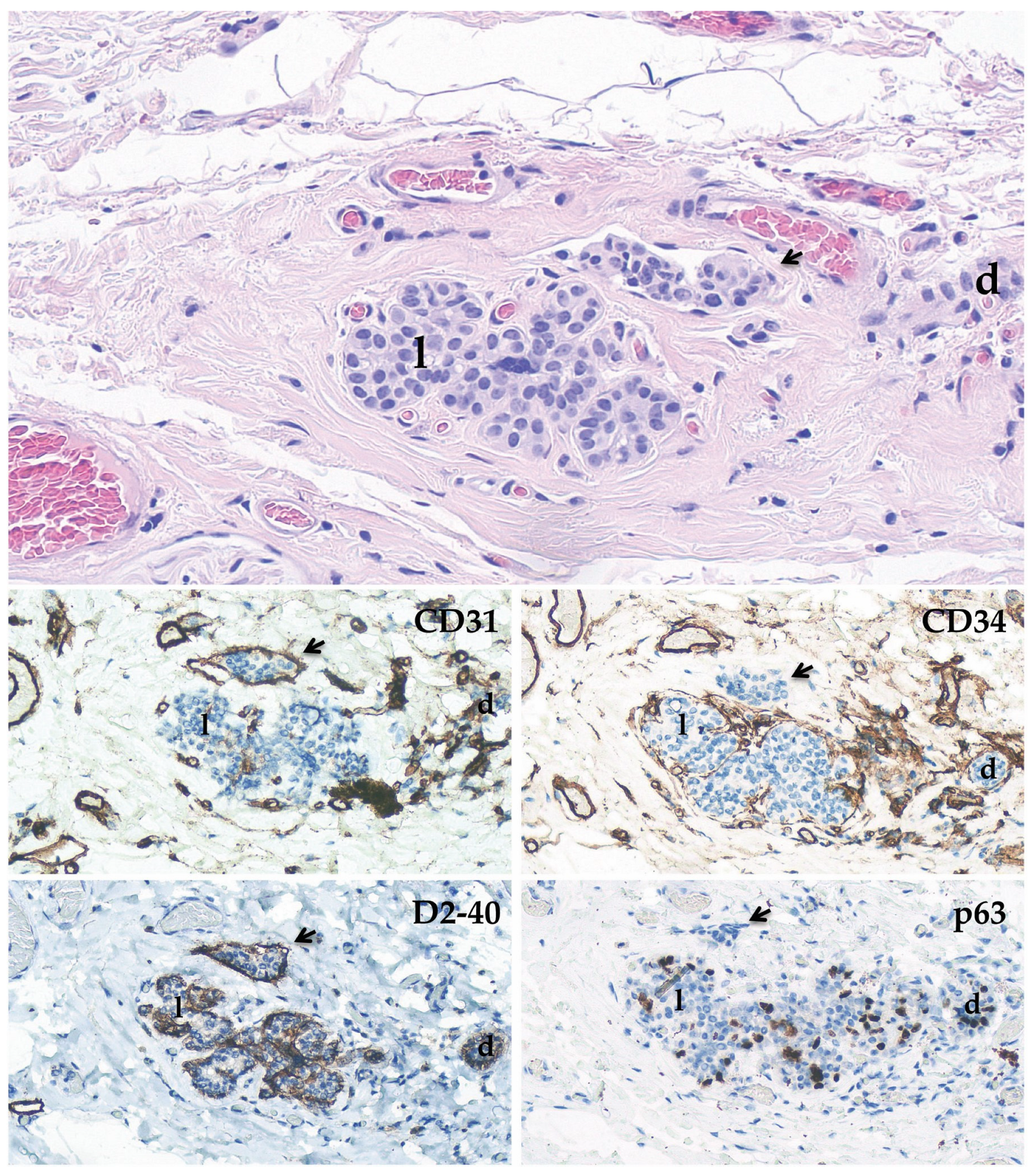
2.3. Immunohistochemical Stainings
3. Biological Overview of Lymphovascular Invasion
4. Clinicopathological Associations
Lymphovascular Invasion in Heredo–Familial Breast Cancer
5. Therapeutic Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammed, R.A.; Martin, S.G.; Mahmmod, A.M.; Macmillan, R.D.; Green, A.R.; Paish, E.C.; Ellis, I.O. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: Findings from a large case series with long-term follow-up. J. Pathol. 2011, 223, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Torous, V.F.; Simpson, R.W.; Balani, J.P.; Baras, A.S.; Berman, M.A.; Birdsong, G.G.; Giannico, G.A.; Paner, G.P.; Pettus, J.R.; Sessions, Z.; et al. College of American Pathologists Cancer Protocols: From Optimizing Cancer Patient Care to Facilitating Interoperable Reporting and Downstream Data Use. JCO Clin. Cancer Inf. 2021, 5, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.P.; Nguyen, J.P.T.; Wang, J.; Ahn, J.S.; Libling, W.A.; Klein, J.M.; Mazumder, P.; Barsky, S.H. Geometric tumor embolic budding characterizes inflammatory breast cancer. Breast Cancer Res. Treat. 2023, 197, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, P.; Connolly, J.; Bose, S. Protocol for the examination of resection specimens from patients with invasive carcinoma of the breast. Coll. Am. Pathol. 2020, 4, 1515–1538. [Google Scholar]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J.; Panel members. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Rosen, P.P. Tumor emboli in intramammary lymphatics in breast carcinoma: Pathologic criteria for diagnosis and clinical significance. Pathol. Annu. 1983, 18, 215–232. [Google Scholar]
- Houvenaeghel, G.; Cohen, M.; Classe, J.M.; Reyal, F.; Mazouni, C.; Chopin, N.; Martinez, A.; Daraï, E.; Coutant, C.; Colombo, P.E.; et al. Lymphovascular invasion has a significant prognostic impact in patients with early breast cancer, results from a large, national, multicenter, retrospective cohort study. ESMO Open 2021, 6, 100316. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Rotmensz, N.; Maisonneuve, P.; Sonzogni, A.; Pruneri, G.; Casadio, C.; Luini, A.; Veronesi, P.; Intra, M.; Galimberti, V.; et al. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann. Oncol. 2007, 18, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.M.; Tong, F.; Shen, J. Lympho-vascular invasion impacts the prognosis in breast-conserving surgery: A systematic review and meta-analysis. BMC Cancer 2022, 22, 102. [Google Scholar] [CrossRef]
- Recht, A.; Comen, E.A.; Fine, R.E.; Fleming, G.F.; Hardenbergh, P.H.; Ho, A.Y.; Hudis, C.A.; Hwang, E.S.; Kirshner, J.J.; Morrow, M.; et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Ann. Surg. Oncol. 2017, 24, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauria, R.; Perrone, F.; Carlomagno, C.; De Laurentiis, M.; Morabito, A.; Gallo, C.; Varriale, E.; Pettinato, G.; Panico, L.; Petrella, G. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer 1995, 76, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.; Connolly, J. Breast. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Gilchrist, K.W.; Gould, V.E.; Hirschl, S.; Imbriglia, J.E.; Patchefsky, A.S.; Penner, D.W.; Pickren, J.; Schwartz, I.S.; Wheeler, J.E.; Barnes, J.M.; et al. Interobserver variation in the identification of breast carcinoma in intramammary lymphatics. Hum. Pathol. 1982, 13, 170–172. [Google Scholar] [CrossRef]
- Sloane, J.P.; Amendoeira, I.; Apostolikas, N.; Bellocq, J.P.; Bianchi, S.; Boecker, W.; Bussolati, G.; Coleman, D.; Connolly, C.E.; Eusebi, V.; et al. Consistency achieved by 23 European pathologists from 12 countries in diagnosing breast disease and reporting prognostic features of carcinomas. European Commission Working Group on Breast Screening Pathology. Virchows Arch. 1999, 434, 3–10. [Google Scholar]
- Nime, F.A.; Rosen, P.P.; Thaler, H.T.; Ashikari, R.; Urban, J.A. Prognostic significance of tumor emboli in intramammary lymphatics in patients with mammary carcinoma. Am. J. Surg. Pathol. 1977, 1, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynden, G.G.; Van der Auwera, I.; Van Laere, S.J.; Colpaert, C.G.; van Dam, P.; Dirix, L.Y.; Vermeulen, P.B.; Van Marck, E.A. Distinguishing blood and lymph vessel invasion in breast cancer: A prospective immunohistochemical study. Br. J. Cancer 2006, 94, 1643–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, B.; Saxena, R.; Morimiya, A.; Mehrotra, S.; Badve, S. Lymphangiogenesis does not occur in breast cancer. Am. J. Surg Pathol. 2005, 29, 1449–1455. [Google Scholar] [CrossRef]
- Williams, C.S.; Leek, R.D.; Robson, A.M.; Banerji, S.; Prevo, R.; Harris, A.L.; Jackson, D.G. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J. Pathol. 2003, 200, 195–206. [Google Scholar] [CrossRef]
- Wu, R.; Sarkar, J.; Tokumaru, Y.; Takabe, Y.; Oshi, M.; Asaoka, M.; Yan, L.; Ishikawa, T.; Takabe, K. Intratumoral lymphatic endothelial cell infiltration reflecting lymphangiogenesis is counterbalanced by immune responses and better cancer biology in the breast cancer tumor microenvironment. Am. J. Cancer Res. 2022, 12, 504–520. [Google Scholar]
- Hoda, S.A.; Hoda, R.S.; Merlin, S.; Shamonki, J.; Rivera, M. Issues relating to lymphovascular invasion in breast carcinoma. Adv. Anat. Pathol. 2006, 13, 308–315. [Google Scholar] [CrossRef]
- Eusebi, V.; Azzopardi, J.G. Vascular infiltration in benign breast disease. J. Pathol. 1976, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Youngson, B.J.; Cranor, M.; Rosen, P.P. Epithelial displacement in surgical breast specimens following needling procedures. Am. J. Surg. Pathol. 1994, 18, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Youngson, B.J.; Liberman, L.; Rosen, P.P. Displacement of carcinomatous epithelium in surgical breast specimens following stereotaxic core biopsy. Am. J. Clin. Pathol. 1995, 103, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Liberman, L.; Vuolo, M.; Dershaw, D.D.; Morris, E.A.; Abramson, A.F.; LaTrenta, L.R.; Polini, N.M.; Rosen, P.P. Epithelial displacement after stereotactic 11-gauge directional vacuum-assisted breast biopsy. AJR Am. J. Roentgenol 1999, 172, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Nagi, C.; Bleiweiss, I.; Jaffer, S. Epithelial displacement in breast lesions: A papillary phenomenon. Arch. Pathol. Lab. Med. 2005, 129, 1465–1469. [Google Scholar] [CrossRef]
