Acellular Biomaterials Associated with Autologous Bone Marrow-Derived Mononuclear Stem Cells Improve Wound Healing through Paracrine Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Isolation and Characterization of BMSCs/Acellular Amniotic Membrane Preparation
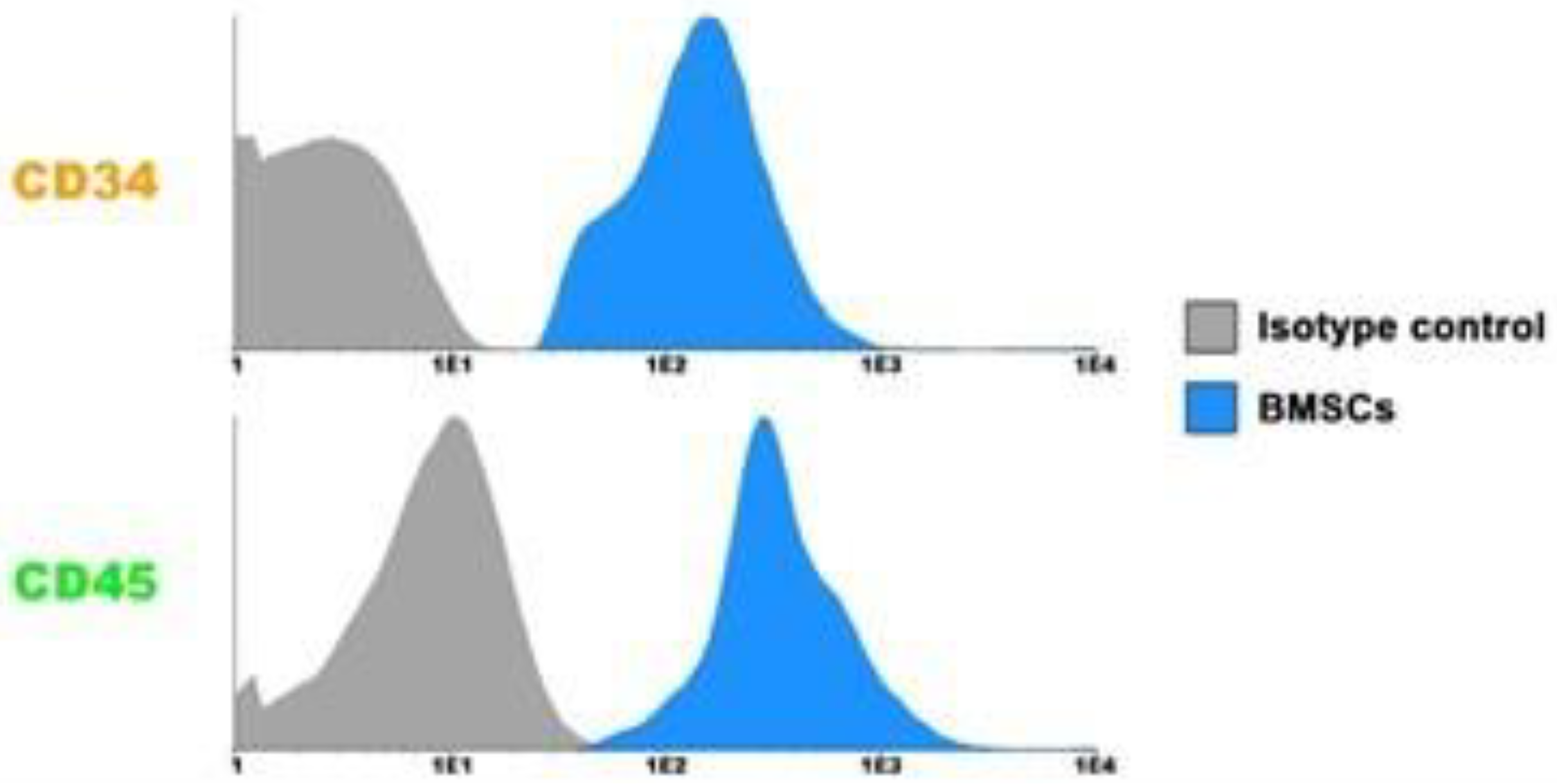
2.4. Flow Cytometry Cell Analysis
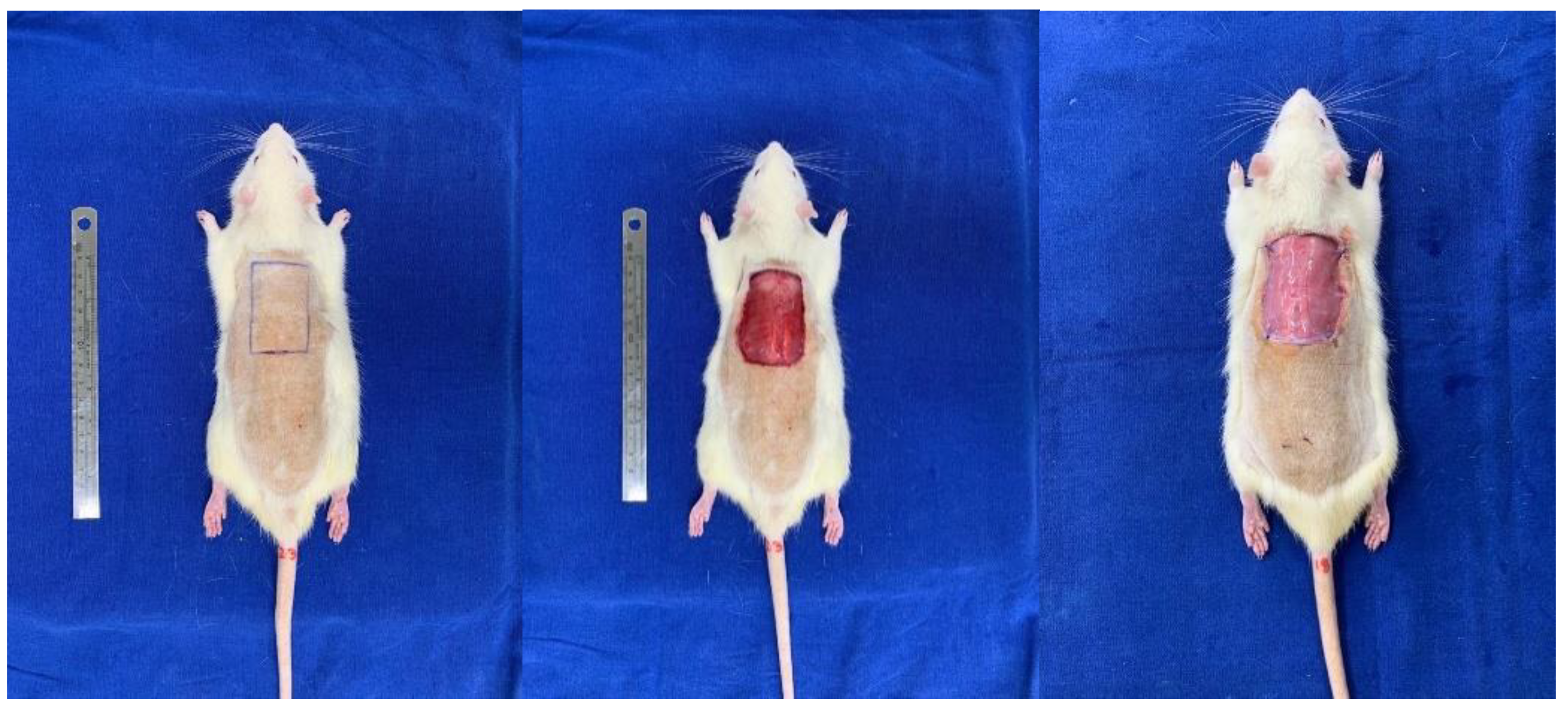
2.5. Rat Flap Skin Model and Membrane Implant
2.6. Measurements of Cytokines, IL-1, IL10, and TGF-β Levels
2.7. Determination of the Oxidative Damage
2.8. Superoxide Dismutase Activity and Glutathione Reductase
2.9. Measurements of Protein
2.10. Euthanasia
2.11. Immunohistochemistry Analysis
2.12. Histopathological Analysis
2.13. Statistical Analysis
3. Results
3.1. Characterization of BMSCs
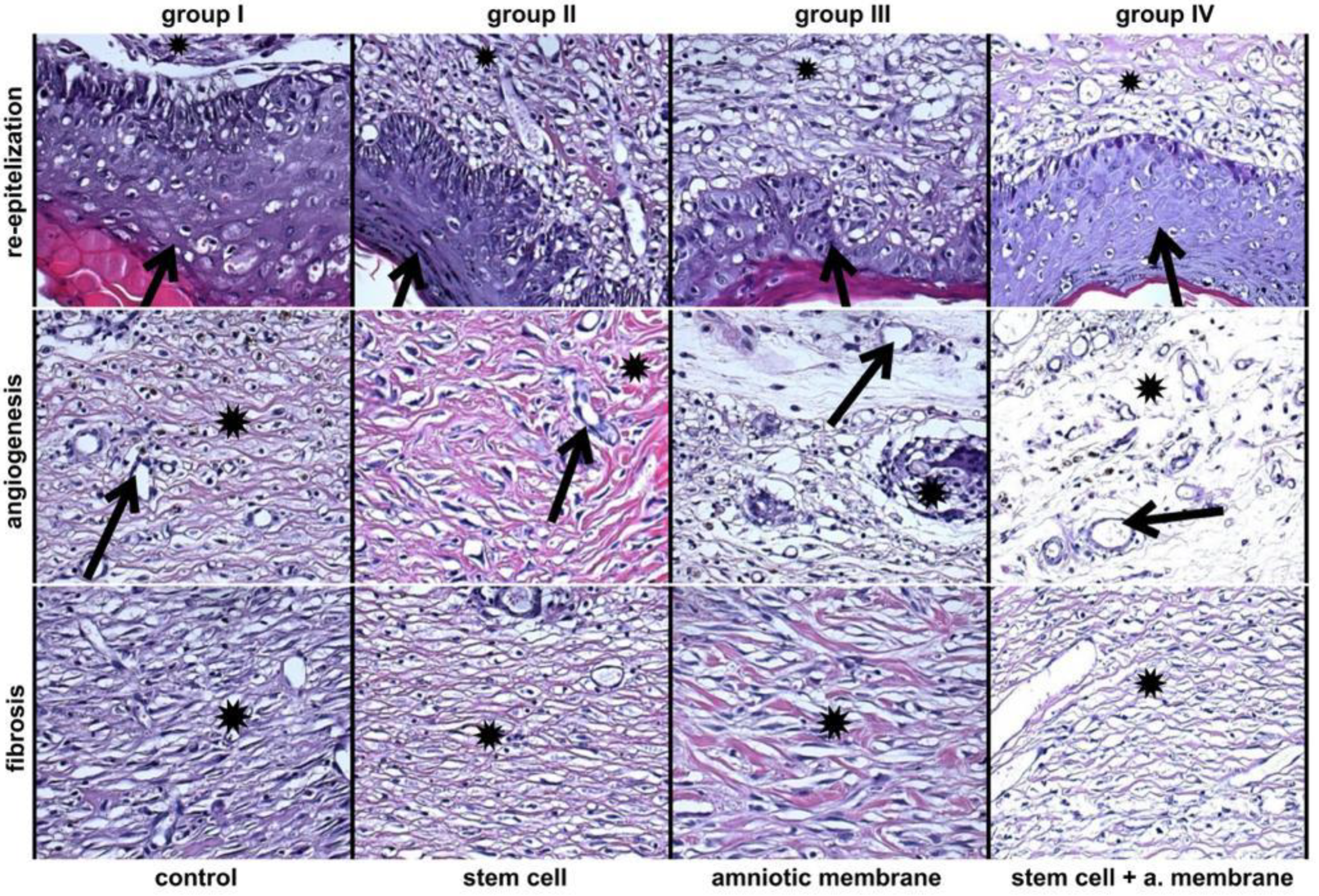
3.2. Assessment of H&E Staining Findings
3.3. Comparison of Interleukin Protein 1 (IL1) Concentration in Tissues Adjacent to the Skin Wound in Rats
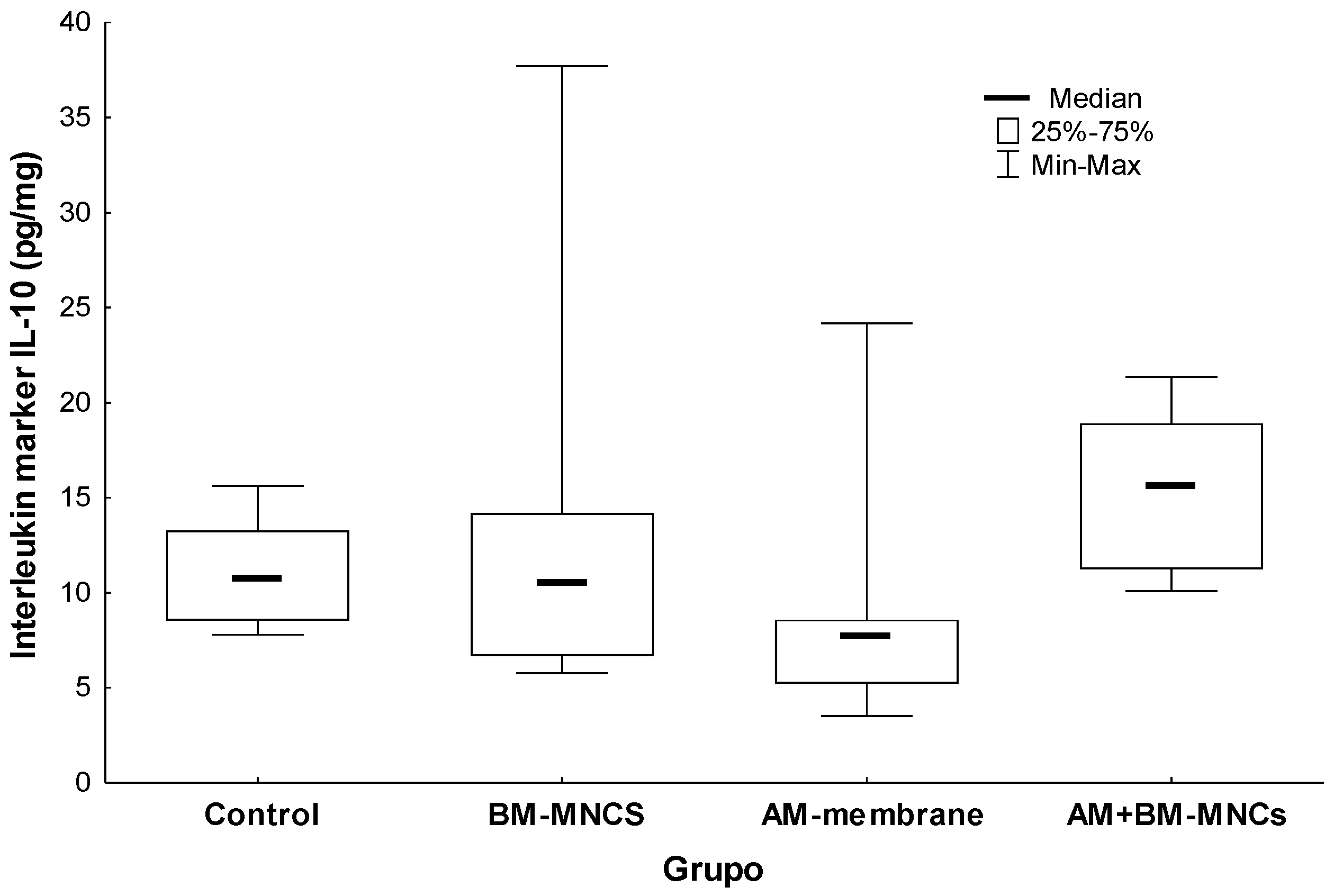
3.4. Comparison of Interleukin Protein 10 (IL10) Concentration in Tissues Adjacent to the Skin Wound in Rats
3.5. Comparison of Interleukin Protein (TGF-β) Concentration in Tissues Adjacent to the Skin Wound in Rats
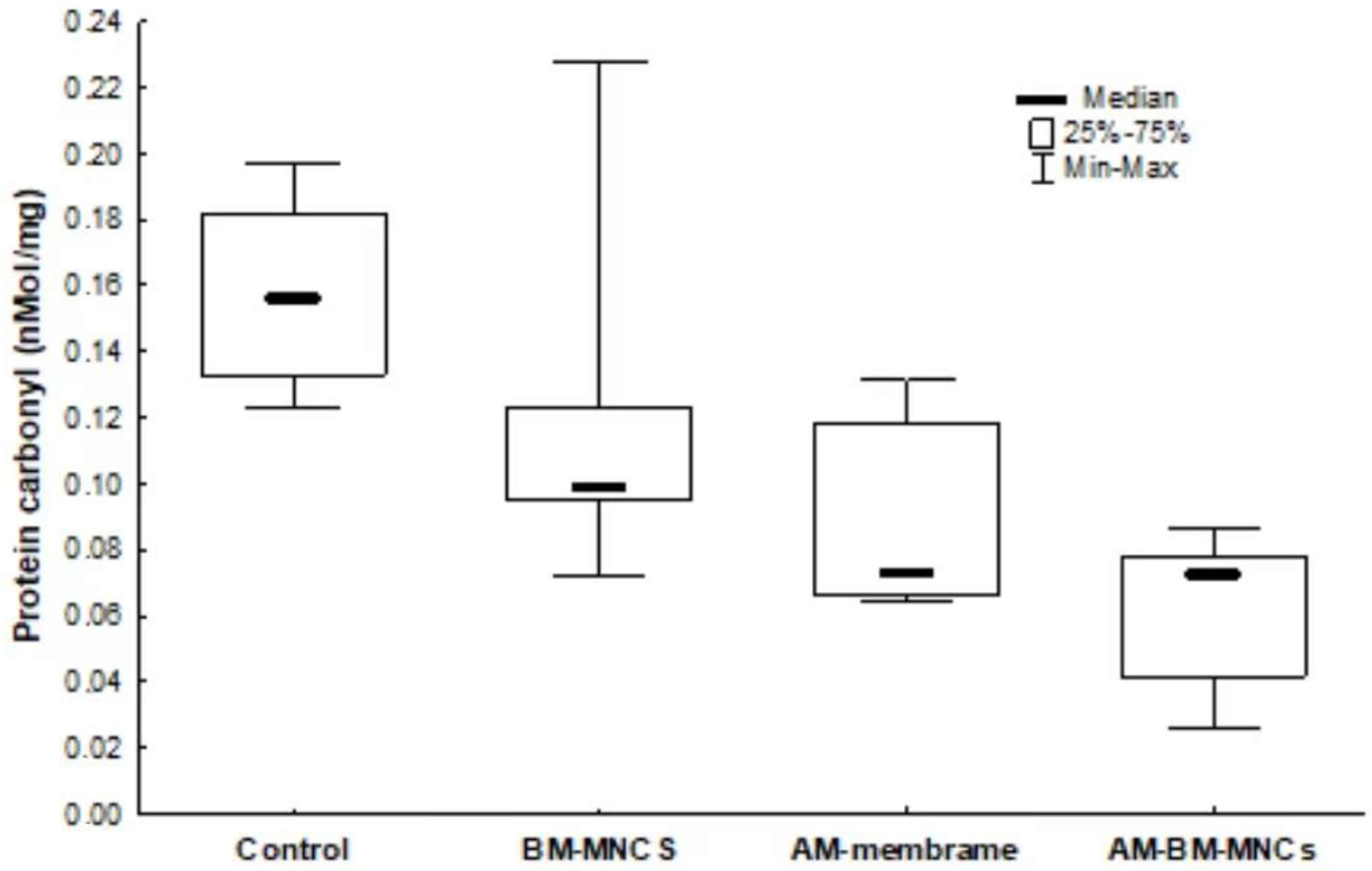
3.6. Oxidative Damage Markers
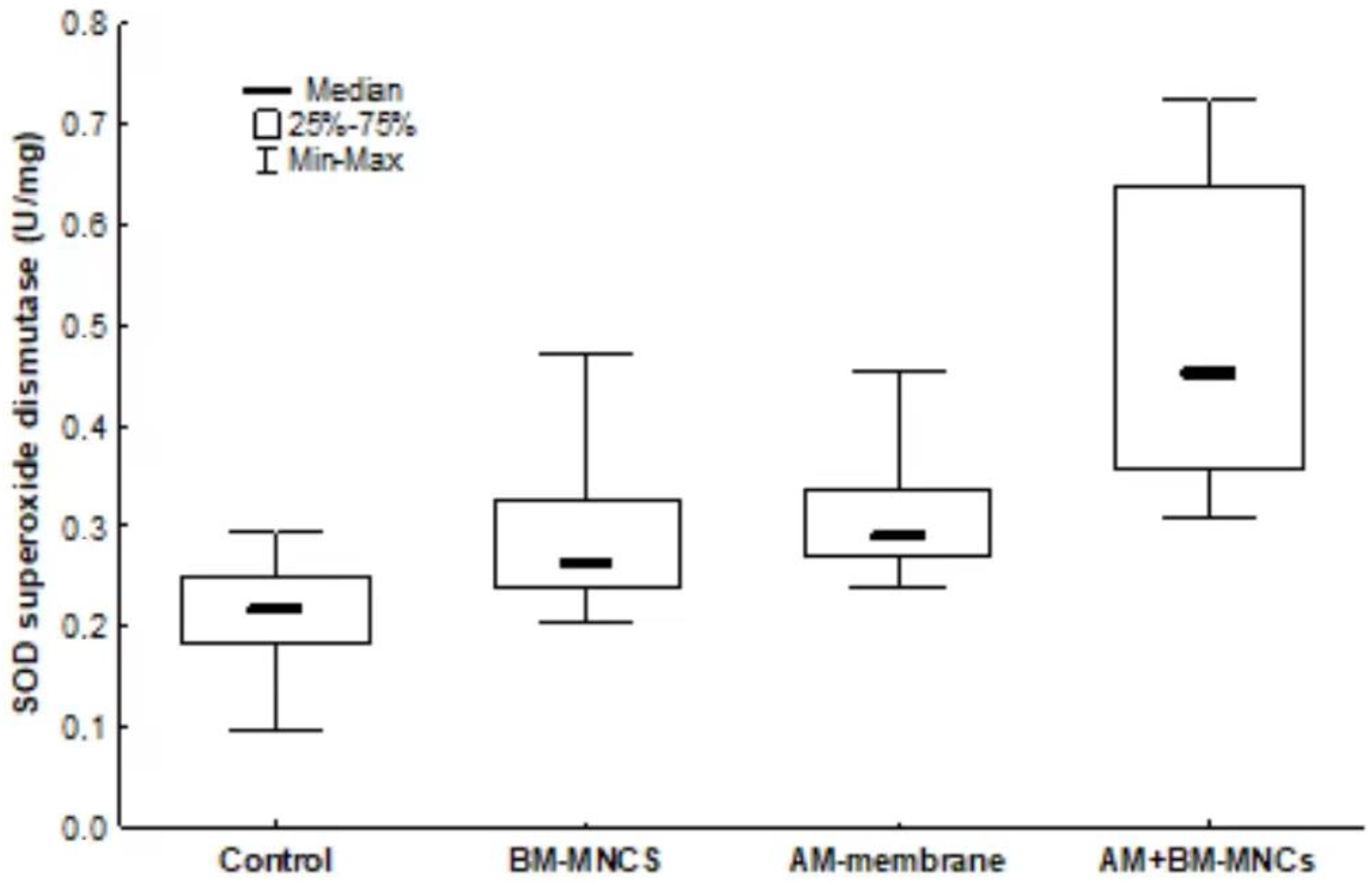
3.7. Antioxidant System
