Outcomes of a QST Protocol in Healthy Subjects and Chronic Pain Patients: A Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Healthy Volunteers
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Selection of Patients with Chronic Pain
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Methods of Data Collection
2.3.1. QST Assessments
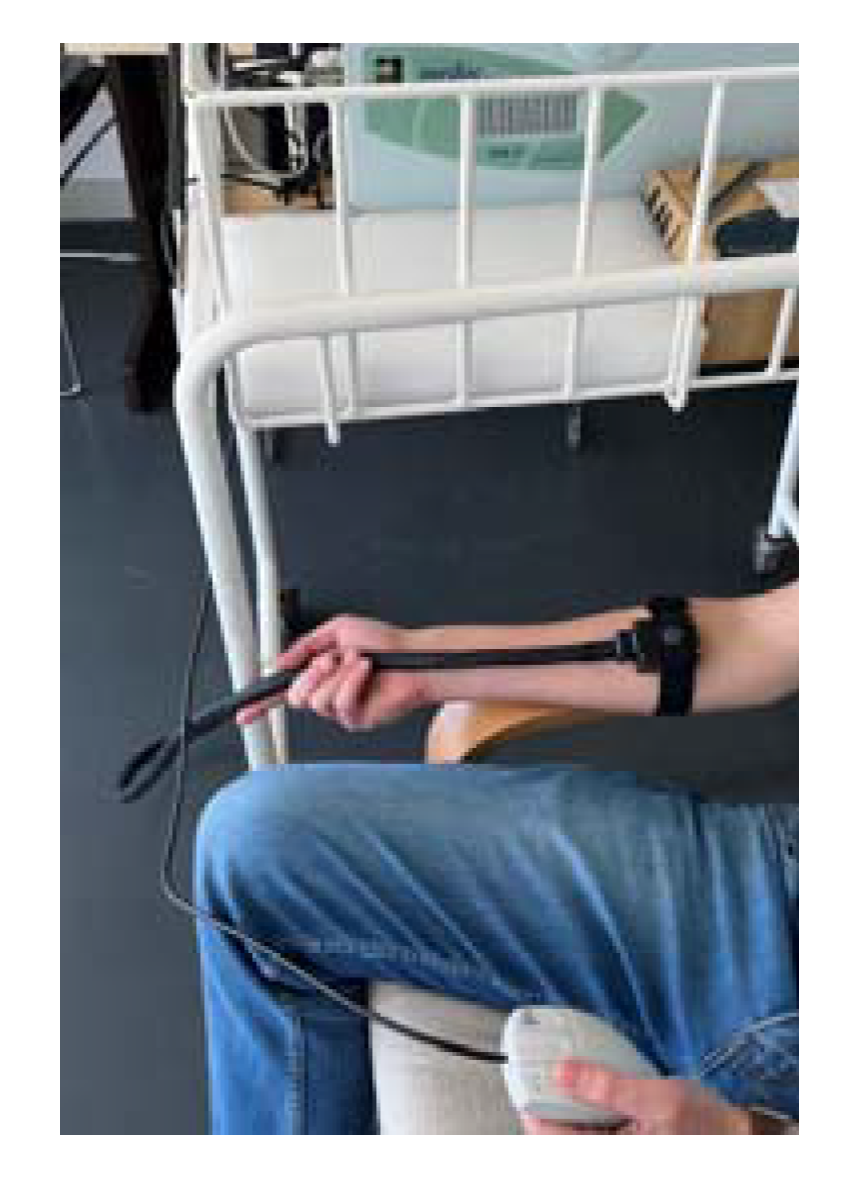
2.3.2. QST Protocol
- Heat Pain Threshold: Application of a baseline temperature (32 °C), which gradually increased at 1 °C/s to a maximum of 50 °C and decreased at 8 °C/s. Volunteers were asked, 6 times, to indicate the transition point at which the nonpainful warm sensation changed into a painful heat sensation, by pressing a button that stopped the temperature-increasing process and electronically recording it. The mean of the responses to the last 3 stimuli was taken as the “heat pain threshold” [45,51,53,62,64].
