Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety
Abstract
:1. Introduction
2. Materials and Methods
3. Results
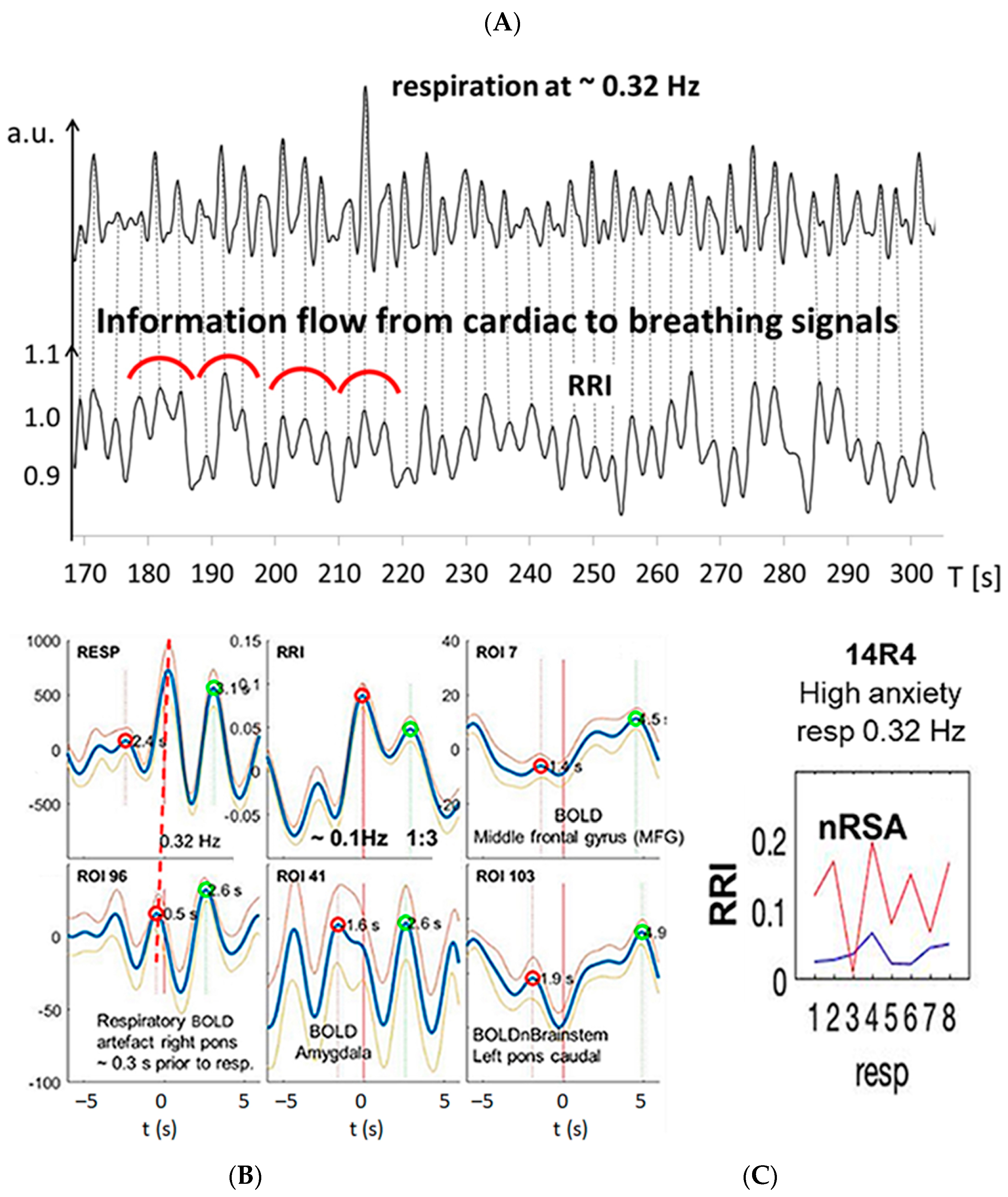
3.1. BOLD Oscillations of Neural and Non-Neural Origin
3.2. Cardiac RRI, Respiratory and BOLD Signals in the 0.05–0.15/0.1–0.2 Hz Band
3.3. Interactions of RRI, Respiratory and BOLD Signals in the 0.2–0.4 Hz Band
3.4. Dominant Information Flow between Cardiac (RRI), Respiratory and BOLD Oscillations in the 0.1–0.2 Hz and 0.2–0.4 Hz Bands
3.5. Management of Anxiety
4. Discussion
4.1. Different Cardio-Respiratory Coupling during High Anxiety
4.1.1. Slow Breathing during High Anxiety (Group 1)
4.1.2. Fast Breathing during High Anxiety (Groups 2 and 3)
4.2. Coupling of Respiration with Heart and Brain Oscillatory Activity
4.2.1. Phase-Shifts in Respiration-Related RRI Oscillations and Activation of a Central Pacemaker
4.2.2. Respiration-Entrained Brain Oscillations and Possible Strategies of Anxiety Management
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckberg, D.L. Human sinus arrhythmia as an index of vagal cardiac outflow. J. Appl. Physiol. 1983, 54, 961–966. [Google Scholar] [CrossRef]
- Hayano, J.; Yasuma, F.; Okada, A.; Mukai, S.; Fujinami, T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation 1996, 94, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Grossman, P.; Taylor, E.W. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 2007, 74, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Yasuma, F.; Hayano, J. Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm? Chest 2004, 125, 683–690. [Google Scholar] [CrossRef]
- Karemaker, J.M. Counterpoint: Respiratory sinus arrhythmia is due to the baroreflex mechanism. J. Appl. Physiol. 2009, 106, 1742–1743. [Google Scholar] [CrossRef]
- Akselrod, S.; Gordon, D.; Ubel, F.; Shannon, D.; Berger, A.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E.; et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef]
- Eckberg, D.L. The human respiratory gate. J. Physiol. 2003, 548, 339–352. [Google Scholar] [CrossRef]
- Eckberg, D.L. Point:counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J. Appl. Physiol. 2009, 106, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Haselton, J.R.; Guyenet, P.G. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am. J. Physiol. 1989, 256, R739–R750. [Google Scholar] [CrossRef] [PubMed]
- Häbler, H.J.; Jänig, W.; Krummel, M.; Peters, O.A. Respiratory modulation of the activity in postganglionic neurons supplying skeletal muscle and skin of the rat hindlimb. J. Neurophysiol. 1993, 70, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Mandel, D.A.; Schreihofer, A.M. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J. Physiol. 2006, 572, 881–896. [Google Scholar] [CrossRef]