3.8. Collagen Level Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [Green Version]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.-C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual growth factor releasing multifunctional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Riau, A.K.; Beuerman, R.W.; Lim, L.S.; Mehta, J.S. Preservation, sterilization and de-epithelialization of human am-niotic membrane for use in ocular surface reconstruction. Biomaterials 2010, 31, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Cargnoni, A.; Di Marcello, M.; Campagnol, M.; Nassuato, C.; Albertini, A.; Parolini, O. Amniotic Membrane Patching Promotes Ischemic Rat Heart Repair. Cell Transpl. 2009, 18, 1147–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume, G.G.; Machado-Júnior, P.A.B.; PaludoBertinato, G.; Simeoni, R.B.; Francisco, J.C.; Guarita-Souza, L.C. Tissue-engineered amniotic membrane in the treatment of myocardial infarction: A systematic review of experimental studies. Am. J. Cardiovasc. Dis. 2021, 11, 1–11. [Google Scholar]
- Han, X.; Ma, Y.; Lu, X.; Li, W.; Xia, E.; Li, T.C.; Zhang, H.; Huang, X. Transplantation of Human Adipose Stem Cells Using Acellular Human Amniotic Membrane Improves Angiogenesis in Injured Endometrial Tissue in a Rat Intrauterine Adhesion Model. Cell Transplant. 2020, 29, 0963689720952055. [Google Scholar] [CrossRef]