- Suprathreshold Pain Magnitude: Subjects were asked to report the level of pain intensity 9 times during three different random 3-s contact heat stimuli. The 3 different temperatures were the mean pain of the first test, a neutral temperature of 36 °C and a suprathreshold temperature of 49 °C. Volunteers were asked to rate discomfort and/or pain in NRS (0 meaning no pain to 10 meaning the worst pain they can imagine) [12,59,60,63,67].
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Kuner, R.; Kuner, T. Cellular Circuits in the Brain and Their Modulation in Acute and Chronic Pain. Physiol. Rev. 2021, 101, 213–258. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2021, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.S.; Tang, S.Y. Can we run away from low back pain? Osteoarthr. Cartil. 2022, 30, 6–8. [Google Scholar] [CrossRef]
- Mendes-Morais, D.; Jantarada, C.; Guimarães-Pereira, L. Translation, Cultural Adaptation and Validation of the Current Opioid Misuse Measure for European Portuguese. Acta Med. Port. 2020, 33, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonça, L.; Dias, C.C.; Castro-Lopes, J. Epidemiology of Chronic Pain: A Population-Based Nationwide Study on Its Prevalence, Characteristics and Associated Disability in Portugal. J. Pain 2012, 13, 773–783. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef]
- Li, Y.; Mai, Y.; Cao, P.; Wen, X.; Fan, T.; Wang, X.; Ruan, G.; Tang, S.; Ding, C.; Zhu, Z. Relative Efficacy and Safety of Anti-Inflammatory Biologic Agents for Osteoarthritis: A Conventional and Network Meta-Analysis. J. Clin. Med. 2022, 11, 3958. [Google Scholar] [CrossRef]
- Wang, J. Efficacy and safety of adalimumab by intra-articular injection for moderate to severe knee osteoarthritis: An open-label randomized controlled trial. J. Int. Med. Res. 2018, 46, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Chan, J.; Chung, F.; Wong, J.; Warner, D.S. Preoperative pain sensitivity and its correlation with postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2011, 114, 445–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arant, K.; Katz, J.; Neogi, T. Quantitative sensory testing: Identifying pain characteristics in patients with osteoarthritis. Osteoarthr. Cartil. 2021, 30, 17–31. [Google Scholar] [CrossRef]
- Mücke, M.; Cuhls, H.; Radbruch, L.; Baron, R.; Maier, C.; Tölle, T.; Treede, R.-D.; Rolke, R. Quantitative sensory testing (QST). English version. Der Schmerz 2021, 35 (Suppl. S3), 153–160. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, Y.; Zhang, J.; Wang, K.; Svensson, P. Quantitative sensory testing for assessment of somatosensory function in human oral mucosa: A review. Acta Odontol. Scand. 2018, 76, 13–20. [Google Scholar] [CrossRef]
- De Vita, M.J.; Buckheit, K.; Gilmour, C.E.; Moskal, D.; Maisto, S.A. Development of a Novel Brief Quantitative Sensory Testing Protocol That Integrates Static and Dynamic Pain Assessments: Test-Retest Performance in Healthy Adults. Pain Med. 2022, 23, 347–351. [Google Scholar] [CrossRef]
- Boettger, M.K.; Grossmann, D.; Bär, K.-J. Thresholds and Perception of Cold Pain, Heat Pain, and the Thermal Grill Illusion in Patients with Major Depressive Disorder. Psychosom. Med. 2013, 75, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Fründt, O.; Grashorn, W.; Buhmann, C.; Forkmann, K.; Mainka, T.; Bingel, U.; Schmidt, K. Quantitative Sensory Testing (QST) in Drug-Naïve Patients with Parkinson’s Disease. J. Park. Dis. 2019, 9, 369–378. [Google Scholar] [CrossRef]
