Abstract
The correlation between diabetes mellitus and infectious diseases is widely recognized. DM patients are characterized by the impaired function of the immune system. This translates into the occurrence of a variety of infections, including urinary tract, skin and surgical site infections, pneumonia, tuberculosis, and, more recently, SARS-CoV-2. Hyperglycemia has been identified as a relevant factor contributing to unfavorable outcomes in hospitalized patients including SARS-CoV-2 patients. Several studies have been performed proving that to maintain the proper and stringent monitoring of glycemia, a balanced diet and physical activity is mandatory to reduce the risk of infections and their associated complications. This review is focused on the mechanisms accounting for the increased susceptibility of DM patients to infections, with particular attention to the impact of newly introduced hypoglycemic drugs in sepsis management.
1. Introduction
Diabetes mellitus (DM) is a chronic disease characterized by abnormal blood glucose levels resulting from impaired insulin action and/or insulin secretion, usually both [1]. DM can be classified as type 1 diabetes, type 2, and gestational diabetes [2]. According to the 10th edition of the IDF Diabetes Atlas, 536.6 million people are currently diagnosed with DM worldwide with a prevalence of 10.5%, and this number is expected to increase to 783.2 million in 2045. The prevalence of DM increases with age, while the incidence of type 2 diabetes is expected to decrease or remain stable in high-income countries [3]. In addition to macro- and microvascular complications, an increased risk of infection is commonly associated with DM [4]. Individuals with DM are at a greater risk of hospitalization and mortality due to viral, bacterial, and fungal infections [5]. However, recent evidence indicates that DM does not represent a significant risk factor for poor survival in patients with sepsis, regardless of intensive care unit (ICU) admission [6,7]. Nevertheless, DM and sepsis remain important causes of morbidity and mortality worldwide, and DM patients represent the largest population experiencing post-sepsis complications [8]. This is mainly due to immunosuppression and uncontrolled hyperglycemia. In fact, high blood glucose impairs innate and adaptive immunity through various mechanisms [9].
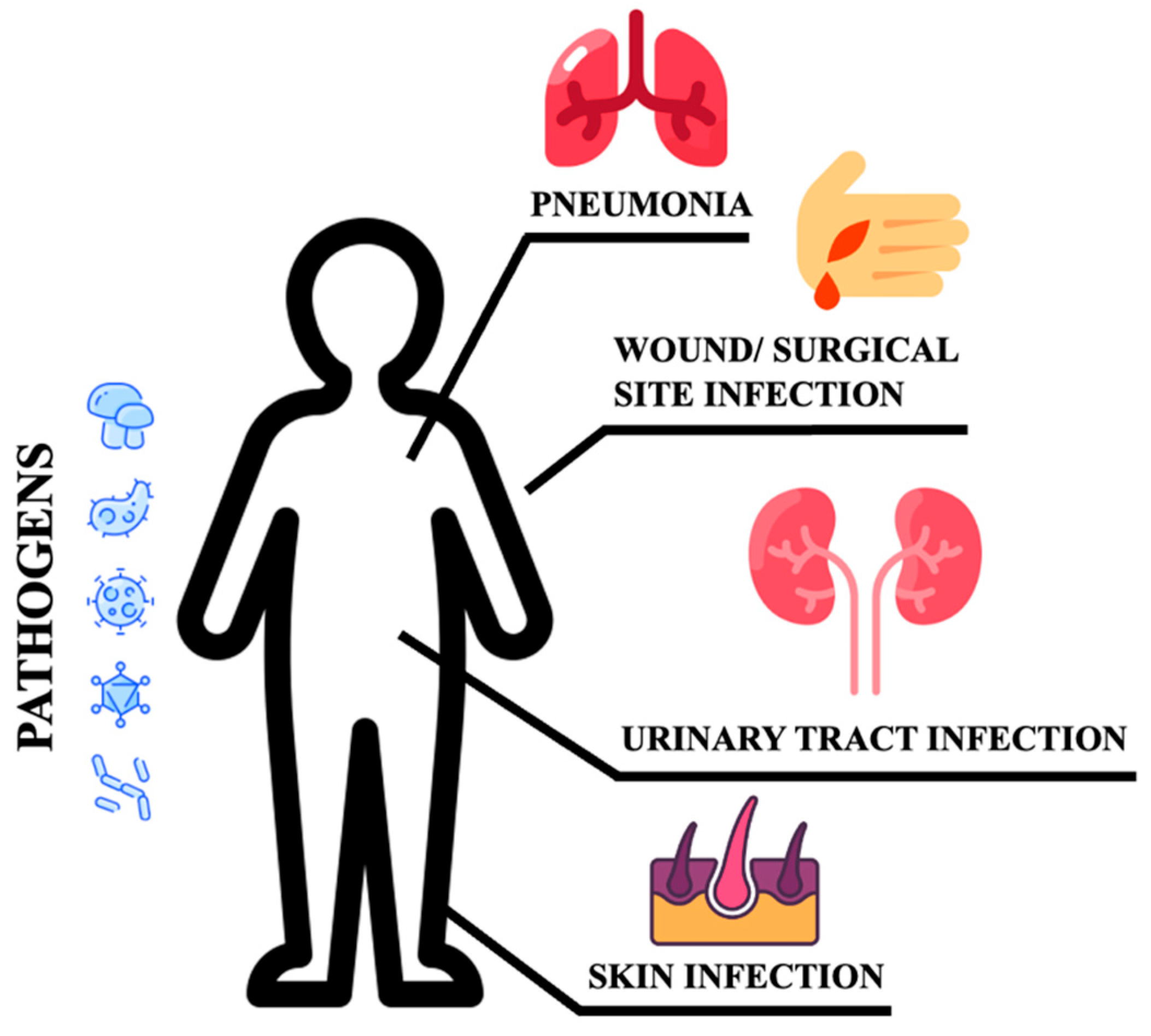
Poor glycemic control increases the risk for skin, bone, eye, ear, gastrointestinal, urinary tract, and respiratory infections [10]. Moreover, impaired healing of diabetic wounds, which affects approximately 25% of all DM patients, is associated with an increased risk of limb amputation, thereby representing a crucial economic and psychosocial issue [11]. Uncommon life-threatening infections are also more frequent among DM patients. These include invasive otitis externa, rhino-cerebral mucormycosis, and emphysematous infections of the gall bladder, kidney, and urinary bladder [12]. Evidence has also been provided on the prevalence of drug resistance in DM patients [13], however, an increased prevalence of resistance to commonly used antibiotics in DM patients is still debated [14]. More recently, several infections have been supposed to rely on newly introduced therapies. As an example, sodium-glucose co-transport 2 inhibitors (SGLT2-i) are associated with the occurrence of urinary and genital tract infections [15,16,17]. Moreover, among infections commonly associated with hospitalization, including urinary tract and skin infections, pneumonias, and surgical infections, the presence of DM confers an increased risk (Figure 1).
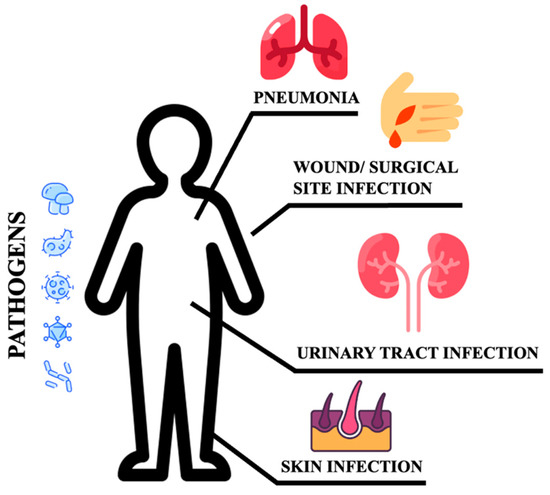
Figure 1.
Sites of infections.
These patients are more susceptible to urinary tract infections than non-DM individuals. They have a higher risk of developing asymptomatic bacteriuria, cystitis, acute pyelonephritis, and complications such as emphysematous pyelonephritis [9,18]. Bacteria isolated from DM patients with an urinary tract infection (UTI) do not differ from those found in non-DM patients with complicated UTI. E. coli are the most common pathogens, while Klebsiella spp., Enterobacter spp., Proteus spp., Group B Streptococci, and Enterococcus faecalis are the most frequently isolated pathogens [19]. DM patients are more likely to develop skin and soft tissue infections, including cellulitis and osteomyelitis [14]. Staphylococcus aureus and Pseudomonas [20] are the most common isolated gram-positive and negative bacteria respectively. Methicillin-resistant Staphylococcus aureus (MRSA) and other antibiotic-resistant pathogens generally account for skin and soft tissue infections in the diabetic foot compared to other tissue sites and populations [14]. DM increases the susceptibility to different respiratory infections, thereby representing an independent risk factor for lower respiratory tract infections, particularly influenza and pneumonia [20,21]. More importantly, DM individuals are at higher risk of pulmonary infections caused by microorganisms such as Mycobacterium tuberculosis, Staphylococcus aureus, gram-negative bacteria, and fungi, and have a high risk of hospitalization upon influenza or flu-like infections. Additionally, infections caused by Streptococcus pneumonia or influenza virus are characterized by high morbidity and mortality rates [20]. Furthermore, pulmonary infections in elderly DM patients remain occult. Advanced age, comorbidities (senile dementia, hypothyroidism), and prolonged bed rest are indeed considered independent risk factors for occult pneumonia [22], resulting in long-term hospitalization and increased mortality. DM also increases community-acquired pneumonia (CAP) compared to non-diabetic individuals, and gram-negative bacteria such as K. pneumoniae and S. aureus are much more commonly isolated [9]. The susceptibility to fungal infections caused by Mucorales has been estimated at 75% in this population. Aspergillus is an additional microorganism causing infections in these patients [23]. It has been reported that S. pneumoniae, Enterobacter, K. pneumoniae, Serratia, E. coli, S. aureus, Proteus, and Haemophilus influenzae are the most common bacteria causing hospital-acquired pneumonia (HAP) during the first four days of hospitalization, while Acinetobacter, MRSA, E. coli, L. pneumophila, Pseudomonas aeruginosa, and K. pneumonia are more prevalent after day five [20,24]. Tuberculosis infections are also frequent and increased with a mortality rate corresponding to 50% [25]. Hyperglycemia also predisposes to superinfection of the surgical site following surgery (SSI), and the association between pre- and post-surgery hyperglycemia remains a significant risk factor for SSI [26]. In conclusion, the risk and mortality associated with infectious diseases are high in DM patients, implying that infections should be considered among the most common DM complications [12]. This review provides the updated results on the benefits potentially associated with newly introduced antidiabetic drugs during infection.
2. Immunity Impairment in DM
Several pre-clinical and clinical studies have revealed a significant defect in both innate and adaptive immunity. Although some mechanisms are glycemia-independent [27], most of them rely on hyperglycemia and its metabolic effects, such as non-enzymatic glycation, generation of reactive oxygen species, and hyperactivity of the polyol pathway [28].
