Invasive Cutaneous Candidiasis, Autoimmune Hemolytic Anemia and Pancytopenia: A Challenging Scenario for Waldenström Macroglobulinemia in an Elderly Patient
Abstract
:1. Introduction
2. Patient Background
3. At Our Hospital
4. The Hematologic Malignancy Diagnosis
5. Treatment Choice
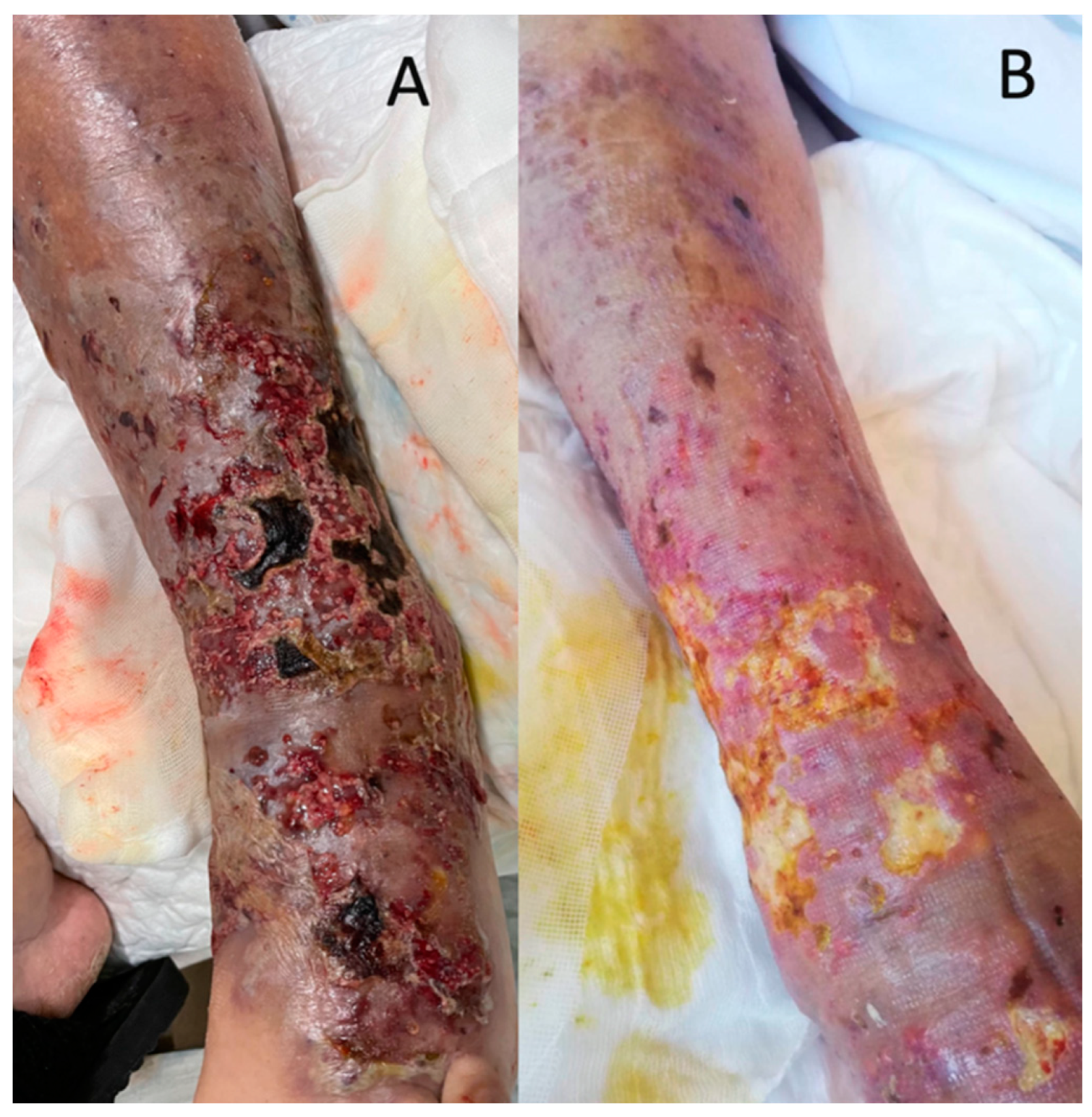
6. Infectious Complications
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, R.G.; Treon, S.P.; Al-Katib, A.; Fonseca, R.; Greipp, P.R.; McMaster, M.L.; Dimopoulos, M.A. Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003, 30, 110–115. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [PubMed] [Green Version]
- Kaiser, L.M.; Hunter, Z.R.; Treon, S.P.; Buske, C. CXCR4 in Waldenström’s Macroglobulinema: Chances and challenges. Leukemia 2021, 35, 333–345. [Google Scholar] [PubMed]
- Castillo, J.J.; Olszewski, A.J.; Kanan, S.; Meid, K.; Hunter, Z.R.; Treon, S.P. Overall survival and competing risks of death in patients with Waldenström macroglobulinaemia: An analysis of the Surveillance, Epidemiology and End Results database. Br. J. Haematol. 2015, 169, 81–89. [Google Scholar]
- Kastritis, E.; Morel, P.; Duhamel, A.; Gavriatopoulou, M.; Kyrtsonis, M.C.; Durot, E.; Symeonidis, A.; Laribi, K.; Hatjiharissi, E.; Ysebaert, L.; et al. A revised international prognostic score system for Waldenström’s macroglobulinemia. Leukemia 2019, 33, 2654–2661. [Google Scholar]
- Ravi, G.; Kapoor, P. Current approach to Waldenström Macroglobulinemia. Cancer Treat. Res. Commun. 2022, 31, 100527. [Google Scholar]
- Askari, E.; Rodriguez, S.; Garcia-Sanz, R. Waldenström’s Macroglobulinemia: An Exploration into the Pathology and Diagnosis of a Complex B-Cell Malignancy. J. Blood Med. 2021, ume 12, 795–807. [Google Scholar]
- Cornely, O.A.; Gachot, B.; Akan, H.; Bassetti, M.; Uzun, O.; Kibbler, C.; Marchetti, O.; De Burghgraeve, P.; Ramadan, S.; Pylkkanen, L.; et al. Epidemiology and Outcome of Fungemia in a Cancer Cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin. Infect. Dis. 2015, 61, 324–331. [Google Scholar]
- Dimopoulos, M.A.; Kastritis, E. How I treat Waldenström macroglobulinemia. Blood 2019, 134, 2022–2035. [Google Scholar]
- Treon, S.P.; Meid, K.; Gustine, J.; Yang, G.; Xu, L.; Liu, X.; Patterson, C.J.; Hunter, Z.R.; Branagan, A.R.; Laubach, J.P.; et al. Long-Term Follow-Up of Ibrutinib Monotherapy in Symptomatic, Previously Treated Patients With Waldenström Macroglobulinemia. J. Clin. Oncol. 2021, 39, 565–575. [Google Scholar] [PubMed]