- Tozza, S.; Severi, D.; Palumbo, G.; Provitera, V.; Ruggiero, L.; Dubbioso, R.; Iodice, R.; Nolano, M.; Manganelli, F. Quantitative Sensory Testing in Late-Onset ATTRv Presymptomatic Subjects: A Single Center Experience. Biomedicines 2022, 10, 2877. [Google Scholar] [CrossRef]
- Walk, D.; Sehgal, N.; Moeller-Bertram, T.; Edwards, R.R.; Wasan, A.; Wallace, M.; Irving, G.; Argoff, C.; Backonja, M.-M. Quantitative sensory testing and mapping: A review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin. J. Pain 2009, 25, 632–640. [Google Scholar] [CrossRef]
- Koulouris, A.E.; Edwards, R.R.; Dorado, K.; Schreiber, K.L.; Lazaridou, A.; Rajan, S.; White, J.; Garcia, J.; Gibbons, C.; Freeman, R. Reliability and Validity of the Boston Bedside Quantitative Sensory Testing Battery for Neuropathic Pain. Pain Med. 2020, 21, 2336–2347. [Google Scholar] [CrossRef]
- Devigili, G.; Cazzato, D.; Lauria, G. Clinical diagnosis and management of small fiber neuropathy: An update on best practice. Expert Rev. Neurother. 2020, 20, 967–980. [Google Scholar] [CrossRef]
- Adler, M.; Taxer, B. Quantitative sensory testing for neuropathic pain and its relevance for physiotherapy. Schmerz 2022, 36, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Adhikari, K.; Aristeguieta, L.M.R.; Chacon-Duque, J.C.; Poletti, G.; Gallo, C.; Rothhammer, F.; Bedoya, G.; Ruiz-Linares, A.; Bennett, D.L. Genetic components of human pain sensitivity: A protocol for a genome-wide association study of experimental pain in healthy volunteers. BMJ Open 2019, 9, e025530. [Google Scholar] [CrossRef] [PubMed]
- Pfau, D.B.; Geber, C.; Birklein, F.; Treede, R.-D. Quantitative Sensory Testing of Neuropathic Pain Patients: Potential Mechanistic and Therapeutic Implications. Curr. Pain Headache Rep. 2012, 16, 199–206. [Google Scholar] [CrossRef]
- Scherens, A.; Maier, C.; Haussleiter, I.; Schwenkreis, P.; Vlckova-Moravcova, E.; Baron, R.; Sommer, C. Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: Comparison of different diagnostic modalities. Eur. J. Pain 2009, 13, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Kokotis, P.; Manios, E.; Schmelz, M.; Fotiou, D.; Dialoupi, I.; Gavriatopoulou, M.; Roussou, M.; Lykka, A.; Dimopoulos, M.A.; Kastritis, E. Involvement of small nerve fibres and autonomic nervous system in AL amyloidosis: Comprehensive characteristics and clinical implications. Amyloid 2020, 27, 103–110. [Google Scholar] [CrossRef]
- Braun, M.; Bello, C.; Riva, T.; Hönemann, C.; Doll, D.; Urman, R.D.; Luedi, M.M. Quantitative Sensory Testing to Predict Postoperative Pain. Curr. Pain Headache Rep. 2021, 25, 3. [Google Scholar] [CrossRef]
- Montes, A.; Roca, G.; Cantillo, J.; Sabate, S.; GENDOLCAT Study Group. Presurgical risk model for chronic postsurgical pain based on 6 clinical predictors: A prospective external validation. Pain 2020, 161, 2611–2618. [Google Scholar] [CrossRef]