- Babaki, D.; Khoshsimaybargard, M.; Yaghoubi, S.; Gholami, M. Comparison of Vestibular Depth Relapse and Wound Healing After Reconstructive Preprosthetic Surgery Using Cryopreserved Amniotic Membrane and Acellular Dermal Matrix—A Comparative Study. Ann. Maxillofac. Surg. 2021, 11, 12–16. [Google Scholar] [CrossRef]
- McQuilling, J.P.; Vines, J.B.; Mowry, K.C. In vitro assessment of a novel, hypothermically stored amniotic membrane for use in a chronic wound environment. Int. Wound J. 2017, 14, 993–1005. [Google Scholar] [CrossRef] [Green Version]
- Tseng, S.C.G.; Espana, E.M.; Kawakita, T.; Di Pascuale, M.A.; Li, W.; He, H.; Liu, T.S.; Cho, T.H.; Gao, Y.Y.; Yeh, L.K.; et al. How does amniotic membrane work? Ocul. Surf. 2004, 2, 177–187. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Zhang, M. Characterization of Ex Vivo Expanded Oral Mucosal Epithelium Cells on Acellular Porcine Corneal Stroma for Ocular Surface Reconstruction. J. Ophthalmol. 2017, 2017, 6761714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.; Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef]
- Seo, M.; Kim, J.C.; Kim, H.K.; Choi, E.W.; Jeong, S.; Nam, K.C.; Jang, M. A Novel Secretory Vesicle from Deer Antlerogenic Mesenchymal Stem Cell-Conditioned Media (DaMSC-CM) Promotes Tissue Regeneration. Stem Cells Int. 2018, 2018, 3891404. [Google Scholar] [CrossRef] [Green Version]
- Takejima, A.L.; Francisco, J.C.; Simeoni, R.B.; de Noronha, L.; Garbers, L.A.F.M.; Foltz, K.M.; Junior, P.A.B.M.; Souza, I.C.; Pinho, R.A.; Carvalho, K.A.T.; et al. Role of mononuclear stem cells and decellularized amniotic membrane in the treatment of skin wounds in rats. Tissue Barriers. 2022, 10, 1982364. [Google Scholar] [CrossRef]