- Mancuso, S.; Carlisi, M.; Santoro, M.; Napolitano, M.; Raso, S.; Siragusa, S. Immunosenescence and lymphomagenesis. Immun. Ageing 2018, 15, 22. [Google Scholar] [PubMed]
- Groarke, E.M.; Young, N.S. Aging and Hematopoiesis. Clin. Geriatr. Med. 2019, 35, 285–293. [Google Scholar] [PubMed]
- Morel, P.; Duhamel, A.; Gobbi, P.; Dimopoulos, M.; Dhodapkar, M.V.; McCoy, J.; Crowley, J.; Ocio, E.M.; Garcia-Sanz, R.; Treon, S.P.; et al. International prognostic scoring system for Waldenström macroglobulinemia. Blood 2009, 113, 4163–4170. [Google Scholar] [PubMed] [Green Version]
- Berentsen, S.; Barcellini, W. Autoimmune Hemolytic Anemias. N. Engl. J. Med. 2021, 385, 1407–1419. [Google Scholar] [PubMed]
- Jäger, U.; Barcellini, W.; Broome, C.M.; Gertz, M.A.; Hill, A.; Hill, Q.A.; Jilma, B.; Kuter, D.J.; Michel, M.; Montillo, M.; et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020, 41, 100648. [Google Scholar]
- Crisp, D.; Pruzanski, W. B-Cell neoplasms with homogeneous cold-reacting antibodies (cold agglutinins). Am. J. Med. 1982, 72, 915–922. [Google Scholar]
- Cohen, Y.; Polliack, A.; Zelig, O.; Goldfarb, A. Monotherapy with rituximab induces rapid remission of recurrent cold agglutinin-mediated hemolytic anemia in a patient with indolent lympho-plasmacytic lymphoma. Leuk Lymphoma. 2001, 42, 1405–1408. [Google Scholar] [CrossRef]
- Gertz, M.A. Waldenström macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2021, 96, 258–269. [Google Scholar]
- Ramchandren, S.; Lewis, R.A. Monoclonal gammopathy and neuropathy. Curr. Opin. Neurol. 2009, 22, 480–485. [Google Scholar]
- Mazzucchelli, M.; Frustaci, A.M.; Deodato, M.; Cairoli, R.; Tedeschi, A. Waldenstrom’s Macroglobulinemia: An Update. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018004. [Google Scholar]
- Moore, D.C. Bruton tyrosine kinase inhibitors for Waldenström macroglobulinemia: A review. J. Oncol. Pharm. Pract. 2021, 27, 1993–1999. [Google Scholar] [CrossRef]
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Warren, D.; Varma, G.; Green, R.; Argyropoulos, K.V.; Yang, G.; Cao, Y.; Xu, L.; et al. Ibrutinib in Previously Treated Waldenström’s Macroglobulinemia. N. Engl. J. Med. 2015, 372, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Sławińska, M.; Barańska-Rybak, W.; Sobjanek, M.; Wilkowska, A.; Mital, A.; Nowicki, R. Ibrutinib-induced pyoderma gangrenosum. Pol. Arch. Med. Wewn. 2016, 126, 710–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.C.; Liu, N.T.; Huang, T.Y. Ulcerative colitis with refractory pyoderma gangrenosum. QJM 2020, 113, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.; Kritharis, A.; Evens, A.M. Paraneoplastic pyoderma gangrenosum with posttransplant lymphoproliferative disorder. Ann. Hematol. 