- Ahmad, S.; Bialek, J.M.; De Oliveira, G.S.; McCarthy, R.J. Thermal Quantitative Sensory Testing to Predict Postoperative Pain Outcomes Following Gynecologic Surgery. Pain Med. 2014, 15, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Schliessbach, J.; Siegenthaler, A.; Bütikofer, L.; Vuilleumier, P.; Jüni, P.; Stamer, U.; Arendt-Nielsen, L.; Curatolo, M. Predicting drug efficacy in chronic low back pain by quantitative sensory tests. Eur. J. Pain 2018, 22, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Fregoso, G.; Wang, A.; Tseng, K.; Wang, J. Transition from Acute to Chronic Pain: Evaluating Risk for Chronic Postsurgical Pain. Pain Physician 2019, 22, 479–488. [Google Scholar]
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef] [Green Version]
- Schug, S.A.; Lavand’Homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. The IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Lopes, A.; Menezes, M.S.; de Barros, G.A.M. Chronic postoperative pain: Ubiquitous and scarcely appraised: Narrative review. Braz. J. Anesthesiol. 2021, 71, 649–655. [Google Scholar] [CrossRef]
- Gómez, M.; Izquierdo, C.E.; Rojals, V.M.; Jr, J.P.; Sanchez, R.P.; Paladini, A.; Varrassi, G. Considerations for Better Management of Postoperative Pain in Light of Chronic Postoperative Pain: A Narrative Review. Cureus 2022, 14, e23763. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Jensen, T.S. Predicting postoperative pain based on preoperative pain perception: Are we doing better than the weatherman? Anesthesiology 2010, 112, 1311–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granot, M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Curr. Opin. Anaesthesiol. 2009, 22, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Siedler, G.; Sommer, C.; Üçeyler, N. Pain-related evoked potentials in patients with large, mixed, and small fiber neuropathy. Clin. Neurophysiol. 2020, 131, 635–641. [Google Scholar] [CrossRef]
- Cruz-Almeida, Y.; King, C.D.; Goodin, B.R.; Sibille, K.T.; Glover, T.L.; Riley, J.L.; Sotolongo, A.; Herbert, M.; Schmidt, J.K.; Fessler, B.J.; et al. Psychological Profiles and Pain Characteristics of Older Adults with Knee Osteoarthritis. Arthritis Care Res. 2013, 65, 1786–1794. [Google Scholar] [CrossRef]
- Dapunt, U.; Gantz, S.; Zhuk, A.; Gather, K.; Wang, H.; Marcus, S. Quantitative sensory testing in physically active individuals and patients who underwent multidisciplinary pain therapy in the longitudinal course. J. Pain Res. 2018, 11, 2323–2330. [Google Scholar] [CrossRef] [Green Version]
- Finan, P.H.; Buenaver, L.F.; Bounds, S.C.; Hussain, S.; Park, R.J.; Haque, U.J.; Campbell, C.M.; Haythornthwaite, J.A.; Edwards, R.R.; Smith, M.T. Discordance between pain and radiographic severity in knee osteoarthritis: Findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013, 65, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Groener, J.B.; Jende, J.M.; Kurz, F.T.; Kender, Z.; Treede, R.-D.; Schuh-Hofer, S.; Nawroth, P.P.; Bendszus, M.; Kopf, S. Understanding Diabetic Neuropathy—From Subclinical Nerve Lesions to Severe Nerve Fiber Deficits: A Cross-Sectional Study in Patients with Type 2 Diabetes and Healthy Control Subjects. Diabetes 2020, 69, 436–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlesso, L.C.; Segal, N.A.; Frey-Law, L.; Zhang, Y.; Na, L.; Nevitt, M.; Lewis, C.E.; Neogi, T. Pain Susceptibility Phenotypes in Those Free of Knee Pain With or at Risk of Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2019, 71, 542–549. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.-D. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 2006, 10, 77–88. [Google Scholar] [CrossRef]