- Porta, A.; Bassani, T.; Bari, V.; Tobaldini, E.; Takahashi, A.C.; Catai, A.M.; Montano, N. Model-based assessment of baroreflex and cardiopulmonary couplings during graded head-up tilt. Comput. Biol. Med. 2012, 42, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.H.; Zoccal, D.B.; Moraes, D.J.A. Neurogenic hypertension and the secrets of respiration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R864–R872. [Google Scholar] [CrossRef] [PubMed]
- Menuet, C.; Connelly, A.A.; Bassi, J.K.; Melo, M.R.; Le, S.; Kamar, J.; Kumar, N.N.; McDougall, S.J.; McMullan, S.; Allen, A.M. PreBötzinger complex neurons drive respiratory modulation of blood pressure and heart rate. eLife 2020, 9, e57288. [Google Scholar] [CrossRef]
- Thayer, J.F.; Friedman, B.H.; Borkovec, T.D. Autonomic characteristics of generalized anxiety disorder and worry. Biol. Psychiatry 1996, 39, 255–266. [Google Scholar] [CrossRef]
- Watkins, L.L.; Grossman, P.; Krishnan, R.; Sherwood, A. Anxiety and vagal control of heart rate. Psychosom. Med. 1998, 60, 498–502. [Google Scholar] [CrossRef]
- Licht, C.M.; de Geus, E.J.; van Dyck, R.; Penninx, B.W. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA). Psychosom. Med. 2009, 71, 508–518. [Google Scholar] [CrossRef]
- Jönsson, P. Respiratory sinus arrhythmia as a function of state anxiety in healthy individuals. Int. J. Psychophysiol. 2007, 63, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 2022, 59, e14122. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.A.; Franquillo, A.C.; Grecucci, A.; Beccia, L.; Caretti, V.; Dadomo, H. Is Low Heart Rate Variability Associated with Emotional Dysregulation, Psychopathological Dimensions, and Prefrontal Dysfunctions? An Integrative View. J. Pers. Med. 2021, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Steffen, P.R.; Austin, T.; DeBarros, A.; Brown, T. The Impact of Resonance Frequency Breathing on Measures of Heart Rate Variability, Blood Pressure, and Mood. Front. Public Health 2017, 5, 222. [Google Scholar] [CrossRef]
- Lehrer, P. How does heart rate variability biofeedback work? Resonance, the baroreflex, and other mechanisms. Biofeedback 2013, 41, 26–31. [Google Scholar] [CrossRef]
- Mather, M.; Thayer, J.F. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Schwerdtfeger, A.; Aigner, C.S.; Pfurtscheller, G. Switch-off” of respiratory sinus arrhythmia can occur in a minority of subjects during functional magnetic resonance imaging (fMRI). Front. Physiol. 2018, 9, 1688. [Google Scholar] [CrossRef]