- Carter, B.A.; Jensen, R.A.; Simpson, J.F.; Page, D.L. Benign transport of breast epithelium into axillary lymph nodes after biopsy. Am. J. Clin. Pathol. 2000, 113, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Diaz, N.M.; Vrcel, V.; Centeno, B.A.; Muro-Cacho, C. Modes of benign mechanical transport of breast epithelial cells to axillary lymph nodes. Adv. Anat. Pathol. 2005, 12, 7–9. [Google Scholar] [CrossRef]
- Khoury, T. Delay to formalin fixation alters morphology and immunohistochemistry for breast carcinoma. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 531–542. [Google Scholar] [CrossRef]
- Acs, G.; Paragh, G.; Rakosy, Z.; Laronga, C.; Zhang, P.J. The extent of retraction clefts correlates with lymphatic vessel density and VEGF-C expression and predicts nodal metastasis and poor prognosis in early-stage breast carcinoma. Mod. Pathol. 2012, 25, 163–177. [Google Scholar] [CrossRef] [Green Version]
- Acs, G.; Khakpour, N.; Kiluk, J.; Lee, M.C.; Laronga, C. The presence of extensive retraction clefts in invasive breast carcinomas correlates with lymphatic invasion and nodal metastasis and predicts poor outcome: A prospective validation study of 2742 consecutive cases. Am. J. Surg. Pathol. 2015, 39, 325–337. [Google Scholar] [CrossRef]
- Damiani, S.; Eusebi, V.; Peterse, J.L. Malignant neoplasms infiltrating pseudoangiomatous’ stromal hyperplasia of the breast: An unrecognized pathway of tumour spread. Histopathology 2002, 41, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G.; Brooks, T.; Thompson, S.; Batsakis, J.G. Use of Ulex europaeus agglutinin I in the identification of lymphatic and blood vessel invasion in previously stained microscopic slides. Am. J. Surg. Pathol. 1987, 11, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 2005, 46, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Ragazzi, M.; Ciarrocchi, A.; Torricelli, F.; de Biase, D.; Zanetti, E.; Bisagni, A.; Corrado, S.; Uccella, S.; La Rosa, S.; et al. Angiosarcoma and anaplastic carcinoma of the thyroid are two distinct entities: A morphologic, immunohistochemical, and genetic study. Mod. Pathol. 2019, 32, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef]
