Real-Life Comparison of Fosfomycin to Nitrofurantoin for the Treatment of Uncomplicated Lower Urinary Tract Infection in Women
Abstract
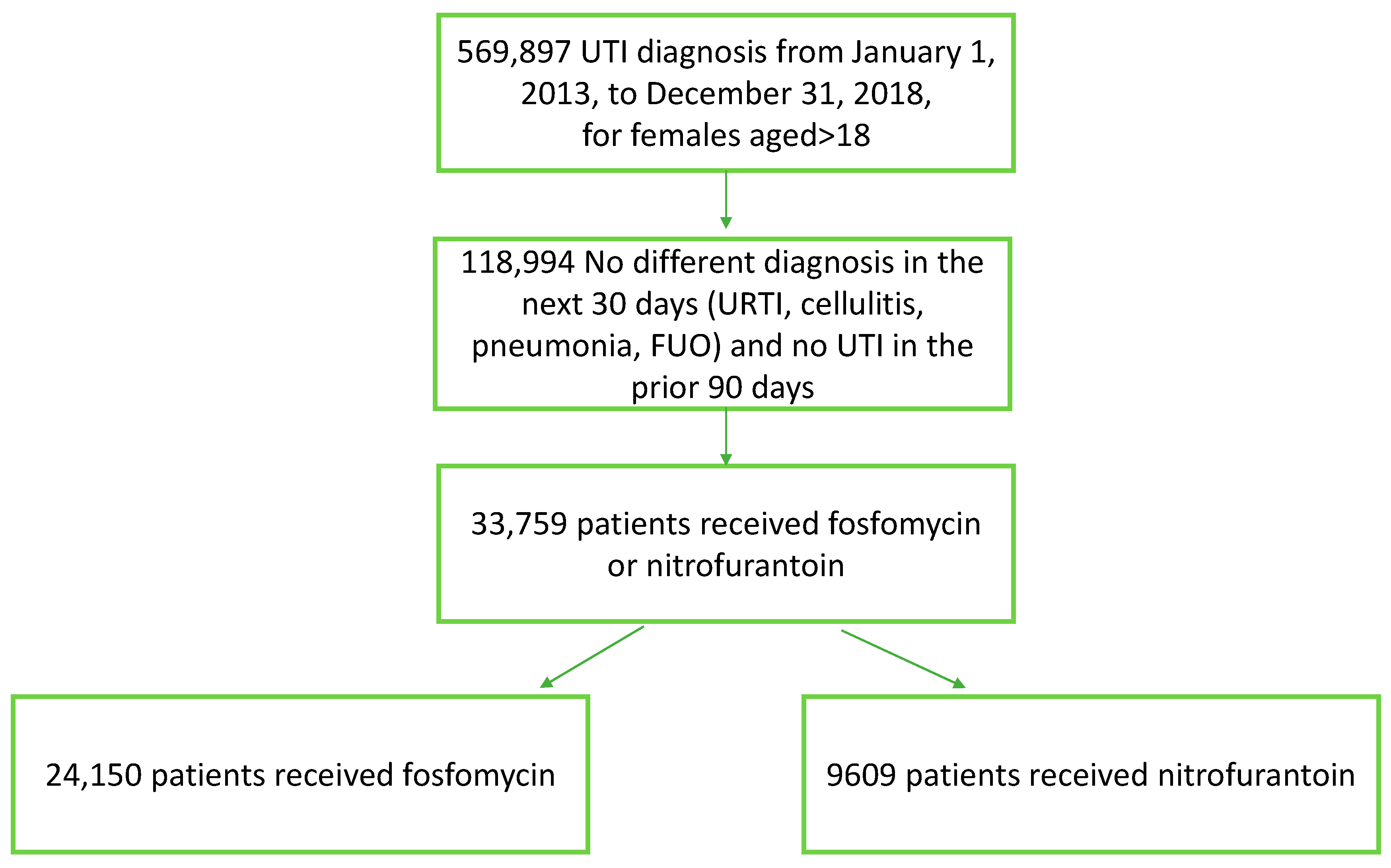
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyrstrup, M.; van der Velden, A.; Engstrom, S.; Goderis, G.; Molstad, S.; Verheij, T.; Coenen, S.; Adriaenssens, N. Antibiotic prescribing in relation to diagnoses and consultation rates in Belgium, the Netherlands and Sweden: Use of European quality indicators. Scand. J. Prim. Health Care 2017, 35, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Mandraka, F.; Kunze, M.; Helbig, S.; Vahlensieck, W.; Naber, K.; Schmiemann, G.; et al. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients. Part II: Therapy and Prevention. Urol. Int. 2018, 100, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Bruyère, F. Re: Angela Huttner, Arina Kowalczyk, Adi Turjeman, et al. Effect of 5-Day Nitrofurantoin vs. Single-dose Fosfomycin on Clinical Resolution of Uncomplicated Lower Urinary Tract Infection in Women: A Randomized Clinical Trial. JAMA 2018;319:1781-9. Eur. Urol. 2018, 74, e124. [Google Scholar] [CrossRef] [PubMed]
- Sadler, S.; Holmes, M.; Ren, S.; Holden, S.; Jha, S.; Thokala, P. Cost-effectiveness of antibiotic treatment of uncomplicated urinary tract infection in women: A comparison of four antibiotics. BJGP Open 2017, 1, bjgpopen17X101097. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Lee, S.H.; Chang, S.S.; Lee, S.H.; Lee, M.; Fang, C.C.; Chen, S.C.; Lee, C.C. Comparative effectiveness of different oral antibiotics regimens for treatment of urinary tract infection in outpatients: An analysis of national representative claims database. Medicine 2014, 93, e304, Erratum in Medicine 2015, 94, 1. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Chateau, D.; Dahl, M.; Zhang, J.; Fisher, A.; Sketris, I.S.; Quail, J.; Marra, F.; Ernst, P.; Bugden, S.; et al. Fluoroquinolone use for uncomplicated urinary tract infections in women: A retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Huttner, A.; Kowalczyk, A.; Turjeman, A.; Babich, T.; Brossier, C.; Eliakim-Raz, N.; Kosiek, K.; Martinez de Tejada, B.; Roux, X.; Shiber, S.; et al. Effect of 5-Day Nitrofurantoin vs. Single-Dose Fosfomycin on