2.1. Neutrophils
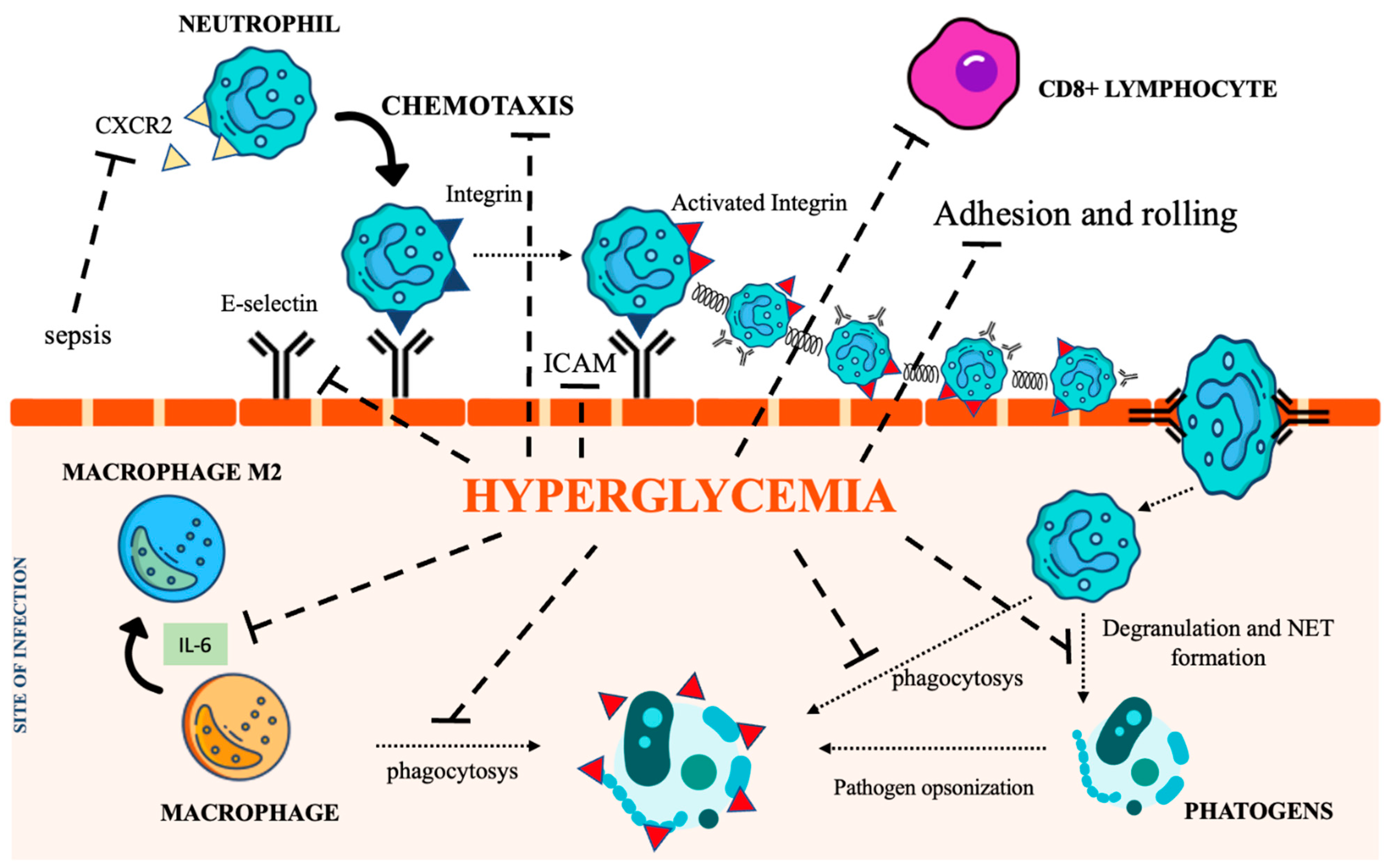
Neutrophils are recognized as key elements to counteract infection, and DM impairs their recruitment as well as their killing capability. Several mechanisms also account for the dysfunction of adhesion, rolling, and chemotaxis [29,30,31]. CXCR2, a chemokine receptor expressed on neutrophils, was found downregulated during sepsis, thereby impairing neutrophil recruitment [27,32]. Moreover, since CXCR2 also controls the expression of the intracellular adhesion molecule-1 (ICAM) on endothelial cells (ECs), its decreased expression further weakens neutrophil recruitment at the inflammatory site [33]. Regarding phagocytosis, the main recognized abnormality is related to C3-mediated opsonization owing to complement glycation [34]. Both intracellular and extracellular killing mechanisms (involving the production of intracellular ROS [35,36], enzymatic degranulation [37], and inhibition of neutrophil extracellular traps (NET) formation [38]) are impaired in the hyperglycemic condition.
2.2. Macrophages
Chronic hyperglycemia also weakens macrophage adhesion and chemotaxis, antibacterial activity, and phagocytosis, damaging both the FCy receptor and the complement cascade [39,40]. Furthermore, the low-grade inflammation caused by hyperglycemia, insulin resistance, and obesity promotes macrophage differentiation towards their anti-inflammatory M2 phenotype, activating the IL-6 signaling pathway [41]. The homeostatic action of IL-6 in limiting inflammation represents a relevant impediment to the control of infections [42]. Figure 2 summarizes the most relevant DM-associated immune cell impairment.
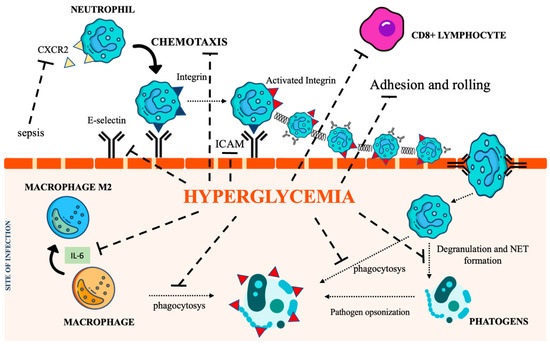
Figure 2.
The immunological pathways under the control of hyperglycemia. CXCR2 (C-X-C Motif Chemokine Receptor 2), IL-6 (interleukin 6), NET (Neutrophil extracellular traps), ICAM (intercellular adhesion molecules). Icon design by icons8.
2.3. Natural Killer Cells
Hyperglycemia-induced oxidative stress also compromises natural killer (NK) cell activity [43]. Importantly, a strong inverse linear relationship between their activity and HbA1c level has been reported [44,45].
2.4. Adaptive Immunity
The impact of DM on adaptive immunity is still debated since a few studies on T lymphocyte dysfunction have reported conflicting results. Preclinical studies have shown that the decreased expression of ICAM and E selectin in ECs impairs the recruitment of cytotoxic CD8+ T lymphocytes to sites of infection, resulting in more severe disease [46]. Dysregulation of the complement cascade has also been reported. As mentioned earlier, non-enzymatic glycation of C3 and C4 decreases opsonization [34]. In addition, the glycation of immunoglobulins can inhibit antigen recognition [47].
In conclusion, DM significantly impacts the immune system, resulting in a higher risk of infections. Several studies have shown defects in both innate and adaptive immunity. Hyperglycemia and insulin resistance are the most relevant factors contributing to the dysfunction of the immune system, which mainly involves neutrophils, macrophages, and natural killer cells.
3. Treatment-Associated Infections
As extensively discussed [8], a clear association between DM and increased infection-related mortality/morbidity is still uncertain. A recent review [6] investigating this specific topic has reported no difference in hospital mortality between diabetic and non-diabetic individuals, concluding that, rather than DM per se, DM-related co-morbidities and long-term complications might drive worse outcomes. Compared to healthy individuals, septic patients are connoted by the increased production of acute phase proteins, such as C reactive protein (CRP). No difference between serum levels of CRP in DM and non-DM patients has been documented [48].
The 2021 International Guidelines for the management of sepsis and septic shock [49], like the previous one (2016) [50], strongly recommended treating patients displaying blood sugar level (BGL) up to 180 mg/dL, thereby underlining the lack of studies on DM patients. Indeed, since hyperglycemia was not linked to increased ICU mortality, a different study proposed a Mean Blood Glucose (MBG) of between 140 and 190 mg/dL to avoid hypoglycemia and its adverse consequences during sepsis (such as increased oxidative stress, platelet aggregation, production of pro-inflammatory cytokines, and the expression of vascular adhesion molecules) [51,52]. In the following section, the relationship between single anti-diabetic agents and outcomes in DM patients with sepsis is discussed.
3.1. Metformin
Metformin, due to its pleiotropic effect [8] and impact on mitochondrial activity, autophagy, and immune modulation [53], appears to be a reliable and safe anti-diabetic drug during infections. A study by Gomez et al. [54] reported that metformin reduces the incidence of sepsis-induced AKI. Moreover, a lower incidence of mortality in patients treated with metformin prior to hospitalization for sepsis has also been shown [55,56,57]. Additionally, investigating the therapeutic impact of metformin against MRS, and multidrug-resistant (MDR) Pseudomonas aeruginosa in combination with antimicrobial agents, it was demonstrated that metformin synergizes with the majority of tested agents, with the highest antibiotic MIC reduction (93% in both cases) when combined with doxycycline and chloramphenicol [58]. The main concern related to metformin in septic patients relies on the risk of lactic acidosis, potentially worsening the already fragile clinical condition. However, a Meta-Analysis by Li et al. [57] analyzing 8195 patients, did not find a statistical difference in the level of serum creatinine and lactic acid between patients treated or not with metformin at pre-admission. Although validation is required, this observation opens a new scenario on metformin in hospitalized patients.
3.2. Insulin
Insulin still represents the first choice to lower BGL towards a safer value [59], however, its effectiveness could depend on specific settings. In fact, its anabolic effect might inhibit autophagy, thereby decreasing the antioxidant action [60], or contribute to antibiotic resistance by affecting biofilm growth. Patel et al. [61] showed that while insulin alone has no effect on the level of biofilm formation or cell growth, the presence of glucose significantly enhances both. This could be a trigger for the expression of biofilm formation and UTI, particularly in the presence of external catheters. Furthermore, this effect is increased by a temperature up to 37 °C, which is commonly experienced by septic patients. Using a preclinical diabetic model, Wei et al. [62] demonstrated that insulin promoted biofilm formation by activating the cyclic-di-GMP signaling pathway. This translated into delayed wound healing and increased antibiotic resistance against P. aeruginosa infection. Due to its effects on T cell proliferation and intermediary metabolism, several studies have proposed that insulin resistance could also impact susceptibility to H1N1 infection and the effectiveness of vaccination [63].