- Bieniasz-Krzywiec, P.; Martín-Pérez, R.; Ehling, M.; García-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019, 30, 917–936.e10. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Szot, C.S.; Wilson, T.D.; Akman, S.; Metheny-Barlow, L.J.; Robertson, J.L.; Freeman, J.W.; Rylander, M.N. Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. J. Cell. Biochem. 2012, 113, 1142–1151. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Chaves, S.; Torres, A.L.; Barrias, C.C.; Bidarra, S.J. Engineering a Vascularized 3D Hybrid System to Model Tumor-Stroma Interactions in Breast Cancer. Front. Bioeng. Biotechnol. 2021, 9, 647031. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Sinha, D.; Saha, P.; Samanta, A.; Bishayee, A. Emerging Concepts of Hybrid Epithelial-to-Mesenchymal Transition in Cancer Progression. Biomolecules 2020, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Bronsert, P.; Enderle-Ammour, K.; Bader, M.; Timme, S.; Kuehs, M.; Csanadi, A.; Kayser, G.; Kohler, I.; Bausch, D.; Hoeppner, J.; et al. Cancer cell invasion and EMT marker expression: A three-dimensional study of the human cancer-host interface. J. Pathol. 2014, 234, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, F.; Seo, B.R.; Fischbach, C.; Chen, W.; Hsu, L.; Gourdon, D. Breast cancer cells alter the dynamics of stromal fibronectin-collagen interactions. Matrix Biol. 2017, 60–61, 86–95. [Google Scholar] [CrossRef]
- Hebert, J.D.; Myers, S.A.; Naba, A.; Abbruzzese, G.; Lamar, J.M.; Carr, S.A.; Hynes, R.O. Proteomic Profiling of the ECM of Xenograft Breast Cancer Metastases in Different Organs Reveals Distinct Metastatic Niches. Cancer Res. 2020, 80, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef] [Green Version]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Evans, R.; Flores-Borja, F.; Nassiri, S.; Miranda, E.; Lawler, K.; Grigoriadis, A.; Monypenny, J.; Gillet, C.; Owen, J.; Gordon, P.; et al. Integrin-Mediated Macrophage Adhesion Promotes Lymphovascular Dissemination in Breast Cancer. Cell Rep. 2019, 27, 1967–1978.e4. [Google Scholar] [CrossRef] [Green Version]
- Valtola, R.; Salven, P.; Heikkilä, P.; Taipale, J.; Joensuu, H.; Rehn, M.; Pihlajaniemi, T.; Weich, H.; deWaal, R.; Alitalo, K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am. J. Pathol. 1999, 154, 1381–1390. [Google Scholar] [CrossRef] [Green Version]
- Joukov, V.; Sorsa, T.; Kumar, V.; Jeltsch, M.; Claesson-Welsh, L.; Cao, Y.; Saksela, O.; Kalkkinen, N.; Alitalo, K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997, 16, 3898–3911. [Google Scholar] [CrossRef] [Green Version]
- Stacker, S.A.; Stenvers, K.; Caesar, C.; Vitali, A.; Domagala, T.; Nice, E.; Roufail, S.; Simpson, R.J.; Moritz, R.; Karpanen, T.; et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 1999, 274, 32127–32136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996, 15, 290–298. [Google Scholar] [CrossRef]
- Dadiani, M.; Kalchenko, V.; Yosepovich, A.; Margalit, R.; Hassid, Y.; Degani, H.; Seger, D. Real-time Imaging of Lymphogenic Metastasis in Orthotopic Human Breast Cancer. Cancer Res. 2006, 66, 8037–8041. [Google Scholar] [CrossRef] [Green Version]
- Jansen, K.A.; Bacabac, R.G.; Piechocka, I.K.; Koenderink, G.H. Cells actively stiffen fibrin networks by generating contractile stress. Biophys. J. 2013, 105, 2240–2251. [Google Scholar] [CrossRef] [Green Version]