Clinical Resolution of Uncomplicated Lower Urinary Tract Infection in Women: A Randomized Clinical Trial. JAMA 2018, 319, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Verma, P.K.; Rawat, V.; Varshney, U.; Singh, R.K. Fosfomycin versus Nitrofurantoin for the Treatment of Lower UTI in Outpatients. J. Lab. Physicians 2021, 13, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Kornfält Isberg, H.; Hedin, K.; Melander, E.; Mölstad, S.; Beckman, A. Uncomplicated urinary tract infection in primary health care: Presentation and clinical outcome. Infect Dis. 2021, 53, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Martischang, R.; Godycki-Ćwirko, M.; Kowalczyk, A.; Kosiek, K.; Turjeman, A.; Babich, T.; Shiber, S.; Leibovici, L.; von Dach, E.; Harbarth, S.; et al. Risk factors for treatment failure in women with uncomplicated lower urinary tract infection. PLoS ONE 2021, 16, e0256464. [Google Scholar] [CrossRef] [PubMed]
- Ten Doesschate, T.; van Haren, E.; Wijma, R.A.; Koch, B.C.P.; Bonten, M.J.M.; van Werkhoven, C.H. The effectiveness of nitrofurantoin, fosfomycin and trimethoprim for the treatment of cystitis in relation to renal function. Clin. Microbiol. Infect. 2020, 26, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Brosh-Nissimov, T.; Navon-Venezia, S.; Keller, N.; Amit, S. Risk analysis of antimicrobial resistance in outpatient urinary tract infections of young healthy adults. J. Antimicrob. Chemother. 2019, 74, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Naamneh, B.; Tkhawkho, L.; Nitzan, O. High Rates of Fosfomycin Resistance in Gram-Negative Urinary Isolates from Israel. Microb. Drug Resist. 2019, 25, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Konwar, M.; Gogtay, N.J.; Ravi, R.; Thatte, U.M.; Bose, D. Evaluation of efficacy and safety of fosfomycin versus nitrofurantoin for the treatment of uncomplicated lower urinary tract infection (UTI) in women—A systematic review and meta-analysis. J. Chemother. 2022, 34, 139–148. [Google Scholar] [CrossRef] [PubMed]
| Fosfomycin n = 24,150 | Nitrofurantoin n = 9609 | p-Value | Effect Size | Number Needed to Treat | |
|---|---|---|---|---|---|
| Age | 45.22 (±18.14) | 50.62 (±20.05) | <0.0001 | 0.282 | |
| BMI | 26.46 (±7.40) | 27.33 (±7.31) | <0.0001 | 0.118 | |