3.3. Glucagon-Like Peptide-1 Receptor Agonists (GLP-1) and Dipeptidyl Peptidase-4 Inhibitor (DPP4 Is)
Glucagon-like peptide-1 receptor agonists (GLP-1) and dipeptidyl Peptidase-4 Inhibitors (DPP4 is) have also been investigated in the last years, demonstrating a direct action on endotoxemia, independently of their glucose-lowering properties [64,65,66,67,68,69]. Specifically, it has shown an increase in biomarkers of inflammation, oxidative stress parameters, and endothelial dysfunction. It has also been shown that activation of GLP-1 receptors can promote B and T cell expansion, particularly toward Treg1 differentiation, thereby contributing to the impaired inflammatory response in patients with sepsis [51]. In addition, the massive activation of the endogenous GLP-1 system during sepsis has been proposed as a predictor of early death or persistent organ dysfunction [70,71], particularly in patients infected by Gram-negative bacteria [51,72].
3.4. SGLT2-Inhibitors (SGLT2i)
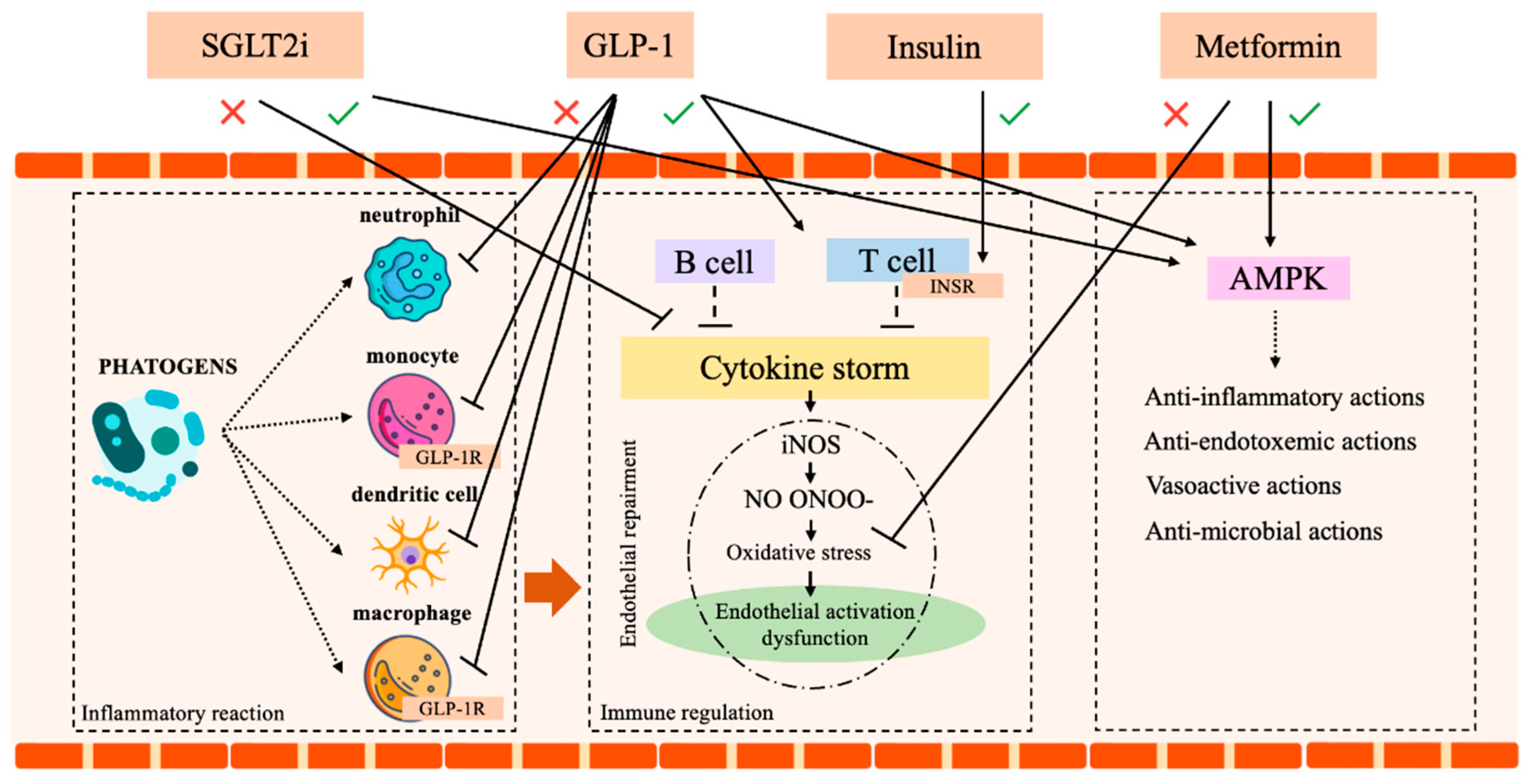
SGLT2-inhibitors (SGLT2i) are a class of antidiabetic drugs, which gained increasing interest for their proven long-term cardio and reno-protective effects [73,74,75]. The original concerns regarding a possible increase in UTIs are currently mitigated since it was limited to Dapaglifozin [76]. Moreover, compared to other active antidiabetic treatments, SGLT2i did not show a difference in the incidence of UTI [76,77,78]. Additionally, a recent systematic review and meta-analysis by Wang et al. [79] demonstrated that SGLT2i displays a powerful anti-inflammatory effect, recognized by the decrease in ferritin, leptin, and plasminogen activator inhibitor (PAI)-1. Although the mechanisms remain unclear, the anti-inflammatory action of Dapagliflozin and Empagliflozin was confirmed by efficacy tests and by the drop in morbidity and mortality observed in preclinical models of sepsis and renal injury [8,80,81,82]. Finally, a large meta-analysis (4568 citations, 26 trials with a total of 59,264 patients) by Li et al. [83] identified a significant reduction of the risk of pneumonia and septic shock in DM patients treated with SGLT2i. Certainly, future studies on SGLT2i in patients with septic shock are needed to better explore this promising antidiabetic class. Table 1 and Figure 3 summarize all these notions.

Table 1.
Effects of anti-diabetic medications on immune modulation and inflammation.
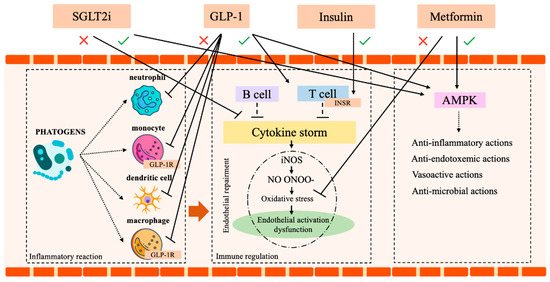
Figure 3.
Immune system and insulin, metformin, SGLT2 and GLP-1 action. AMPK (AMP-activated protein kinase), SGLT2 (sodium-glucose co-transport 2 inhibitors), GLP-1 (Glucagon-like peptide-1 receptor agonists), GLP-1R (Glucagon-like peptide-1 receptor), T reg1 (T regulator 1 lymphocytes), INSR (Insulin receptor). Icon design by icons8.
Since SARS-CoV-2 and tuberculosis frequently occur in DM patients, a detailed description of their link will be approached.
4. Diabetes and SARS-CoV-2 Infection
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first identified at the beginning of 2020, has led to a global pandemic known as COVID-19. COVID-19 has affected more than 752 million people and caused more than 6.8 million deaths worldwide (updated to 28 January 2023) [84]. Since the outbreak of the pandemic, several medications, including vaccines, were administered to decrease transmission, hospitalization, and infection-associated death. Despite these efforts, a significant number of patients, particularly those with DM, hypertension, chronic obstructive pulmonary disease, obesity, and cardiovascular disease continue to experience fatal outcomes [85]. DM is recognized as a risk factor for poor outcomes, including progression to acute respiratory distress syndrome (ARDS) and mortality [86]. These patients are generally characterized by the presence of comorbidities such as retinopathy, kidney injury, poor metabolic control, or have a history of hospitalization for diabetic ketoacidosis or hypoglycemia in the past 5 years and are mostly treated with several anti-diabetic medications [87]. DM patients, particularly those with poor metabolic control, are at a higher risk of severe complications and death from COVID-19. SARS-CoV-2 infection also increases the risk of thromboembolism and is more likely to induce cardiorespiratory failure in DM patients than in non-DM individuals [88]. Additionally, the occurrence of DM onset after COVID-19 hospitalization has been reported [89,90].