- Masgoret, P.; de Soto, I.; Caballero, A.; Ríos, J.; Gomar, C. Incidence of contralateral neurosensitive changes and persistent postoperative pain 6 months after mastectomy: A prospective, observational investigation. Medicine 2020, 99, e19101. [Google Scholar] [CrossRef] [PubMed]
- Dukefoss, T.T.; Kleggetveit, I.P.; Helås, T.; Jørum, E. Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP). Scand. J. Pain 2019, 20, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D.; Crispel, Y.; Eisenberg, E.; Granovsky, Y.; Ben-Nun, A.; Sprecher, E.; Best, L.-A.; Granot, M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008, 138, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.C.; Pinto, J.M.; Almeida, A.; Pinto, P.R. Predictive value of quantitative sensory testing for acute and chronic postsurgical pain after total joint arthroplasty: A systematic review. Pain 2022, 163, e385–e400. [Google Scholar] [CrossRef]
- Sangesland, A.; Støren, C.; Vaegter, H.B. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand. J. Pain 2017, 15, 44–52. [Google Scholar] [CrossRef]
- Agostinho, C.M.S.; Scherens, A.; Richter, H.; Schaub, C.; Rolke, R.; Treede, R.-D.; Maier, C. Habituation and short-term repeatability of thermal testing in healthy human subjects and patients with chronic non-neuropathic pain. Eur. J. Pain 2009, 13, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Vivian, H.Y.; Abrishami, A.; Peng, P.W.H.; Wong, J.; Chung, F. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2009, 111, 657–677. [Google Scholar]
- Granot, M.; Lowenstein, L.; Yarnitsky, D.; Tamir, A.; Zimmer, E.Z. Postcesarean Section Pain Prediction by Preoperative Experimental Pain Assessment. Anesthesiology 2003, 98, 1422–1426. [Google Scholar] [CrossRef]
- Stanhope, J. Brief Pain Inventory review. Occup. Med. 2016, 66, 496–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleeland, C.S. The Brief Pain Inventory—User Guide; The University of Texas: Houston, TX, USA, 2009; p. 66. [Google Scholar]
- Hsu, Y.-W.; Somma, J.; Hung, Y.-C.; Tsai, P.-S.; Yang, C.-H.; Chen, C.-C. Predicting Postoperative Pain by Preoperative Pressure Pain Assessment. Anesthesiology 2005, 103, 613–618. [Google Scholar] [CrossRef]
- Nielsen, P.R.; Nørgaard, L.N.; Rasmussen, L.S.; Kehlet, H. Prediction of post-operative pain by an electrical pain stimulus. Acta Anaesthesiol. Scand. 2007, 51, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, C.H.; Hill, L.; Dyer, R.A.; Torr, G.; Coetzee, E. Postoperative Sensitization and Pain After Cesarean Delivery and the Effects of Single IM Doses of Tramadol and Diclofenac Alone and in Combination. Obstet. Anesthesia Dig. 2003, 97, 526–533. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Averbeck, B.; Seitz, L.; Kolb, F.P.; Kutz, D.F. Sex differences in thermal detection and thermal pain threshold and the thermal grill illusion: A psychophysical study in young volunteers. Biol. Sex Differ. 2017, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Schestatsky, P.; Algaba, R.; Pérez, D.; Casanova-Molla, J.; León, L.; Costa, J.; Valls-Solé, J. Transient decrease of sensory perception after thermoalgesic stimuli for quantitative sensory testing. Muscle Nerve 2007, 36, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, E.; Midbari, A.; Haddad, M.; Pud, D. Predicting the analgesic effect to oxycodone by ‘static’ and ‘dynamic’ quantitative sensory testing in healthy subjects. Pain 2010, 151, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Heldestad, V.; Linder, J.; Sellersjö, L.; Nordh, E. Reproducibility and influence of test modality order on thermal perception and thermal pain thresholds in quantitative sensory testing. Clin. Neurophysiol. 2010, 121, 1878–1885. [Google Scholar] [CrossRef]