| Last UTI (in days) | 506.32 (±406.50) | 407.45 (±380.24) | <0.0001 | 0.251 | |
| Creatinine | 0.76 (±0.26) | 0.77 (±0.26) | <0.0001 | 0.067 | |
| TREATMENT FAILURE (Within 7 days from UTI diagnosis) | 1970 (8.16%) | 660 (6.87%) | <0.0001 | 0.049 | 78 |
| Hospital admission | 121 (0.50%) | 94 (0.98%) | <0.0001 | 0.057 | 210 |
| ER | 214 (0.89%) | 110 (1.14%) | 0.030 | 0.048 | 387 |
| Emergency clinic | 334 (1.38%) | 88 (0.92%) | 0.0004 | 0.043 | 214 |
| IV antibiotics | 19 (0.08%) | 13 (0.14%) | 0.17 | 0.018 | 1766 |
| Change in antibiotic regimen | 1351 (5.59%) | 364 (3.79%) | <0.0001 | 0.086 | 55 |
| Pyelonephritis | 66 (0.27%) | 73 (0.76%) | <0.0001 | 0.071 | 206 |
| TREATMENT FAILURE excluding antibiotic change | 690 (2.9%) | 334 (3.5%) | 0.003 | 0.034 | 162 |
| REINFECTION (8th–30th day from UTI diagnosis) | 1873 (7.76%) | 885 (9.21%) | <0.0001 | 0.052 | 69 |
| UTI diagnosis in days 8–30 | 1173 (4.86%) | 592 (6.16%) | <0.0001 | 0.057 | 77 |
| Hospital admissions in 30 days | 210 (0.87%) | 144 (1.50%) | <0.0001 | 0.059 | 159 |
| ER in 30 days | 361 (1.49%) | 155 (1.61%) | 0.43 | 0.010 | 846 |
| Emergency clinic in 30 days | 447 (1.85%) | 162 (1.69%) | 0.32 | 0.012 | 606 |
| COMBINED FAILURE | 3843 (15.9%) | 1545 (16.1%) | 0.72 | 0.005 | 604 |
| Treatment Failure | Reinfection | Combined Failure | |
|---|---|---|---|
| Total | OR 0.74 95% CI 0.59–0.92 p-value = 0.008 | OR = 0.967 (0.8–1.16) p-value = 0.71 | OR 0.85 (0.733–0.994) p-value = 0.4 |
| Less than 40 years | OR = 1.16 (0.7–1.87) p-value = 0.56 | OR 1.25 (0.794–1.92) p-value = 0.327 | OR 1.13 (0.786–1.62) p-value = 0.49 |
| 40 and older | OR = 0.66 (0.51–0.85) p-value = 0.001 | OR = 0.92 (0.75–1.12) p-value = 0.4 | OR = 0.8 (0.678–0.948) p-value = 0.01 |
| AGE ≤ 40 Years | AGE > 40 Years | |||||
|---|---|---|---|---|---|---|
| Fosfomycin | Nitrofurantoin | p-Value | Fosfomycin | Nitrofurantoin | p-Value | |
| n = 10,823 | n = 3469 | n = 13,327 | n = 6140 | |||
| TREATMENT FAILURE | 862 (7.96%) | 276 (7.96%) | 1 | 1108 (8.31%) | 384 (6.25%) | <0.0001 |
| (Within 7 days from UTI diagnosis) | ||||||
| Hospital admission | 49 (0.45%) | 35 (1.01%) | 0.0005 | 94 (0.71%) | 48 (0.78%) | 0.59 |
| ER | 120 (1.11%) | 62 (1.79%) | 0.003 | 72 (0.54%) | 59 (0.96%) | 0.001 |