4.1. Diabetes and Increased Susceptibility to COVID-19 Infection
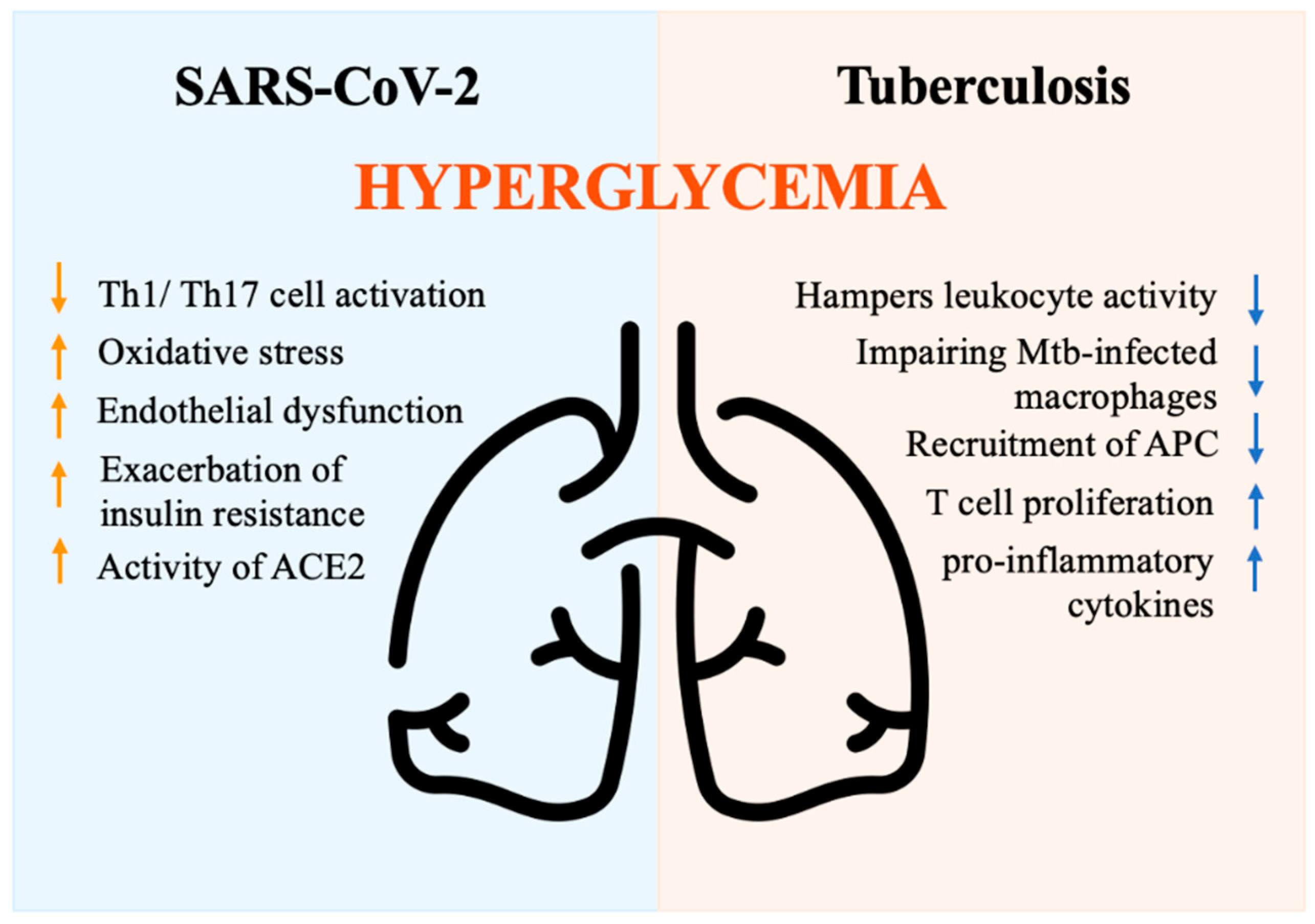
Multiple mechanisms have been proposed to explain the increased complications and mortality observed in DM individuals. DM is associated with decreased phagocytic activity, neutrophil chemotaxis, T cell function, and lower innate and adaptive immune activities [91]. Hyperglycemia is an independent factor associated with a severe prognosis in individuals hospitalized for COVID-19 [92]. Hyperglycemia may rely on stress, inflammation, and disruption of beta cells, as well as steroid administration [93]. Hyperglycemia negatively impacts innate cell-mediated immunity [91] and perturbs the antiviral response by suppressing Th1/Th17 cell activation, inducing oxidative stress, and causing endothelial dysfunction [94]. Moreover, the presence of hyperglycemia at the time of hospital admission may result from the exacerbation of insulin resistance driven by the release of counter-regulatory hormones and cytokines, which in turn impact the immune response [95]. Glycation of proteins, microangiopathy of alveolar capillaries, and proteolysis of connective tissue, finally translate into the collapse of small airways during expiration [96]. Moreover, acute hyperglycemia increases the activity of the urinary angiotensin-converting enzyme 2 (ACE2), which in turn enhances the virulence of SARS-CoV-2 [97,98]. Indeed, SARS-CoV-2 binds to the ACE2 receptor, which plays a role in multiple molecular processes and regulates glucose levels [99]. ACE2 degrades angiotensin II and angiotensin I into the smaller peptides angiotensin-(1–7) and angiotensin-(1–9). Angiotensin-(1–7) has antioxidant and anti-inflammatory effects through the Mas receptor pathway, which can be altered in DM individuals [100]. In non-survivors, the pathway that regulates inflammation appears to be imbalanced, with a drop in angiotensin-(1–7) levels [101]. In conclusion, hyperglycemia activates inflammatory pathways and exacerbates oxidative stress, weakening the immune system [102]. DM patients with COVID-19 exhibit an imbalanced anti-inflammatory and pro-inflammatory T cell ratio, characterized by the over-activation of the Th1 and Th17 subsets [103]. This results in a high level of C-reactive protein (CRP), pro-calcitonin, ferritin, and IL-6, which contribute to the hyper-immune response denoted as a cytokine storm [104] (Figure 4).
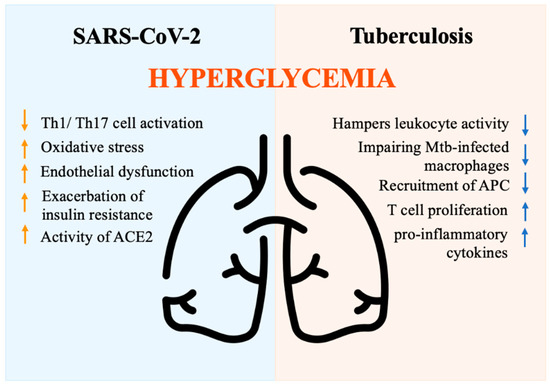
Figure 4.
Immune response driven by hyperglycemia during SARS-CoV-2 and tuberculosis infections. ACE2 (Angiotensin-converting enzyme 2), APC (Antigen-presenting cells), Mtb (Mycobacterium tuberculosis bacilli), Th1/Th17 (Lymphocytes T helper 1 e 17), Icon design by icons8.
4.2. Antidiabetic Agents and SARS-CoV-2
Prognostic benefits in DM patients can be accomplished by proper glycemic control, which also results from a balanced diet, physical activity, and consistent monitoring of blood glucose and blood pressure levels [102,105]. Increasing evidence indicates that glycemic control is crucial for COVID-19 hospitalized patients [86]. Among the antidiabetics, metformin, DPP4is and GLP-1Ras, SGLT2is, and insulin have been administered in patients with COVID-19. Administration of metformin has been shown to lower the incidence of mortality and hospitalization in COVID-19 DM patients [106]. Among the pleiotropic effect of metformin, its anti-inflammatory action is included [107]. Specifically, it improves ACE2 stability by hampering its ubiquitination and proteasome-mediated degradation [108] and leads to the reduced production of reactive oxygen species, oxidative stress, and DNA damage [102]. Researchers have suggested a therapeutic effect of DPP4is in SARS-CoV-2 infection [102]. Recent studies demonstrated that DPP4 inhibitors possess anti-inflammatory, immunomodulatory, and anti-fibrotic features [109]. However, other studies have reported that DPP4is significantly increases the risk of hospitalization and intensive care unit admission [110]. GLP-1Ras act on the ACE2 and Mas receptor pathways and may prevent SARS-CoV-2 infection and modulate inflammation and fibrosis [111] Studies showed a significant reduction in mortality and hospital admission in patients treated with GLP-1Ras or GLP-1RAs pre-admission [110,112,113]. However, the introduction of GLP-1RAs in critically ill patients is not fully recommended based on their potential side effects, the need for titration, and the therapeutic window [114]. The impact of SGLT2 inhibitors on COVID-19 has yet to be fully established, and the occurrence of diabetic ketoacidosis during gliflozin administration must be considered with caution [115]. Recent studies have shown a statistically significant decrease in hospitalization in patients treated with SGLT-2 inhibitors [110]. However, in acutely ill hospitalized patients with COVID-19 and DM, insulin is recommended [116], based on its anti-inflammatory action and the ability to suppress ACE2 expression [117]. Patients with severe COVID-19 and DM frequently require higher doses of insulin [102], thereby multi-injection insulin therapy is considered the most appropriate therapeutic option [118].
5. DM and Tuberculosis
The link between DM and Tuberculosis (TB) has been investigated by immunological and epidemiology studies. Chronic hyperglycemia represents a major risk factor for TB infection, disease severity, and treatment response by weakening the host immune response. TB still represents a major problem in low- and middle-income countries, particularly in the area of ongoing HIV epidemics, overcrowded living conditions, and inadequate healthcare systems [119]. At the same time, increasing industrialization, rapid urbanization, and aging populations are the major causes of the growing incidence of obesity and DM in emerging nations [3]. The association between DM and TB is becoming a global health problem and can be better understood using a syndemic model [120]. The interaction between DM and TB and the need for chronic care represents a significant burden for public healthcare systems. Poor glycemic control reflects inadequate access to effective diabetes care [121], which is associated with high rates of undiagnosed DM in low- and middle-income countries [122]. According to multiple observational studies, DM increases the risk of progression from latent TB to active disease threefold [123]. Therefore, socioeconomic disparities in DM diagnosis and lack of adequate patient care enhance TB susceptibility. Furthermore, DM not only increases the incidence of active TB but also increases the disease severity, with higher death rates and relapse after the accomplishment of antibiotic treatment [124]. Moreover, the presence of DM is associated with greater severity and delayed sputum conversion [125], which boost TB transmission. Finally, growing evidence indicates that DM is also associated with an increased risk of multi-drug resistant TB (MDR-TB) [126,127], further impairing the control of both epidemics.
5.1. Immune Mechanisms
A pathogenic model for TB susceptibility in DM patients has been proposed based on several studies [128]. Resident alveolar macrophages serve as the first line of immune defense against Mycobacterium tuberculosis bacilli (Mtb) in the lungs. Despite their inability to control Mtb replication, macrophages activate signals that recruit macrophages, dendritic cells, and neutrophils to present antigens to T-cells in lung-draining lymph nodes and prime the adaptive immune response. However, chronic hyperglycemia hampers leukocyte activity, particularly impairing the sentinel role of Mtb-infected macrophages [129]. Due to defective phagocytosis, reduced chemokine production, delayed recruitment of antigen-presenting cells (APC), and prolonged priming of adaptive immunity are commonly found. Therefore, Mtb replication occurs before T-cell activation. Once activated, the immune response promotes the release of pro-inflammatory cytokines and increases T cell proliferation [130,131,132], leading to increased disease severity, tissue damage, and poor outcomes (Figure 4). Overall, different mechanisms play a crucial role in increasing TB susceptibility, shortening survival, and increasing disease severity and recurrence rates in DM patients [133,134]. Future studies are needed to identify specific immune-metabolic pathways involved in the defective anti-tubercular response to be exploited as targeted approaches in DM patients.
5.2. Management of Tuberculosis in DM Patients
Combined diagnosis of DM and TB poses a significant challenge for the management of the disease. Studies on screening for active TB in DM patients have demonstrated weak results in terms of cost-effectiveness, with a major impact in high TB prevalence areas [135]. For this reason, a better risk stratification (including TB prevalence, history of TB, glycemic control, socioeconomic variables, and symptoms) is required for TB screening in DM patients. Additionally, screening for latent TB in DM patients, particularly in those with poor glycemic control, would identify a specific high-risk population who may benefit from prophylaxis. Unfortunately, the efficacy of preventive treatment in DM patients compared to non-diabetics remains undetermined, making it a priority and a future challenge. On the other hand, DM screening in TB patients is essential to control both infection and chronic complications associated with hyperglycemia. The availability of an adequate healthcare system and local conditions influence the choice of screening tests for DM in low- and middle-income countries [136,137]. Repeat fasting or random glucose tests are widely available, but results are commonly altered by transient hyperglycemia caused by active infection [138]. Point-of-cares for HbA1c measurement, as well as non-invasive advanced glycation end-product evaluation, could represent valid alternative approaches to screening DM, deserving a more accurate evaluation of the TB population [139]. The optimal treatment strategy for TB infection in DM patients is not yet established, and the standard antibiotic regimen is not tailored to comorbidities. As previously mentioned, DM patients have a higher rate of treatment failure, recurrence, and death. Some studies have shown lower serum concentrations of rifampicin [140] in diabetics and overweight patients, suggesting hidden pharmacokinetic variations of TB drugs. Increasing the dose of rifampicin or extending the time of treatment could improve TB outcomes [141,142], however, drug toxicity should be considered. Additionally, anti-diabetic drugs are influenced by TB antibiotic regimens. For example, rifampicin enhances the hepatic metabolism of all sulphonylureas, leading to complex dosing and increased adverse effects such as hypoglycemia. Nevertheless, sulphonylureas remain the most prescribed hypoglycemic drug in low- and middle-income countries due to their cost-effectiveness [143]. Metformin effects could be also impaired by rifampicin, owing to the increase in hepatic uptake and major glucose-lowering effects [144]. New oral antidiabetic drugs, such as GLP-1RAs, DPP4is, and SGLT2, have not shown clinically significant interactions with anti-TB drugs [145]. In contrast, even if insulin does not undergo hepatic metabolism, its availability, storage, delivery, and cost represent the main drawbacks to its administration in poor economic areas.