- RLevine, D.; Garland, C. Oliver, Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Bannister, J.V.; Calabrese, L. Assays for superoxide dismutase. Methods Biochem. Anal. 1987, 32, 279–312. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Bancroft, J.D.; Gamble, M. Connective tissues and stains. In Theory and Practice of Histological Techniques, 6th ed.; Bancroft, J.D., Gamble, M., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2008; pp. 135–160. [Google Scholar]
- Kogan, S.; Sood, A.; Granick, M.S. Amniotic Membrane Adjuncts and Clinical Applications in Wound Healing: A Review of the Literature. Wounds Compend. Clin. Res. Pract. 2018, 30, 168–173. [Google Scholar]
- Chehelcheraghi, F.; Eimani, H.; Homayoonsadraie, S.; Torkaman, G.; Amini, A.; AlaviMajd, H.; Shemshadi, H. Effects of Acellular Amniotic Membrane Matrix and Bone Marrow-Derived Mesenchymal Stem Cells in Improving Random Skin Flap Survival in Rats. Iran Red Crescent Med. J. 2016, 18, e25588. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhou, H.; Du, W.; Huang, X.; Zheng, X.; Zhang, C.; Hu, H.; Wang, J.; Quan, R. Hair follicle stem cells combined with human allogeneic acellular amniotic membrane for repair of full thickness skin defects in nude mice. J. Tissue Eng. Regen. Med. 2020, 14, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Koper-Lenkiewicz, O.M.; Sutkowska, K.; Wawrusiewicz-Kurylonek, N.; Kowalewska, E.; Matowicka-Karna, J. Proinflammatory Cytokines (IL-1, -6, -8, -15, -17, -18, -23, TNF-α) Single Nucleotide Polymorphisms in Rheumatoid Arthritis—A Literature Review. Int. J. Mol. Sci. 2022, 23, 2106. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.; Khan, M.A.; Shamma, T.; Altuhami, A.; Ahmed, H.A.; Mohammed Assiri, A.; Broering, D.C. Targeting Interleukin-10 Restores Graft Microvascular Supply and Airway Epithelium in Rejecting Allografts. Int. J. Mol. Sci. 2022, 23, 1269. [Google Scholar] [CrossRef]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Xu, Z.X.; Hao, Y.H.; Gao, Y.B.; Yao, B.W.; Zhang, J.; Wang, B.; Hu, Z.Q.; Peng, R.Y. A novel microcurrent dressing for wound healing in a rat skin defect model. Mil. Med. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Manuelpillai, U.; Tchongue, J.; Lourensz, D.; Vaghjiani, V.; Samuel, C.S.; Liu, A.; Sievert, W.; Sievert, W. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4—Treated mice. Cell Transplant. 2010, 19, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S. Paracrine role for TGF-β-induced CTGF and VEGF in mesangial matrix expansion in progressive glomerular disease. Histol. Histopathol. 2012, 27, 1131–1141. [Google Scholar] [CrossRef]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Terapias antioxidantes para cicatrização de feridas: Um guia clínico para produtos atualmente disponíveis comercialmente. Ski. Pharm. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef]