| Emergency clinic | 200 (1.85%) | 51 (1.47%) | 0.16 | 134 (1.01%) | 37 (0.60%) | 0.005 |
| Pyelonephritis | 41 (0.38%) | 42 (1.21%) | <0.0001 | 25 (0.19%) | 31 (0.50%) | 0.0002 |
| IV antibiotics | 8 (0.07%) | 6 (0.17%) | 0.12 | 11 (0.08%) | 7 (0.11%) | 0.61 |
| Change in antibiotic regimen | 531 (4.91%) | 129 (3.72%) | 0.003 | 820 (6.15%) | 235 (3.83%) | <0.0001 |
| TREATMENT FAILURE excluding antibiotic change | 374 (3.5%) | 166 (4.8%) | <0.001 | 316(2.4%) | 168 (2.7) | 0.14 |
| REINFECTION | 809 (7.47%) | 301 (8.68%) | 0.024 | 1064 (7.98%) | 584 (9.51%) | 0.0004 |
| (8th–30th day from UTI diagnosis) | ||||||
| UTI diagnosis in days 8–30 | 437 (4.04%) | 159 (4.58%) | 0.17 | 736 (5.52%) | 433 (7.05%) | <0.0001 |
| Hospital admissions in 30 days | 59 (0.55%) | 47 (1.35%) | <0.0001 | 151 (1.13%) | 97 (1.58%) | 0.011 |
| ER in 30 days | 181 (1.67%) | 92 (2.65%) | 0.0005 | 180 (1.35%) | 63 (1.03%) | 0.061 |
| Emergency clinic in 30 days | 275 (2.54%) | 92 (2.65%) | 0.71 | 172 (1.29%) | 70 (1.14%) | 0.4 |
| COMBINED FAILURE | 1671 (15.4%) | 577(16.6%) | 0.098 | 2172 (16.3%) | 968 (15.8%) | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafrir, A.; Oster, Y.; Shauly-Aharonov, M.; Strahilevitz, J. Real-Life Comparison of Fosfomycin to Nitrofurantoin for the Treatment of Uncomplicated Lower Urinary Tract Infection in Women. Biomedicines 2023, 11, 1019. https://doi.org/10.3390/biomedicines11041019
Shafrir A, Oster Y, Shauly-Aharonov M, Strahilevitz J. Real-Life Comparison of Fosfomycin to Nitrofurantoin for the Treatment of Uncomplicated Lower Urinary Tract Infection in Women. Biomedicines. 2023; 11(4):1019. https://doi.org/10.3390/biomedicines11041019
Chicago/Turabian StyleShafrir, Asher, Yonatan Oster, Michal Shauly-Aharonov, and Jacob Strahilevitz. 2023. "Real-Life Comparison of Fosfomycin to Nitrofurantoin for the Treatment of Uncomplicated Lower Urinary Tract Infection in Women" Biomedicines 11, no. 4: 1019. https://doi.org/10.3390/biomedicines11041019
APA StyleShafrir, A., Oster, Y., Shauly-Aharonov, M., & Strahilevitz, J. (2023). Real-Life Comparison of Fosfomycin to Nitrofurantoin for the Treatment of Uncomplicated Lower Urinary Tract Infection in Women. Biomedicines, 11(4), 1019. https://doi.org/10.3390/biomedicines11041019