6. Conclusions and Future Perspective
In conclusion, DM is a world-spreading health problem and is associated with an increased risk of several infections. Chronic hyperglycemia impairs the function of several components of the immune system, thereby increasing the risk of infection-related morbidity and mortality. TB represents a long-lasting recognized infection associated with DM, while the SARS-CoV-2 pandemic has highlighted the risk of severe complications and death in DM patients. Attaining an early DM diagnosis and its related infections, managing patients to obtain proper glycemic control, and improving the selection of drugs lacking clinical interactions appear mandatory to reduce the burden of infection in DM patients. No definitive data are available on the safety of using newly introduced antidiabetic drugs in sepsis, thereby this topic should be a future challenge. Finally, the impact of hyperglycemia in impairing the immune system has spurred clinicians to search for and ascertain the beneficial effects of drugs administered to DM patients during sepsis in rescuing the immune defense. Data so far available have suggested that the prevention and reduction of infection severity may be considered the most relevant benefits. However, since randomized clinical trials are still an unmet need, tailored therapeutic strategies to manage infections and improve patient outcomes in DM still remain an open question.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Aschner, P.; Basit, A.; Fawwad, A.; Guariguata, L.; James, S.; Karuranga, S.; Malanda, B.; Mbanya, J.C.; O’neill, S.; Ogle, G.; et al. IDF Guide for Diabetes Epidemiology Studies IDF Guide for Diabetes Epidemiology Studies i Acknowledgements Authors. Available online: https://diabetesatlas.org/idf-guide-for-epidemiology-studies/ (accessed on 28 January 2023).
- IDF Diabetes Atlas 10th Edition. Available online: www.diabetesatlas.org (accessed on 28 January 2023).
- Schuetz, P.; Castro, P.; Shapiro, N.I. Diabetes and Sepsis: Preclinical Findings and Clinical Relevance. Diabetes Care. 2011, 34, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Erener, S. Diabetes, Infection Risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Kapsokosta, G.; Mouktaroudi, M.; Rovina, N.; Kaldis, V.; Stefos, A.; Kontogiorgi, M.; Giamarellos-Bourboulis, E.; Gogos, C. Diabetes on Sepsis Outcomes in Non-ICU Patients: A Cohort Study and Review of the Literature. J Diabetes Complicat. 2021, 35, 107765. [Google Scholar] [CrossRef]
- Jiang, L.; Cheng, M. Impact of Diabetes Mellitus on Outcomes of Patients with Sepsis: An Updated Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2022, 14, 1–17. [Google Scholar] [CrossRef]
- Costantini, E.; Carlin, M.; Porta, M.; Brizzi, M.F. Type 2 Diabetes Mellitus and Sepsis: State of the Art, Certainties and Missing Evidence. Acta Diabetol. 2021, 58, 1139–1151. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Fiayyaz, F.; Sabir, S.; Khurshid, M. Diabetes-Associated Infections: Development of Antimicrobial Resistance and Possible Treatment Strategies. Arch. Microbiol. 2020, 202, 953–965. [Google Scholar] [CrossRef]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Hosking, F.J.; Cook, D.G. Glycemic Control and Risk of Infections among People with Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care 2018, 41, 2127–2135. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Shah, B.R.; Hux, J.E. Quantifying the Risk of Infectious Diseases for People with Diabetes. Available online: http://diabetesjournals.org/care/article-pdf/26/2/510/648543/dc0203000510.pdf (accessed on 28 January 2023).
- Toniolo, A.; Cassani, G.; Puggioni, A.; Rossi, A.; Colombo, A.; Onodera, T.; Ferrannini, E. The Diabetes Pandemic and Associated Infections: Suggestions for Clinical Microbiology. Rev. Res. Med. Microbiol. 2019, 30, 1–17. [Google Scholar] [CrossRef]
- Polk, C.; Sampson, M.M.; Roshdy, D.; Davidson, L.E. Skin and Soft Tissue Infections in Patients with Diabetes Mellitus. In Infectious Disease Clinics of North America; W.B. Saunders: Philadelphia, PA, USA, 2021; pp. 183–197. [Google Scholar] [CrossRef]
- Chowdhury, T.; Gousy, N.; Bellamkonda, A.; Dutta, J.; Zaman, C.F.; Zakia, U.B.; Tasha, T.; Dutta, P.; Deb Roy, P.; Gomez, A.M.; et al. Fournier’s Gangrene: A Coexistence or Consanguinity of SGLT-2 Inhibitor Therapy. Cureus 2022, 14, e27773. [Google Scholar] [CrossRef] [PubMed]
- Ellegård, L.; Prytz, M. Fournier’s Gangrene under SGLT-2 Inhibitor Therapy: A Literature Review and Case Report. Int. J. Surg. Case Rep. 2020, 77, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Zhu, Q.Q.; Chen, Y.H.; Li, X.L.; Chen, F.; Huang, J.A.; Xu, B. Cardiovascular Safety, Long-Term Noncardiovascular Safety, and Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis with Trial Sequential Analysis. J. Am. Heart Assoc. 2018, 7, e007165. [Google Scholar] [CrossRef] [PubMed]
- Kamei, J.; Yamamoto, S. Complicated Urinary Tract Infections with Diabetes Mellitus. J. Infect. Chemother. 2021, 27, 1131–1136. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Chazan, B.; Saliba, W. Urinary Tract Infections in Patients with Type 2 Diabetes Mellitus: Review of Prevalence, Diagnosis, and Management. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 129–136. [Google Scholar] [CrossRef]
- Klekotka, R.B.; Mizgała, E.; Król, W. The Etiology of Lower Respiratory Tract Infections in People with Diabetes. Pneumonol. I Alergol. Pol. 2015, 83, 401–408. [Google Scholar] [CrossRef]
- Knapp, S. Diabetes and Infection: Is There a Link?—A Mini-Review. Gerontology 2013, 59, 99–104. [Google Scholar] [CrossRef]
- Hua, J.; Huang, P.; Liao, H.; Lai, X.; Zheng, X. Prevalence and Clinical Significance of Occult Pulmonary Infection in Elderly Patients with Type 2 Diabetes Mellitus. Biomed. Res. Int. 2021, 2021, 3187388. [Google Scholar] [CrossRef]
- Jones, R.N. Microbial Etiologies of Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia. Clin. Infect. Dis. 2010, 51, S81–S87. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Di, X.; Liu, B.; Liu, Y. Different Microbiological and Clinical Aspects of Lower Respiratory Tract Infections between China and European/American Countries. J. Thorac. Dis. 2014, 6, 134–142. [Google Scholar] [CrossRef]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in Patients with Diabetes Mellitus: A Review of Pathogenesis. Indian J. Endocrinol. Metab. 2012, 16, 27. [Google Scholar] [CrossRef]
- Martin, E.T.; Kaye, K.S.; Knott, C.; Nguyen, H.; Santarossa, M.; Evans, R.; Bertran, E.; Jaber, L. Diabetes and Risk of Surgical Site Infection: A Systematic Review and Meta-Analysis. Infect. Control. Hosp. Epidemiol. 2016, 37, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Carlos, D.; Spiller, F.; Souto, F.O.; Trevelin, S.C.; Borges, V.F.; de Freitas, A.; Alves-Filho, J.C.; Silva, J.S.; Ryffel, B.; Cunha, F.Q. Histamine H 2 Receptor Signaling in the Pathogenesis of Sepsis: Studies in a Murine Diabetes Model. J. Immunol. 2013, 191, 1373–1382. [Google Scholar] [CrossRef]
- Alba-Loureiro, T.C.; Munhoz, C.D.; Martins, J.O.; Cerchiaro, G.A.; Scavone, C.; Curi, R.; Sannomiya, P. Neutrophil Function and Metabolism in Individuals with Diabetes Mellitus. Volume 40. Available online: www.bjournal.com.br (accessed on 28 January 2023).