- Dilsiz, O.Y.; Akhundzada, I.; Bilkay, U.; Uyanikgil, Y.; Teymur, H.; Ates, U.; Baka, M. Effects of metoclopramide and ranitidine on survival of flat template mcfarlane skin flaps in a rat wound healing model. Drug Res. 2013, 63, 91–97. [Google Scholar]
- Kumar, P.; Kumar, D.; Sikkaa, P.; Singh, P. Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress during cryopreservation. Anim. Reprod. Sci. 2015, 152, 26–31. [Google Scholar] [CrossRef]
- Feng, C.; Yang, M.; Lan, M.; Liu, C.; Zhang, Y.; Huang, B.; Liu, H.; Zhou, Y. ROS: Crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxidative Med. Cell. Longev. 2017, 2017, 5601593. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Huang, L.; Shen, M.; Liu, Y.; Liu, G.; Wu, Y.; Ding, F.; Ma, K.; Wang, W.; Zhang, Y.; et al. Pioglitazone protects compression-mediated apoptosis in nucleus pulposus mesenchymal stem cells by suppressing oxidative stress. Oxidative Med. Cell. Longev. 2019, 2019, 4764071. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Y.; Liu, L.; Wang, H.; Shen, P.; Yang, H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: Therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle 2020, 19, 1727–1739. [Google Scholar] [CrossRef]