- Hayasaka, H.; Yoshida, J.; Kuroda, Y.; Nishiguchi, A.; Matsusaki, M.; Kishimoto, K.; Nishimura, H.; Okada, M.; Shimomura, Y.; Kobayashi, D.; et al. CXCL12 promotes CCR7 ligand-mediated breast cancer cell invasion and migration toward lymphatic vessels. Cancer Sci. 2022, 113, 1338–1351. [Google Scholar] [CrossRef]
- Pang, M.F.; Georgoudaki, A.M.; Lambut, L.; Johansson, J.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016, 35, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Garmy-Susini, B.; Avraamides, C.J.; Schmid, M.C.; Foubert, P.; Ellies, L.G.; Barnes, L.; Feral, C.; Papayannopoulou, T.; Lowy, A.; Blair, S.L.; et al. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010, 70, 3042–3051. [Google Scholar] [CrossRef] [Green Version]
- Dieterich, L.C.; Kapaklikaya, K.; Cetintas, T.; Proulx, S.T.; Commerford, C.D.; Ikenberg, K.; Bachmann, S.B.; Scholl, J.; Detmar, M. Transcriptional profiling of breast cancer-associated lymphatic vessels reveals VCAM-1 as regulator of lymphatic invasion and permeability. Int. J. Cancer 2019, 145, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef] [Green Version]
- Adami, H.O.; Malker, B.; Holmberg, L.; Persson, I.; Stone, B. The relation between survival and age at diagnosis in breast cancer. N. Engl. J. Med. 1986, 315, 559–563. [Google Scholar] [CrossRef]
- Chung, M.; Chang, H.R.; Bland, K.I.; Wanebo, H.J. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer 1996, 77, 97–103. [Google Scholar] [CrossRef]
- Walker, R.A.; Lees, E.; Webb, M.B.; Dearing, S.J. Breast carcinomas occurring in young women (<35 years) are different. Br. J. Cancer 1996, 74, 1796–1800. [Google Scholar]
- Winchester, D.P.; Osteen, R.T.; Menck, H.R. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer 1996, 78, 1838–1843. [Google Scholar] [CrossRef]
- Kollias, J.; Elston, C.W.; Ellis, I.O.; Robertson, J.F.; Blamey, R.W. Early-onset breast cancer—Histopathological and prognostic considerations. Br. J. Cancer 1997, 75, 1318–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, C.; Smith, I.E. Breast cancer in adolescents and young women. Eur. J. Cancer 2003, 39, 2632–2642. [Google Scholar] [CrossRef]
- Rakha, E.A.; Martin, S.; Lee, A.H.; Morgan, D.; Pharoah, P.D.; Hodi, Z.; Macmillan, D.; Ellis, I.O. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012, 118, 3670–3680. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Bayer, G.; Aumayr, K.; Taucher, S.; Geleff, S.; Rudas, M.; Kubista, E.; Hausmaninger, H.; Samonigg, H.; Gnant, M. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann. Surg. 2004, 240, 306–312. [Google Scholar] [CrossRef]
- Mohammed, R.A.; Martin, S.G.; Gill, M.S.; Green, A.R.; Paish, E.C.; Ellis, I.O. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am. J. Surg. Pathol. 2007, 31, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Corti, C.; Lopez, G.; Michelotti, A.; Despini, L.; Gambini, D.; Lorenzini, D.; Guerini-Rocco, E.; Maggi, S.; Noale, M.; et al. Lymphovascular invasion and extranodal tumour extension are risk indicators of breast cancer related lymphoedema: An observational retrospective study with long-term follow-up. BMC Cancer 2018, 18, 935. [Google Scholar] [CrossRef] [Green Version]