- Strulov, L.; Zimmer, E.Z.; Granot, M.; Tamir, A.; Jakobi, P.; Lowenstein, L. Pain Catastrophizing, Response to Experimental Heat Stimuli, and Post–Cesarean Section Pain. J. Pain 2007, 8, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Schestatsky, P.; Stefani, L.C.; Sanches, P.R.; Júnior, D.P.D.S.; Torres, I.L.S.; Dall-Agnol, L.; Balbinot, L.F.; Caumo, W. Validation of a Brazilian quantitative sensory testing (QST) device for the diagnosis of small fiber neuropathies. Arq. Neuro-Psiquiatr. 2011, 69, 943–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautenbacher, S.; Huber, C.; Schöfer, D.; Kunz, M.; Parthum, A.; Weber, P.G.; Roman, C.; Griessinger, N.; Sittl, R. Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: A comparison with other psychological and physiological predictors. Pain 2010, 151, 722–731. [Google Scholar] [CrossRef]
- Courtin, A.S.; Slootjes, S.M.; Caty, G.; Hermans, M.P.; Plaghki, L.; Mouraux, A. Assessing thermal sensitivity using transient heat and cold stimuli combined with a Bayesian adaptive method in a clinical setting: A proof of concept study. Eur. J. Pain 2020, 24, 1812–1821. [Google Scholar] [CrossRef]
- Ezenwa, M.O.; Molokie, R.E.; Wang, Z.J.; Yao, Y.; Suarez, M.L.; Pullum, C.; Schlaeger, J.M.; Fillingim, R.B.; Wilkie, D.J. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic Pain? Pain Pract. 2016, 16, 282–293. [Google Scholar] [CrossRef] [Green Version]
- NCBI. Age Groups. 1998. Available online: https://www.ncbi.nlm.nih.gov/mesh/68000328 (accessed on 12 August 2022).
- Hirschfeld, G.; Zernikow, B. Variability of “optimal” cut points for mild, moderate, and severe pain: Neglected problems when comparing groups. Pain 2013, 154, 154–159. [Google Scholar] [CrossRef]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Miettinen, T.; Kautiainen, H.; Mäntyselkä, P.; Linton, S.J.; Kalso, E. Pain interference type and level guide the assessment process in chronic pain: Categorizing pain patients entering tertiary pain treatment with the Brief Pain Inventory. PLoS ONE 2019, 14, e0221437. [Google Scholar] [CrossRef]
- Adams, M.H.; Dobscha, S.K.; Smith, N.X.; Yarborough, B.J.; Deyo, R.A.; Morasco, B.J. Prevalence and Correlates of Low Pain Interference Among Patients with High Pain Intensity Who Are Prescribed Long-Term Opioid Therapy. J. Pain 2018, 19, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Anekar, A.A.; Cascella, M. WHO Analgesic Ladder; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yap, B.W. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Pan, P.H.; Coghill, R.; Houle, T.; Seid, M.H.; Lindel, W.M.; Parker, R.L.; Washburn, S.A.; Harris, L.; Eisenach, J.C. Multifactorial Preoperative Predictors for Postcesarean Section Pain and Analgesic Requirement. Anesthesiology 2006, 104, 417–425. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Shefi, T.; Schneider, V.J.; Li, D.; Ding, L.; King, C.D.; Coghill, R.C. Quantitative sensory testing in patients with migraine: A systematic review and meta-analysis. Pain 2018, 159, 1202–1223. [Google Scholar] [CrossRef]