- Hopper, R.A.; Woodhouse, K.; Semple, J.L. Acellularization of Human Placenta with Preservation of the Basement Membrane. Ann. Plast. Surg. 2003, 51, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Li, Y.; You, Q.; Yang, J.; Jin, Y.; Zou, G.; Tang, J.; Ge, Z.; Liu, Y. FGF-2-Induced Human Amniotic Mesenchymal Stem Cells Seeded on a Human Acellular Amniotic Membrane Scaffold Accelerated Tendon-to-Bone Healing in a Rabbit Extra-Articular Model. Stem Cells Int. 2020, 2020, 4701476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthukumar, T.; Anbarasu, K.; Prakash, D.; Sastry, T.P. Effect of growth factors and pro-inflammatory cytokines by the collagen biocomposite dressing material containing Macrotyloma uniflorum plant extract-in vivo wound healing. Colloids Surf. B Biointerfaces 2014, 121, 178–188. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.W.; Du, L.Q.; Wu, X.Y. Acellular porcine corneal matrix as a carrier scaffold for cultivating human corneal epithelial cells and fibroblasts in vitro. Int. J. Ophthalmol. 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hatzfeld, A.S.; Pasquesoone, L.; Germain, N.; Danzé, P.; Drucbert, A.; Tardivel, M.; Marchetti, P.; DuquennoyMartinot, V.; Guerreschi, P.; Marchetti, P. Benefits of cryopreserved human amniotic membranes in association with conventional treatments in the management of full-thickness burns. Int. Wound J. 2019, 16, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.C.; Uemura, L.; Simeoni, R.B.; da Cunha, R.C.; Mogharbel, B.F.; Simeoni, P.R.B.; Naves, G.; Napimoga, M.H.; No-ronha, L.; Carvalho, K.A.T.; et al. Acellular Human Amniotic Membrane Scaffold with 15d-PGJ2 Nanoparticles in Postinfarct Rat Model. Tissue Eng. Part A 2020, 26, 1128–1137. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, I.C.; Takejima, A.L.; Simeoni, R.B.; Gamba, L.K.; Ribeiro, V.S.T.; Foltz, K.M.; de Noronha, L.; de Almeida, M.B.; Neto, J.R.F.; de Carvalho, K.A.T.; et al. Acellular Biomaterials Associated with Autologous Bone Marrow-Derived Mononuclear Stem Cells Improve Wound Healing through Paracrine Effects. Biomedicines 2023, 11, 1003. https://doi.org/10.3390/biomedicines11041003
de Souza IC, Takejima AL, Simeoni RB, Gamba LK, Ribeiro VST, Foltz KM, de Noronha L, de Almeida MB, Neto JRF, de Carvalho KAT, et al. Acellular Biomaterials Associated with Autologous Bone Marrow-Derived Mononuclear Stem Cells Improve Wound Healing through Paracrine Effects. Biomedicines. 2023; 11(4):1003. https://doi.org/10.3390/biomedicines11041003
Chicago/Turabian Stylede Souza, Isio Carvalho, Aline Luri Takejima, Rossana Baggio Simeoni, Luize Kremer Gamba, Victoria Stadler Tasca Ribeiro, Katia Martins Foltz, Lucia de Noronha, Meila Bastos de Almeida, Jose Rocha Faria Neto, Katherine Athayde Teixeira de Carvalho, and et al. 2023. "Acellular Biomaterials Associated with Autologous Bone Marrow-Derived Mononuclear Stem Cells Improve Wound Healing through Paracrine Effects" Biomedicines 11, no. 4: 1003. https://doi.org/10.3390/biomedicines11041003