- Pinder, S.E.; Ellis, I.O.; Galea, M.; O’Rouke, S.; Blamey, R.W.; Elston, C.W. Pathological prognostic factors in breast cancer. III. Vascular invasion: Relationship with recurrence and survival in a large study with long-term follow-up. Histopathology 1994, 24, 41–47. [Google Scholar] [CrossRef]
- Ejlertsen, B.; Jensen, M.B.; Rank, F.; Rasmussen, B.B.; Christiansen, P.; Kroman, N.; Kvistgaard, M.E.; Overgaard, M.; Toftdahl, D.B.; Mouridsen, H.T. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J. Natl. Cancer Inst. 2009, 101, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Fisher, E.R.; Gregorio, R.M.; Fisher, B.; Redmond, C.; Vellios, F.; Sommers, S.C. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer 1975, 36, 1–85. [Google Scholar] [CrossRef]
- Gajdos, C.; Tartter, P.I.; Bleiweiss, I.J. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann. Surg. 1999, 230, 692–696. [Google Scholar] [CrossRef]
- Davis, B.W.; Gelber, R.D.; Goldhirsch, A.; Hartmann, W.H.; Locher, G.W.; Reed, R.; Golouh, R.; Säve-Söderbergh, J.; Holloway, L.; Russell, I. Prognostic significance of peritumoral vessel invasion in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Hum. Pathol. 1985, 16, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Viale, G.; Zurrida, S.; Maiorano, E.; Mazzarol, G.; Pruneri, G.; Paganelli, G.; Maisonneuve, P.; Veronesi, U. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 2005, 103, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Osako, T.; Okumura, Y.; Nakano, M.; Ohtsuka, H.; Fujisue, M.; Arima, N. An evaluation of lymphovascular invasion in relation to biology and prognosis according to subtypes in invasive breast cancer. Oncol. Lett. 2022, 24, 245. [Google Scholar] [CrossRef]
- Heerma van Voss, M.R.; van der Groep, P.; Bart, J.; van der Wall, E.; van Diest, P.J. Lympho-vascular invasion in BRCA related breast cancer compared to sporadic controls. BMC Cancer 2010, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Atcı, M.M.; Geredeli, Ç.; Ay, S.; Sakin, A.; Ertürk, B.; Seçmeler, Ş.; Arıcı, S.; Çekin, R.; Yaşar, N.; Can, O.; et al. Clinical and Pathological Characteristics of Patients with High-Risk Breast Cancer Based on BRCA Mutation Profiles: A Retrospective Study. Eur. J. Breast Health 2021, 17, 123–127. [Google Scholar] [CrossRef]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packwood, K.; Martland, G.; Sommerlad, M.; Shaw, E.; Moutasim, K.; Thomas, G.; Bateman, A.C.; Jones, L.; Haywood, L.; Evans, D.G.; et al. Breast cancer in patients with germline TP53 pathogenic variants have typical tumour characteristics: The Cohort study of TP53 carrier early onset breast cancer (COPE study). J. Pathol. Clin. Res. 2019, 5, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Colorectal Version. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1436). (accessed on 28 February 2023).