- Andersen, B.; Goldsmith, G.H.; Spagnuolo, P.J. Neutrophil Adhesive Dysfunction in Diabetes Mellitus: The Role of Cellular and Plasma Factors. J. Lab. Clin. Med. 1988, 111, 267–274. [Google Scholar]
- Delamaire, M.; Maugendre, D.; Moreno, M.; le Goff, M.C.; Allannic, H.; Genetet, B. Impaired Leucocyte Functions in Diabetic Patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]
- Tater, D.; Tepaut, B.; Bercovici, J.P.; Youinou, P. Polymorphonuclear cell derangements in type i diabetes. Horm. Metab. Res. 1987, 19, 642–647. [Google Scholar] [CrossRef]
- Rios-Santos, F.; Alves-Filho, J.C.; Souto, F.O.; Spiller, F.; Freitas, A.; Lotufo, C.M.C.; Soares, M.B.P.; dos Santos, R.R.; Teixeira, M.M.; Cunha, F.D.Q. Down-Regulation of CXCR2 on Neutrophils in Severe Sepsis Is Mediated by Inducible Nitric Oxide Synthase-Derived Nitric Oxide. Am. J. Respir. Crit. Care Med. 2007, 175, 490–497. [Google Scholar] [CrossRef]
- Vieira, S.M.; Lemos, H.P.; Grespan, R.; Napimoga, M.H.; Dal-Secco, D.; Freitas, A.; Cunha, T.M.; Verri, W.A.; Souza, D.A.; Jamur, M.C.; et al. A Crucial Role for TNF-α in Mediating Neutrophil Influx Induced by Endogenously Generated or Exogenous Chemokines, KC/CXCL1 and LIX/CXCL5: RESEARCH PAPER. Br. J. Pharmacol. 2009, 158, 779–789. [Google Scholar] [CrossRef]
- Hair, P.S.; Echague, C.G.; Rohn, R.D.; Krishna, N.K.; Nyalwidhe, J.O.; Cunnion, K.M. Hyperglycemic Conditions Inhibit C3-Mediated Immunologic Control of Staphylococcus Aureus. J. Transl. Med. 2012, 10, 1–16. [Google Scholar] [CrossRef]
- Chao, W.C.; Yen, C.L.; Wu, Y.H.; Chen, S.Y.; Hsieh, C.Y.; Chang, T.C.; Ou, H.Y.; Shieh, C.C. Increased Resistin May Suppress Reactive Oxygen Species Production and Inflammasome Activation in Type 2 Diabetic Patients with Pulmonary Tuberculosis Infection. Microbes Infect. 2015, 17, 195–204. [Google Scholar] [CrossRef]
- Shah, S.V.; Wallin, J.D.; Eilenj, S.D. Chemiluminescence and superoxide anion production by leukocytes from diabetic patients. J. Clin. Endocrinol. Metab. 1983, 57, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Stegenga, M.E.; van der Crabben, S.N.; Blümer, R.M.; Levi, M.; Meijers, J.C.; Serlie, M.J.; Tanck, M.W.; Sauerwein, H.P.; van der Poll, T. Hyperglycemia Enhances Coagulation and Reduces Neutrophil Degranulation, Whereas Hyperinsulinemia Inhibits Fibrinolysis during Human Endotoxemia. Blood J. Am. Soc. Hematol. 2008, 112, 82–89. [Google Scholar] [CrossRef]
- Joshi, M.B.; Lad, A.; Bharath Prasad, A.S.; Balakrishnan, A.; Ramachandra, L.; Satyamoorthy, K. High Glucose Modulates IL-6 Mediated Immune Homeostasis through Impeding Neutrophil Extracellular Trap Formation. FEBS Lett. 2013, 587, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, B.I.; Twahirwa, M.; Rahbar, M.H.; Schlesinger, L.S. Phagocytosis via Complement or Fc-Gamma Receptors Is Compromised in Monocytes from Type 2 Diabetes Patients with Chronic Hyperglycemia. PLoS ONE 2014, 9, e92977. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.; Chen, M. Sustained High Glucose Exposure Sensitizes Macrophage Responses to Cytokine Stimuli but Reduces Their Phagocytic Activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhang, H.J.; Hu, Q.X.; Liu, X.Y.; Wang, Z.Q.; Fan, J.Y.; Zhan, M.; Chen, F.L. Altered Polarization, Morphology, and Impaired Innate Immunity Germane to Resident Peritoneal Macrophages in Mice with Long-Term Type 2 Diabetes. J. Biomed. Biotechnol. 2012, 2012, 867023. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S.; et al. Signaling by IL-6 Promotes Alternative Activation of Macrophages to Limit Endotoxemia and Obesity-Associated Resistance to Insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From Endoplasmic-Reticulum Stress to the Inflammatory Response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef]
- Whalen, M.M. Inhibition of Human Natural Killer Cell Function in Vitro by Glucose Concentrations Seen in Poorly Controlled Diabetes. Cell. Physiol. Biochem. 1997, 7, 53–60. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, K.; Lee, S.B.; Kang, S.; Park, J.S.; Ahn, C.W.; Nam, J.S. Relationship between Natural Killer Cell Activity and Glucose Control in Patients with Type 2 Diabetes and Prediabetes. J. Diabetes Investig. 2019, 10, 1223–1228. [Google Scholar] [CrossRef]
- Kumar, M.; Roe, K.; Nerurkar, P.V.; Orillo, B.; Thompson, K.S.; Verma, S.; Nerurkar, V.R. Reduced Immune Cell Infiltration and Increased Pro-Inflammatory Mediators in the Brain of Type 2 Diabetic Mouse Model Infected with West Nile Virus. 2014. Available online: http://www.jneuroinflammation.com/content/11/1/80 (accessed on 28 January 2023).
- Lapolla, A.; Tonani, R.; Fedele, D.; Garbeglio, M.; Senesi, A.; Seraglia, R.; Favretto, D.; Traldi, P. Non-Enzymatic Glycation of IgG: An In Vivo Study. Horm. Metab. Res. 2002, 34, 260–264. [Google Scholar] [CrossRef]
- van Vught, L.A.; Scicluna, B.P.; Hoogendijk, A.J.; Wiewel, M.A.; Klein Klouwenberg, P.M.C.; Cremer, O.L.; Horn, J.; Nürnberg, P.; Bonten, M.M.J.; Schultz, M.J.; et al. Association of Diabetes and Diabetes Treatment with the Host Response in Critically Ill Sepsis Patients. Crit. Care 2016, 20, 252. [Google Scholar] [CrossRef] [PubMed]
- Ruiqiang, Z.; Yifen, Z.; Ziqi, R.; Wei, H.; Xiaoyun, F. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021, Interpretation and Expectation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 1159–1164. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zeng, F.; Luo, X.; Lei, Y.; Li, J.; Lu, S.; Huang, X.; Lan, Y.; Liu, R. GLP-1 Receptor: A New Target for Sepsis. Front. Pharmacol. 2021, 12, 706908. [Google Scholar] [CrossRef]
- Lu, Z.; Tao, G.; Sun, X.; Zhang, Y.; Jiang, M.; Liu, Y.; Ling, M.; Zhang, J.; Xiao, W.; Hua, T.; et al. Association of Blood Glucose Level and Glycemic Variability with Mortality in Sepsis Patients During ICU Hospitalization. Front. Public Health 2022, 10, 857368. [Google Scholar] [CrossRef]
- Bharath, L.P.; Nikolajczyk, B.S. The Intersection of Metformin and Inflammation. Am. J. Physiol. Cell Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef]
- Gómez, H.; del Rio-Pertuz, G.; Priyanka, P.; Manrique-Caballero, C.L.; Chang, C.C.H.; Wang, S.; Liu, Q.; Zuckerbraun, B.S.; Murugan, R.; Angus, D.C.; et al. Association of Metformin Use During Hospitalization and Mortality in Critically Ill Adults with Type 2 Diabetes Mellitus and Sepsis. Crit. Care Med. 2022, 50, 935–944. [Google Scholar] [CrossRef]
- Montoya, C. Clarification of Key Points in a Study Evaluating the Association of Metformin and Mortality in Patients with Sepsis and Type 2 Diabetes. Crit. Care Med. 2023, 51, E60. [Google Scholar] [CrossRef]
- Yen, F.S.; Wei, J.C.C.; Shih, Y.H.; Pan, W.L.; Hsu, C.C.; Hwu, C.M. Role of Metformin in Morbidity and Mortality Associated with Urinary Tract Infections in Patients with Type 2 Diabetes. J. Pers. Med. 2022, 12, 702. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Guo, Y.; Duan, Y.; Guo, Y.; Ding, X. Association of Preadmission Metformin Use and Prognosis in Patients with Sepsis and Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 811776. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alzoubi, K.H.; Masadeh, M.M.; Aburashed, Z.O. Metformin as a Potential Adjuvant Antimicrobial Agent against Multidrug Resistant Bacteria. Clin. Pharmacol. 2021, 13, 83–90. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, G.; Davis, T.; Engelbrecht, A.M. Hyperglycaemia in Critically Ill Patients: The Immune System’s Sweet Tooth. Crit. Care 2017, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Curtis, J.C.; Plotkin, B.J. Insulin Regulation of Escherichia Coli Abiotic Biofilm Formation: Effect of Nutrients and Growth Conditions. Antibiotics 2021, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, Z.; Luo, J.; Kong, J.; Ding, Y.; Chen, Y.; Wang, K. Insulin Treatment Enhances Pseudomonas Aeruginosa Biofilm Formation by Increasing Intracellular Cyclic Di-GMP Levels, Leading to Chronic Wound Infection and Delayed Wound Healing. 2019, Volume 11. Available online: www.ajtr.org (accessed on 28 January 2023).
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018, 28, 922–934.e4. [Google Scholar] [CrossRef]
- Steven, S.; Hausding, M.; Kröller-Schön, S.; Mader, M.; Mikhed, Y.; Stamm, P.; Zinßius, E.; Pfeffer, A.; Welschof, P.; Agdauletova, S.; et al. Gliptin and GLP-1 Analog Treatment Improves Survival and Vascular Inflammation/Dysfunction in Animals with Lipopolysaccharide-induced Endotoxemia. Basic Res. Cardiol. 2015, 110, 1–14. [Google Scholar] [CrossRef]
- Helmstädter, J.; Keppeler, K.; Aust, F.; Küster, L.; Frenis, K.; Filippou, K.; Vujacic-Mirski, K.; Tsohataridis, S.; Kalinovic, S.; Kröller-Schön, S.; et al. GLP-1 Analog Liraglutide Improves Vascular Function in Polymicrobial Sepsis by Reduction of Oxidative Stress and Inflammation. Antioxidants 2021, 10, 1175. [Google Scholar] [CrossRef]
- Steven, S.; Jurk, K.; Kopp, M.; Kröller-Schön, S.; Mikhed, Y.; Schwierczek, K.; Roohani, S.; Kashani, F.; Oelze, M.; Klein, T.; et al. Themed Section: Redox Biology and Oxidative Stress in Health and Disease. Br. J. Pharm. 2017, 174, 1620. [Google Scholar] [CrossRef]
- Kröller-Schön, S.; Knorr, M.; Hausding, M.; Oelze, M.; Schuff, A.; Schell, R.; Sudowe, S.; Scholz, A.; Daub, S.; Karbach, S.; et al. Glucose-Independent Improvement of Vascular Dysfunction in Experimental Sepsis by Dipeptidyl-Peptidase 4 Inhibition. Cardiovasc. Res. 2012, 96, 140–149. [Google Scholar] [CrossRef]
- al Zoubi, S.; Chen, J.; Murphy, C.; Martin, L.; Chiazza, F.; Collotta, D.; Yaqoob, M.M.; Collino, M.; Thiemermann, C. Linagliptin Attenuates the Cardiac Dysfunction Associated with Experimental Sepsis in Mice with Pre-Existing Type 2 Diabetes by Inhibiting NF-ΚB. Front. Immunol. 2018, 9, 2996. [Google Scholar] [CrossRef]
- Wang, S.C.; Wang, X.Y.; Liu, C.T.; Chou, R.H.; Chen, Z.B.; Huang, P.H.; Lin, S.J. The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Ameliorates Endothelial Inflammation and Microvascular Thrombosis in a Sepsis Mouse Model. Int. J. Mol. Sci. 2022, 23, 3065. [Google Scholar] [CrossRef] [PubMed]
- Brakenridge, S.C.; Moore, F.A.; Mercier, N.R.; Cox, M.; Wu, Q.; Moldawer, L.L.; Mohr, A.M.; Efron, P.A.; Smith, R.S. Persistently Elevated Glucagon-Like Peptide-1 Levels among Critically Ill Surgical Patients after Sepsis and Development of Chronic Critical Illness and Dismal Long-Term Outcomes. J. Am. Coll. Surg. 2019, 229, 58–67.e1. [Google Scholar] [CrossRef] [PubMed]