- Griffioen, M.A.; Greenspan, J.D.; Johantgen, M.; Von Rueden, K.; O’Toole, R.V.; Dorsey, S.G.; Renn, C.L. Quantitative Sensory Testing and Current Perception Threshold Testing in Patients with Chronic Pain Following Lower Extremity Fracture. Biol. Res. Nurs. 2018, 20, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hainsworth, K.R.; Simpson, P.M.; Ali, O.; Varadarajan, J.; Rusy, L.; Weisman, S.J. Quantitative Sensory Testing in Adolescents with Co-Occurring Chronic Pain and Obesity: A Pilot Study. Children 2020, 7, 55. [Google Scholar] [CrossRef]
- Guo, S.; Wang, W.; Huang, H.; Bi, Y.; Jin, Y.; Li, C.; Zhu, D.; Wang, L. Quantitative sensory testing can effectively predict cardiovascular autonomic neuropathy in patients with type 2 diabetes mellitus. Acta Diabetol. 2021, 58, 1541–1549. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Nie, H.; Laursen, M.B.; Laursen, B.S.; Madeleine, P.; Simonsen, O.H.; Graven-Nielsen, T. Sensitization in patients with painful knee osteoarthritis. Pain 2010, 149, 573–581. [Google Scholar] [CrossRef]
- van Griensven, H.; Schmid, A.; Trendafilova, T.; Low, M. Central Sensitization in Musculoskeletal Pain: Lost in Translation? J. Orthop. Sports Phys. Ther. 2020, 50, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Nackley, A.; Huh, B.Y.; Terrando, N.; Maixner, D.W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Staud, R.; Robinson, M.E.; Price, D. Temporal Summation of Second Pain and Its Maintenance Are Useful for Characterizing Widespread Central Sensitization of Fibromyalgia Patients. J. Pain 2007, 8, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Thompson, H.D.; Tang, S.; Jarrell, J.F. Temporal Summation in Chronic Pelvic Pain. J. Obstet. Gynaecol. Can. 2020, 42, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Humo, M.; Lu, H.; Yalcin, I. The molecular neurobiology of chronic pain–induced depression. Cell Tissue Res. 2019, 377, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, A.; Zis, P. Depression, anxiety and acute pain: Links and management challenges. Postgrad. Med. 2019, 131, 438–444. [Google Scholar] [CrossRef]
- Gupta, K.K.; Attri, J.P.; Singh, A.; Kaur, H.; Kaur, G. Basic concepts for sample size calculation: Critical step for any clinical trials. Saudi J. Anaesth. 2016, 10, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mitra, K.; Mandal, M. Sample size calculation: Basic principles. Indian J. Anaesth. 2016, 60, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Gierthmühlen, J.; Enax-Krumova, E.K.; Attal, N.; Bouhassira, D.; Cruccu, G.; Finnerup, N.B.; Haanpää, M.; Hansson, P.; Jensen, T.S.; Freynhagen, R.; et al. Who is healthy? Aspects to consider when including healthy volunteers in QST-based studies—A consensus statement by the EUROPAIN and NEUROPAIN consortia. Pain 2015, 156, 2203–2211. [Google Scholar] [CrossRef]




| Variable | Control (n = 40) | CPG (n = 50) | p * Value |
|---|---|---|---|
| 1—Females n (%) | (24) 60.0 | (35) 70.0 | 0.984 |
| 2—Age distribution n (%) | |||
| Young (<18 years) | 3 (7.5) | 0 (0.0) | 0.001 |
| Adults (18–65 years) | 37 (92.5) | 8 (32.0) | |
| Elderly (>65 years) | 0 (0.0) | 17(68.0) | |
| 3—Predominant pain location n (%) | |||
| Musculoskeletal | 4 (50.0) | 50 (100.0) | 0.001 |