2015, 94, 893–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, N.; Patel, B.; Palfrey, W.; Isache, C. Rapidly progressive necrotizing cellulitis secondary to Candida tropicalis infection in an immunocompromised host. IDCases 2020, 19, e00691. [Google Scholar] [CrossRef] [PubMed]
- Maffei, R.; Maccaferri, M.; Arletti, L.; Fiorcari, S.; Benatti, S.; Potenza, L.; Luppi, M.; Marasca, R. Immunomodulatory effect of ibrutinib: Reducing the barrier against fungal infections. Blood Rev. 2020, 40, 100635. [Google Scholar] [CrossRef]
- Cheng, M.P.; Kusztos, A.E.; Gustine, J.N.; Dryden-Peterson, S.L.; Dubeau, T.E.; Woolley, A.E.; Hammond, S.P.; Baden, L.R.; Treon, S.P.; Castillo, J.J.; et al. Low risk of Pneumocystis jirovecii pneumonia and invasive aspergillosis in patients with Waldenström macroglobulinaemia on ibrutinib. Br. J. Haematol. 2019, 185, 788–790. [Google Scholar] [CrossRef] [Green Version]
- Guarana, M.; Nucci, M. Acute disseminated candidiasis with skin lesions: A systematic review. Clin. Microbiol. Infect. 2018, 24, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [Green Version]
- Tortorano, A.M.; Prigitano, A.; Morroni, G.; Brescini, L.; Barchiesi, F. Candidemia: Evolution of Drug Resistance and Novel Therapeutic Approaches. Infect. Drug Resist. 2021, 14, 5543–5553. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, E. Pulmonary Langerhans’ cell histiocytosis in adults. Adv. Respir. Med. 2017, 85, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Hirabayashi, K.; Zamboni, G. IgG4-related disease. Pathologica 2012, 104, 43–55. [Google Scholar] [PubMed]
- Meshram, R.M.; Gajimwar, V.S.; Gholap, S.; Jhanwar, M. Bone marrow involvement: Atypical presentation of early-onset childhood sarcoidosis. Eur. J. Rheumatol. 2020, 7, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Crispin, P.; Holmes, A. Clinical and pathological feature of bone marrow granulomas: A modern Australian series. Int. J. Lab. Hematol. 2018, 40, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Pease, G.L. Granulomatous Lesions in Bone Marrow. Blood 1956, 11, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Zahr, A.A.; Salama, M.E.; Carreau, N.; Tremblay, D.; Verstovsek, S.; Mesa, R.; Hoffman, R.; Mascarenhas, J. Bone marrow fibrosis in myelofibrosis: Pathogenesis, prognosis and targeted strategies. Haematologica 2016, 101, 660–671. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.A.; García-Sanz, R.; Gavriatopoulou, M.; Morel, P.; Kyrtsonis, M.C.; Michalis, E.; Kartasis, Z.; Leleu, X.; Palladini, G.; Tedeschi, A.; et al. Primary therapy of Waldenström macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): Long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood 2013, 122, 3276–3282. [Google Scholar] [CrossRef] [Green Version]



| Relevant Findings | |
|---|---|
| Bone marrow morphological evaluation | Infiltration by monoclonal lymphoplasmacytic cells (>75%) and small plasma cells with lambda restriction. |
| Bone marrow flow cytometry |
|
| Histology | Lymphoid neoplastic population with a diffuse pattern with more than 75% of tumor cells with scattered plasma cells and increased reticulin deposition. |
| Molecular testing | MYD88L265P mutation CXCR4S342mutation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, J.C.; Askari, E.; Carrasco, N.; Piris, M.A.; Perez de Camino, B.; Pardo, L.; Cornago, J.; Lopez-Lorenzo, J.L.; Llamas, P.; Solan, L. Invasive Cutaneous Candidiasis, Autoimmune Hemolytic Anemia and Pancytopenia: A Challenging Scenario for Waldenström Macroglobulinemia in an Elderly Patient. Biomedicines 2023, 11, 1007. https://doi.org/10.3390/biomedicines11041007
Caballero JC, Askari E, Carrasco N, Piris MA, Perez de Camino B, Pardo L, Cornago J, Lopez-Lorenzo JL, Llamas P, Solan L. Invasive Cutaneous Candidiasis, Autoimmune Hemolytic Anemia and Pancytopenia: A Challenging Scenario for Waldenström Macroglobulinemia in an Elderly Patient. Biomedicines. 2023; 11(4):1007. https://doi.org/10.3390/biomedicines11041007
Chicago/Turabian StyleCaballero, Juan Carlos, Elham Askari, Nerea Carrasco, Miguel Angel Piris, Begoña Perez de Camino, Laura Pardo, Javier Cornago, Jose Luis Lopez-Lorenzo, Pilar Llamas, and Laura Solan. 2023. "Invasive Cutaneous Candidiasis, Autoimmune Hemolytic Anemia and Pancytopenia: A Challenging Scenario for Waldenström Macroglobulinemia in an Elderly Patient" Biomedicines 11, no. 4: 1007. https://doi.org/10.3390/biomedicines11041007
APA StyleCaballero, J. C., Askari, E., Carrasco, N., Piris, M. A., Perez de Camino, B., Pardo, L., Cornago, J., Lopez-Lorenzo, J. L., Llamas, P., & Solan, L. (2023). Invasive Cutaneous Candidiasis, Autoimmune Hemolytic Anemia and Pancytopenia: A Challenging Scenario for Waldenström Macroglobulinemia in an Elderly Patient. Biomedicines, 11(4), 1007. https://doi.org/10.3390/biomedicines11041007