- Perl, S.H.; Bloch, O.; Zelnic-Yuval, D.; Love, I.; Mendel-Cohen, L.; Flor, H.; Rapoport, M.J. Sepsis-Induced Activation of Endogenous GLP-1 System Is Enhanced in Type 2 Diabetes. Diabetes/Metab. Res. Rev. 2018, 34, e2982. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Perl, S.H.; Lazarovitch, T.; Zelnik-Yovel, D.; Love, I.; Mendel-Cohen, L.; Goltsman, G.; Flor, H.; Rapoport, M.J. Hyper-Activation of Endogenous GLP-1 System to Gram-Negative Sepsis Is Associated with Early Innate Immune Response and Modulated by Diabetes. Shock 2021, 55, 796–805. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diabetes Rep. 2022, 22, 39–52. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Cui, Q.; Zhou, Y.; Zhao, B.; Mei, D.; Xuemei. Acute Kidney Injury Following SGLT2 Inhibitors among Diabetic Patients: A Pharmacovigilance Study. Int. Urol. Nephrol. 2022, 54, 2949–2957. [Google Scholar] [CrossRef]
- Donnan, J.R.; Grandy, C.A.; Chibrikov, E.; Marra, C.A.; Aubrey-Bassler, K.; Johnston, K.; Swab, M.; Hache, J.; Curnew, D.; Nguyen, H.; et al. Comparative Safety of the Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e022577. [Google Scholar] [CrossRef]
- Dave, C.V.; Schneeweiss, S.; Kim, D.; Fralick, M.; Tong, A.; Patorno, E. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Severe Urinary Tract Infections. Ann. Intern. Med. 2019, 171, 248–256. [Google Scholar] [CrossRef]
- Wiegley, N.; So, P.N. Sodium-Glucose Cotransporter 2 Inhibitors and Urinary Tract Infection: Is There Room for Real Concern? Kidney360 2022, 3, 1991–1993. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, J.; Zhong, L.; Li, S.; Zhou, L.; Zhang, Q.; Li, M.; Xiao, X. The Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Biomarkers of Inflammation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharm. 2022, 13, 4779. [Google Scholar] [CrossRef] [PubMed]
- Kıngır, Z.B.; Özdemir Kumra, Z.N.; Çam, M.E.; Çilingir, Ö.T.; Şekerler, T.; Ercan, F.; Özakpınar, Ö.B.; Özsavcı, D.; Sancar, M.; Okuyan, B. Effects of Dapagliflozin in Experimental Sepsis Model in Rats. Ulus. Travma Ve Acil Cerrahi Derg. 2019, 25, 213–221. [Google Scholar] [CrossRef]
- Chi, P.J.; Lee, C.J.; Hsieh, Y.J.; Lu, C.W.; Hsu, B.G. Dapagliflozin Ameliorates Lipopolysaccharide Related Acute Kidney Injury in Mice with Streptozotocin-Induced Diabetes Mellitus. Int. J. Med. Sci. 2022, 19, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Soni, S.; Dyck, J.R.B. Empagliflozin Suppresses Inflammation and Protects against Acute Septic Renal Injury. Inflammopharmacology 2021, 29, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Tse, Y.-K.; Chandramouli, C.; Hon, N.W.-L.; Cheung, C.-L.; Lam, L.-Y.; Wu, M.; Huang, J.-Y.; Yu, S.-Y.; Leung, K.-L.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and the Risk of Pneumonia and Septic Shock. J. Clin. Endocrinol. Metab. 2022, 107, 3442–3451. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. 2023. WHO Coronavirus (COVID-19) Dashboard. Available online: https://Covid19.Who.Int/ (accessed on 28 January 2023).
- Wang, B.; Li, R.; Lu, Z. Does Comorbidity Increase the Risk of Patients with COVID-19- Evidence from Meta-Analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Pranata, R.; Henrina, J.; Raffaello, W.M.; Lawrensia, S.; Huang, I. Diabetes and COVID-19: The Past, the Present, and the Future. In Metabolism: Clinical and Experimental; W.B. Saunders: Philadelphia, PA, USA, 2021. [Google Scholar] [CrossRef]
- McGurnaghan, S.J.; Weir, A.; Bishop, J.; Kennedy, S.; Blackbourn, L.A.K.; McAllister, D.A.; Hutchinson, S.; Caparrotta, T.M.; Mellor, J.; Jeyam, A.; et al. Risks of and Risk Factors for COVID-19 Disease in People with Diabetes: A Cohort Study of the Total Population of Scotland. Lancet Diabetes Endocrinol. 2021, 9, 82–93. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and Diabetes Mellitus: From Pathophysiology to Clinical Management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Singh, A.K.; Khunti, K. COVID-19 and diabetes. Annu. Rev. Med. 2022, 73, 129–147. [Google Scholar] [CrossRef]
- Khunti, K.; del Prato, S.; Mathieu, C.; Kahn, S.E.; Gabbay, R.A.; Buse, J.B. COVID-19, Hyperglycemia, and New-Onset Diabetes. Diabetes Care 2021, 44, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Jafar, N.; Edriss, H.; Nugent, K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef]
- Coppelli, A.; Giannarelli, R.; Aragona, M.; Penno, G.; Falcone, M.; Tiseo, G.; Ghiadoni, L.; Barbieri, G.; Monzani, F.; Virdis, A.; et al. Hyperglycemia at Hospital Admission Is Associated with Severity of the Prognosis in Patients Hospitalized for COVID-19: The Pisa COVID-19 Study. Diabetes Care 2020, 43, 2345–2348. [Google Scholar] [CrossRef]
- Brooks, D.; Schulman-Rosenbaum, R.; Griff, M.; Lester, J.; Low Wang, C.C. Glucocorticoid-Induced Hyperglycemia Including Dexamethasone-Associated Hyperglycemia in COVID-19 Infection: A Systematic Review. Endocr. Pract. 2022, 28, 1166–1177. [Google Scholar] [CrossRef]
- Chai, C.; Chen, K.; Li, S.; Cheng, G.; Wang, W.; Wang, H.; Wei, D.; Peng, C.; Sun, Q.; Tang, Z. Effect of Elevated Fasting Blood Glucose Level on the 1-Year Mortality and Sequelae in Hospitalized COVID-19 Patients: A Bidirectional Cohort Study. J. Med. Virol. 2022, 94, 3240–3250. [Google Scholar] [CrossRef]
- Mifsud, S.; Schembri, E.L.; Gruppetta, M. Stress-Induced Hyperglycaemia. Br. J. Hosp. Med. 2018, 79, 634–639. [Google Scholar] [CrossRef]
- Kolahian, S.; Leiss, V.; Nürnberg, B. Diabetic Lung Disease: Fact or Fiction? In Endocrine and Metabolic Disorders; Springer: New York, NY, USA, 2019; pp. 303–319. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Xiao, F.; Zimpelmann, J.; Har, R.L.H.; Lai, V.; Scholey, J.W.; Reich, H.N.; Burns, K.D. Urinary ACE2 in Healthy Adults and Patients with Uncomplicated Type 1 Diabetes. Can. J. Physiol. Pharmacol. 2014, 92, 703–706. [Google Scholar] [CrossRef]
- Rao, S.; Lau, A.; So, H.C. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of SARS-CoV-2: A Mendelian Randomization Analysis Highlights Tentative Relevance of Diabetes-Related Traits. Diabetes Care 2020, 43, 1416–1426. [Google Scholar] [CrossRef]
- Pugliese, G.; Vitale, M.; Resi, V.; Orsi, E. Is Diabetes Mellitus a Risk Factor for COronaVIrus Disease 19 (COVID-19)? Acta Diabetol. 2020, 57, 1275–1285. [Google Scholar] [CrossRef]
- Pal, R.; Bhansali, A. COVID-19, Diabetes Mellitus and ACE2: The Conundrum. Diabetes Res. Clin. Pract. 2020, 162, 108132. [Google Scholar] [CrossRef]
- South, A.M.; Tomlinson, L.; Edmonston, D.; Hiremath, S.; Sparks, M.A. Controversies of Renin–Angiotensin System Inhibition during the COVID-19 Pandemic. Nat. Rev. Nephrol. 2020, 16, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Pelle, M.C.; Zaffina, I.; Provenzano, M.; Moirano, G.; Arturi, F. COVID-19 and Diabetes—Two Giants Colliding: From Pathophysiology to Management. Front. Endocrinol. 2022, 13, 974540. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Rao, X.; Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J. Diabetes Res. 2017, 2017, 6494795. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Marre, M.; Thivolet, C. Prognostic Factors in Patients with Diabetes Hospitalized for COVID-19: Findings from the CORONADO Study and Other Recent Reports. Diabetes Metab. 2020, 46, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.; Joshi, A.D.; Nguyen, L.H.; Leeming, E.R.; Mazidi, M.; Drew, D.A.; Gibson, R.; Graham, M.S.; Lo, C.H.; Capdevila, J.; et al. Diet Quality and Risk and Severity of COVID-19: A Prospective Cohort Study. Gut 2021, 70, 2096–2104. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Yan, P.; Sun, T.; Zeng, Z.; Li, S. Metformin in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 704666. [Google Scholar] [CrossRef]
- Scheen, A.J. Metformin and COVID-19: From Cellular Mechanisms to Reduced Mortality. Diabetes Metab. 2020, 46, 423–426. [Google Scholar] [CrossRef]
- Ursini, F.; Ciaffi, J.; Landini, M.P.; Meliconi, R. COVID-19 and Diabetes: Is Metformin a Friend or Foe? Diabetes Res. Clin. Pract. 2020, 164, 108167. [Google Scholar] [CrossRef]
- Narayanan, N.; Naik, D.; Sahoo, J.; Kamalanathan, S. Dipeptidyl Peptidase 4 Inhibitors in COVID-19: Beyond Glycemic Control. World J. Virol. 2022, 11, 399–410. [Google Scholar] [CrossRef]
- Nassar, M.; Abosheaishaa, H.; Singh, A.K.; Misra, A.; Bloomgarden, Z. Noninsulin-Based Antihyperglycemic Medications in Patients with Diabetes and COVID-19: A Systematic Review and Meta-Analysis. J. Diabetes 2023. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Dalan, R.; Hopkins, D.; Mingrone, G.; Boehm, B.O. Endocrine and Metabolic Link to Coronavirus Infection. Nat. Rev. Endocrinol. 2020, 16, 297–298. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Intan, D.; Hananto, J.E.; Putri, C.; Kurniawan, A. Pre-Admission Glucagon-like Peptide-1 Receptor Agonist (GLP-1RA) and Mortality from Coronavirus Disease 2019 (COVID-19): A Systematic Review, Meta-Analysis, and Meta-Regression. Diabetes Res. Clin. Pract. 2021, 179, 109031. [Google Scholar] [CrossRef]
- Nyland, J.E.; Raja-Khan, N.T.; Bettermann, K.; Haouzi, P.A.; Leslie, D.L.; Kraschnewski, J.L.; Parent, L.J.; Grigson, P.S. Diabetes, Drug Treatment, and Mortality in COVID-19: A Multinational Retrospective Cohort Study. Diabetes 2021, 70, 2903–2916. [Google Scholar] [CrossRef]
- Popovic, D.S.; Papanas, N.; Pantea Stoian, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Use of Novel Antidiabetic Agents in Patients with Type 2 Diabetes and COVID-19: A Critical Review. Diabetes Ther. 2021, 12, 3037–3054. [Google Scholar] [CrossRef]
- Bielka, W.; Przezak, A.; Pawlik, A. Therapy of Type 2 Diabetes in Patients with SARS-CoV-2 Infection. Int. J. Mol. Sci. 2021, 22, 7605. [Google Scholar] [CrossRef]
- Drucker, D.J. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr. Rev. 2021, 41, 457–470. [Google Scholar] [CrossRef]
- Roca-Ho, H.; Riera, M.; Palau, V.; Pascual, J.; Soler, M.J. Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse. Int. J. Mol. Sci. 2017, 18, 563. [Google Scholar] [CrossRef]
- Ricchio, M.; Tassone, B.; Pelle, M.C.; Mazzitelli, M.; Serapide, F.; Fusco, P.; Lionello, R.; Cancelliere, A.; Procopio, G.; Lio, E.; et al. Characteristics, Management, and Outcomes of Elderly Patients with Diabetes in a COVID-19 Unit: Lessons Learned from a Pilot Study. Medicina 2021, 57, 341. [Google Scholar] [CrossRef]
- Global Tuberculosis ReporT 2022. 2022. Available online: http://apps.who.int/bookorders (accessed on 28 January 2023).