| Others | 4 (50.0) | 0 (0.0) | |
| 4—Pain intensity in the interview n (%) | |||
| No pain | 40 (100.0) | 17 (34.0) | 0.001 |
| Mild pain | 0 (0.0) | 5 (10.0) | |
| Moderate pain | 0 (0.0) | 19 (38.0) | |
| Severe pain | 0 (0.0) | 9 (18.0) | |
| Mean (NRS ± SD) | 0.00 ± 0.00 | 3.58 ± 3.15 | 0.001 |
| 5—Average of past pain intensity n (%) | |||
| No pain | 32 (80.0) | 0 (0.0) | 0.001 |
| Mild pain | 1 (2.5) | 12 (24.0) | |
| Moderate pain | 6 (15.0) | 24 (48.0) | |
| Severe pain | 1 (2.5) | 14 (28.0) | |
| Mean (NRS ± SD) | 0.98 ± 2.06 | 5.14 ± 2.12 | |
| 6—Pain at its maximum n (%) | |||
| No pain | 32 (80.0) | 0 (0.0) | 0.001 |
| Mild pain | 0 (0.0) | 5 (10.0) | |
| Moderate pain | 5 (12.5) | 16 (32.0) | |
| Severe pain | 3 (7.5) | 29 (58.0) | |
| Mean (NRS ± SD) | 1.23 ± 2.56 | 6.76 ± 2.32 | 0.001 |
| 7—Pain interference on the subjects’ activity n (%) | |||
| None | 1 (12.5) | 5 (10.2) | 0.254 |
| High | 1 (12.5) | 21 (42.9) | |
| Low | 6 (75.0) | 23 (46.9) | |
| Mean (NRS ± SD) | 3.75 ± 2.32 | 5.80 ± 2.79 | 0.644 |
| 8—Pain interference on the subjects’ humor n (%) | |||
| None | 0 (0.0) | 9 (18.4) | 0.060 |
| High | 1 (12.5) | 19 (38.8) | |
| Low | 7 (87.5) | 21 (42.9) | |
| Mean (NRS ± SD) | 2.50 ± 2.14 | 5.35 ± 3.40 | 0.043 |
| 9—Pain interference on the subjects’ ability to sleep n (%) | |||
| None | 5 (62.5) | 13 (26.5) | |
| High | 1 (12.5) | 11 (22.4) | 0.127 |
| Low | 2 (25.0) | 25 (51.0) | |
| Mean (NRS ± SD) | 2.25 ± 3.28 | 3.88 ± 3.31 | 0.873 |
| Variable | Control (n = 40) | CPG (n = 50) | p * Value |
|---|---|---|---|
| 1—Threshold mean temperature, T ± SD (°C) | 45.19 ± 3.43 | 48.32 ± 2.29 | 0.001 |
| 2—Perception of pain intensity at 36 °C, NRS ± SD | 0.28 ± 0.73 | 0.67 ± 1.19 | 0.162 |
| 3—Perception of pain at threshold mean temperature, NRS ± SD | 3.04 ± 2.76 | 4.97 ± 2.98 | 0.003 |
| 4—Perception of pain intensity at 49 °C, NRS ± SD | 6.80 ± 1.92 | 6.28 ± 2.99 | 0.792 |
| 5—Perception of pain at 10 s of tonic stimulation, NRS ± SD | 4.20 ± 2.13 | 4.04 ± 3.33 | 0.694 |
| 6—Perception of pain at 20 s of tonic stimulation, NRS ± SD | 3.65 ± 1.98 | 4.28 ± 3.26 | 0.323 |
| 7—Perception of pain at 40 s of tonic stimulation, NRS ± SD | 3.93 ± 2.26 | 4.58 ± 3.31 | 0.362 |
| 8—Perception of pain at 55 s of tonic stimulation, NRS ± SD | 4.13 ± 2.19 | 4.86 ± 3.31 | 0.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, P.; Tavares, I.; Fonseca, S.; Pozza, D.H. Outcomes of a QST Protocol in Healthy Subjects and Chronic Pain Patients: A Controlled Clinical Trial. Biomedicines 2023, 11, 1023. https://doi.org/10.3390/biomedicines11041023
Dias P, Tavares I, Fonseca S, Pozza DH. Outcomes of a QST Protocol in Healthy Subjects and Chronic Pain Patients: A Controlled Clinical Trial. Biomedicines. 2023; 11(4):1023. https://doi.org/10.3390/biomedicines11041023
Chicago/Turabian StyleDias, Patrícia, Isaura Tavares, Sara Fonseca, and Daniel Humberto Pozza. 2023. "Outcomes of a QST Protocol in Healthy Subjects and Chronic Pain Patients: A Controlled Clinical Trial" Biomedicines 11, no. 4: 1023. https://doi.org/10.3390/biomedicines11041023
APA StyleDias, P., Tavares, I., Fonseca, S., & Pozza, D. H. (2023). Outcomes of a QST Protocol in Healthy Subjects and Chronic Pain Patients: A Controlled Clinical Trial. Biomedicines, 11(4), 1023. https://doi.org/10.3390/biomedicines11041023