- Makower, D.; Lin, J.; Xue, X.; Sparano, J.A. Lymphovascular invasion, race, and the 21-gene recurrence score in early estrogen receptor-positive breast cancer. npj Breast Cancer 2021, 7, 20. [Google Scholar] [CrossRef]
- Mutai, R.; Goldvaser, H.; Shochat, T.; Peretz, I.; Sulkes, A.; Yerushalmi, R. Prognostic Value of the Detection of Lymphovascular Invasion in Hormone Receptor-Positive Early Breast Cancer in the Era of Molecular Profiling. Oncology 2019, 96, 14–24. [Google Scholar] [CrossRef]
- Al-Zawi, A.S.A.; Yin, S.L.; Aladili, Z. Lymphovascular invasion in hormone-positive, human epidermal growth factor-negative, low-burden axillary disease in early breast cancer patients tested for oncotype DX recurrence score. Contemp. Oncol. 2022, 26, 139–143. [Google Scholar]
- Klahan, S.; Wong, H.S.; Tu, S.H.; Chou, W.H.; Zhang, Y.F.; Ho, T.F.; Liu, C.Y.; Yih, S.Y.; Lu, H.F.; Chen, S.C.; et al. Identification of genes and pathways related to lymphovascular invasion in breast cancer patients: A bioinformatics analysis of gene expression profiles. Tumour Biol. 2017, 39, 5573. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Saraf, A.; Lee, S.M.; Zhong, X.; Hibshoosh, H.; Kalinsky, K.; Connolly, E.P. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2016, 157, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.J.; Kang, S.J.; Cho, J.S.; Yoon, J.H.; Park, M.H. Lymphovascular invasion can be better than pathologic complete response to predict prognosis in breast cancer treated with neoadjuvant chemotherapy. Medicine 2018, 97, e11647. [Google Scholar] [CrossRef]
- Hamy, A.S.; Lam, G.T.; Laas, E.; Darrigues, L.; Balezeau, T.; Guerin, J.; Livartowski, A.; Sadacca, B.; Pierga, J.Y.; Vincent-Salomon, A.; et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res Treat. 2018, 169, 295–304. [Google Scholar] [CrossRef] [PubMed]
| Criteria |
|---|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, E.; Gambini, D.; Despini, L.; Asnaghi, D.; Runza, L.; Ferrero, S. Updates on Lymphovascular Invasion in Breast Cancer. Biomedicines 2023, 11, 968. https://doi.org/10.3390/biomedicines11030968
Kuhn E, Gambini D, Despini L, Asnaghi D, Runza L, Ferrero S. Updates on Lymphovascular Invasion in Breast Cancer. Biomedicines. 2023; 11(3):968. https://doi.org/10.3390/biomedicines11030968
Chicago/Turabian StyleKuhn, Elisabetta, Donatella Gambini, Luca Despini, Dario Asnaghi, Letterio Runza, and Stefano Ferrero. 2023. "Updates on Lymphovascular Invasion in Breast Cancer" Biomedicines 11, no. 3: 968. https://doi.org/10.3390/biomedicines11030968
APA StyleKuhn, E., Gambini, D., Despini, L., Asnaghi, D., Runza, L., & Ferrero, S. (2023). Updates on Lymphovascular Invasion in Breast Cancer. Biomedicines, 11(3), 968. https://doi.org/10.3390/biomedicines11030968