- Mendenhall, E.; Kohrt, B.A.; Norris, S.A.; Ndetei, D.; Prabhakaran, D. Non-Communicable Disease Syndemics: Poverty, Depression, and Diabetes among Low-Income Populations. Lancet 2017, 389, 951–963. [Google Scholar] [CrossRef]
- Gakidou, E.; Mallinger, L.; Abbott-Klafter, J.; Guerrero, R.; Villalpando, S.; Ridaura, R.L.; Aekplakorn, W.; Naghavi, M.; Lim, S.; Lozano, R.; et al. Management of Diabetes and Associated Cardiovascular Risk Factors in Seven Countries: A Comparison of Data from National Health Examination Surveys. Bull. World Health Organ. 2011, 89, 172–183. [Google Scholar] [CrossRef]
- Riza, A.L.; Pearson, F.; Ugarte-Gil, C.; Alisjahbana, B.; van de Vijver, S.; Panduru, N.M.; Hill, P.C.; Ruslami, R.; Moore, D.; Aarnoutse, R.; et al. Clinical Management of Concurrent Diabetes and Tuberculosis and the Implications for Patient Services. Lancet Diabetes Endocrinol. 2014, 2, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.Y.; Murray, M.B. Diabetes Mellitus Increases the Risk of Active Tuberculosis: A Systematic Review of 13 Observational Studies. PLoS Med. 2008, 5, 1091–1101. [Google Scholar] [CrossRef]
- Baker, M.A.; Harries, A.D.; Jeon, C.Y.; Hart, J.E.; Kapur, A.; Lönnroth, K.; Ottmani, S.E.; Goonesekera, S.D.; Murray, M.B. The Impact of Diabetes on Tuberculosis Treatment Outcomes: A Systematic Review. BMC Med. 2011, 9, 81. [Google Scholar] [CrossRef]
- Jiménez-Corona, M.E.; Cruz-Hervert, L.P.; García-García, L.; Ferreyra-Reyes, L.; Delgado-Sánchez, G.; Bobadilla-Del-Valle, M.; Canizales-Quintero, S.; Ferreira-Guerrero, E.; Báez-Saldaña, R.; Téllez-Vázquez, N.; et al. Association of Diabetes and Tuberculosis: Impact on Treatment and Post-Treatment Outcomes. Thorax 2013, 68, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, P.; Ugarte-Gil, C.; Golub, J.; Pearson, F.; Critchley, J. The Effects of Diabetes on Tuberculosis Treatment Outcomes: An Updated Systematic Review and Meta-Analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, B.S.; Mengesha, M.M.; Teferra, A.A.; Awoke, M.A.; Habtewold, T.D. Association between Diabetes Mellitus and Multi-Drug-Resistant Tuberculosis: Evidence from a Systematic Review and Meta-Analysis 11 Medical and Health Sciences 1117 Public Health and Health Services. Syst. Rev. 2018, 7, 161. [Google Scholar] [CrossRef]
- Martinez, N.; Kornfeld, H. Diabetes and Immunity to Tuberculosis. In European Journal of Immunology; Wiley-VCH: Weinheim, Germany, 2014; pp. 617–626. [Google Scholar] [CrossRef]
- Vallerskog, T.; Martens, G.W.; Kornfeld, H. Diabetic Mice Display a Delayed Adaptive Immune Response to Mycobacterium Tuberculosis. J. Immunol. 2010, 184, 6275–6282. [Google Scholar] [CrossRef]
- Kumar, N.P.; Sridhar, R.; Banurekha, V.V.; Jawahar, M.S.; Nutman, T.B.; Babu, S. Expansion of Pathogen-Specific T-Helper 1 and T-Helper 17 Cells in Pulmonary Tuberculosis with Coincident Type 2 Diabetes Mellitus. J. Infect. Dis. 2013, 208, 739–748. [Google Scholar] [CrossRef]
- Martinez, N.; Vallerskog, T.; West, K.; Nunes-Alves, C.; Lee, J.; Martens, G.W.; Behar, S.M.; Kornfeld, H. Chromatin Decondensation and T Cell Hyperresponsiveness in Diabetes-Associated Hyperglycemia. J. Immunol. 2014, 193, 4457–4468. [Google Scholar] [CrossRef]
- Kumar, N.P.; Sridhar, R.; Banurekha, V.V.; Jawahar, M.S.; Fay, M.P.; Nutman, T.B.; Babu, S. Type 2 Diabetes Mellitus Coincident with Pulmonary Tuberculosis Is Associated with Heightened Systemic Type 1, Type 17, and Other Proinflammatory Cytokines. Ann. Am. Thorac. Soc. 2013, 10, 441–449. [Google Scholar] [CrossRef]
- Podell, B.K.; Ackart, D.F.; Obregon-Henao, A.; Eck, S.P.; Henao-Tamayo, M.; Richardson, M.; Orme, I.M.; Ordway, D.J.; Basaraba, R.J. Increased Severity of Tuberculosis in Guinea Pigs with Type 2 Diabetes: A Model of Diabetes-Tuberculosis Comorbidity. Am. J. Pathol. 2014, 184, 1104–1118. [Google Scholar] [CrossRef]
- Martens, G.W.; Arikan, M.C.; Lee, J.; Ren, F.; Greiner, D.; Kornfeld, H. Tuberculosis Susceptibility of Diabetic Mice. Am. J. Respir. Cell Mol. Biol. 2007, 37, 518–524. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Harries, A.D.; Baker, M.A.; Hart, J.E.; Kapur, A.; Lönnroth, K.; Ottmani, S.E.; Goonesekera, S.; Murray, M.B. Bi-Directional Screening for Tuberculosis and Diabetes: A Systematic Review. Trop. Med. Int. Health 2010, 15, 1300–1314. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, D.; Nagaraja, S.B.; Nair, A.; Satyanarayana, S.; Kumar, A.M.V.; Chadha, S.S.; Wilson, N.; Sharma, S.K.; Soneja, M.; et al. Screening of Patients with Diabetes Mellitus for Tuberculosis in India. Trop. Med. Int. Health 2013, 18, 646–654. [Google Scholar] [CrossRef]
- Lin, Y.; Li, L.; Mi, F.; Du, J.; Dong, Y.; Li, Z.; Qi, W.; Zhao, X.; Cui, Y.; Hou, F.; et al. Screening Patients with Diabetes Mellitus for Tuberculosis in China. Trop. Med. Int. Health 2012, 17, 1302–1308. [Google Scholar] [CrossRef]
- Oluboyo, P.O.; Erasmus, R.T. The Significance of Glucose Intolerance in Pulmonary Tuberculosis. Tubercle 1990, 71, 135–138. [Google Scholar] [CrossRef]
- Adepoyibi, T.; Weigl, B.; Greb, H.; Neogi, T.; McGuire, H. New Screening Technologies for Type 2 Diabetes Mellitus Appropriate for Use in Tuberculosis Patients. Public Health Action 2013, 3, 10–17. [Google Scholar] [CrossRef]
- Nijland, H.M.J.; Ruslami, R.; Stalenhoef, J.E.; Nelwan, E.J.; Alisjahbana, B.; Nelwan, R.H.H.; van der Ven, A.J.A.M.; Danusantoso, H.; Aarnoutse, R.E.; van Crevel, R. Exposure to Rifampicin Is Strongly Reduced in Patients with Tuberculosis and Type 2 Diabetes. Clin. Infect. Dis. 2006, 43, 848–854. [Google Scholar] [CrossRef]
- van Ingen, J.; Aarnoutse, R.E.; Donald, P.R.; Diacon, A.H.; Dawson, R.; Plemper Van Balen, G.; Gillespie, S.H.; Boeree, M.J. Why Do We Use 600 Mg of Rifampicin in Tuberculosis Treatment? Clin. Infect. Dis. 2011, 52, e194–e199. [Google Scholar] [CrossRef]
- Ruslami, R.; Ganiem, A.R.; Dian, S.; Apriani, L.; Achmad, T.H.; van der Ven, A.J.; Borm, G.; Aarnoutse, R.E.; van Crevel, R. Intensified Regimen Containing Rifampicin and Moxifloxacin for Tuberculous Meningitis: An Open-Label, Randomised Controlled Phase 2 Trial. Lancet Infect. Dis. 2013, 13, 27–35. [Google Scholar] [CrossRef]
- Mohan, V.; Saboo, B.; Khader, J.; Modi, K.D.; Jindal, S.; Wangnoo, S.K.; Amarnath, S. Position of Sulfonylureas in the Current ERA: Review of National and International Guidelines. In Clinical Medicine Insights: Endocrinology and Diabetes; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2022. [Google Scholar] [CrossRef]
- Cho, S.K.; Yoon, J.S.; Lee, M.G.; Lee, D.H.; Lim, L.A.; Park, K.; Park, M.S.; Chung, J.Y. Rifampin Enhances the Glucose-Lowering Effect of Metformin and Increases OCT1 MRNA Levels in Healthy Participants. Clin. Pharm. 2011, 89, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Tornio, A.; Niemi, M.; Neuvonen, P.J.; Backman, J.T. Drug Interactions with Oral Antidiabetic Agents: Pharmacokinetic Mechanisms and Clinical Implications. Trends Pharmacol. Sci. 2012, 33, 312–322. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).