- Rassler, B.; Schwerdtfeger, A.R.; Schwarz, G.; Pfurtscheller, G. Negative respiratory sinus arrhythmia (nRSA) in the MRI-scanner—A physiologic phenomenon observed during elevated anxiety in healthy persons. Physiol. Behav. 2022, 245, 113676. [Google Scholar] [CrossRef]
- Yli-Hankala, A.; Porkkala, T.; Kaukinen, S.; Häkkinen, V.; Jäntti, V. Respiratory sinus arrhythmia is reversed during positive pressure ventilation. Acta Physiol. Scand. 1991, 141, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.C.; Beda, A.; de Abreu, M.G.; Spieth, P.M.; Granja-Filho, P.; Jandre, F.C.; Giannella-Neto, A. Comparison of objective methods to classify the pattern of respiratory sinus arrhythmia during mechanical ventilation and paced spontaneous breathing. Physiol. Meas. 2009, 30, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Schwerdtfeger, A.; Rassler, B.; Andrade, A.; Schwarz, G.; Klimesch, W. Verification of a central pacemaker in brain stem by phase-coupling analysis between HR interval- and BOLD-oscillations in the 0.10–0.15 Hz frequency band. Front. Neurosci. 2020, 14, 922. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Birn, R.M.; Bandettini, P.A. Resting-state fMRI confounds and cleanup. Neuroimage 2013, 80, 349–359. [Google Scholar] [CrossRef]
- Huneau, C.; Benali, H.; Chabriat, H. Investigating Human Neurovascular Coupling Using Functional Neuroimaging: A Critical Review of Dynamic Models. Front. Neurosci. 2015, 9, 467. [Google Scholar] [CrossRef]
- Bari, V.; De Maria, B.; Mazzucco, C.E.; Rossato, G.; Tonon, D.; Nollo, G.; Faes, L.; Porta, A. Cerebrovascular and cardiovascular variability interactions investigated through conditional joint transfer entropy in subjects prone to postural syncope. Physiol. Meas. 2017, 38, 976–991. [Google Scholar] [CrossRef]
- Zelano, C.; Jiang, H.; Zhou, G.; Arora, N.; Schuele, S.; Rosenow, J.; Gottfried, J.A. Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J. Neurosci. 2016, 36, 12448–12467. [Google Scholar] [CrossRef]
- Valenza, G.; Passamonti, L.; Duggento, A.; Toschi, N.; Barbieri, R. Uncovering complex central autonomic networks at rest: A functional magnetic resonance imaging study on complex cardiovascular oscillations. J. R. Soc. Interface 2020, 17, 20190878. [Google Scholar] [CrossRef]
- Faes, L.; Porta, A.; Cucino, R.; Cerutti, S.; Antolini, R.; Nollo, G. Causal transfer function analysis to describe closed loop interactions between cardiovascular and cardiorespiratory variability signals. Biol. Cybern. 2004, 90, 390–399. [Google Scholar] [CrossRef]
- Faes, L.; Nollo, G.; Porta, A. Non-uniform multivariate embedding to assess the information transfer in cardiovascular and cardiorespiratory variability series. Comput. Biol. Med. 2011, 42, 290–297. [Google Scholar] [CrossRef]
- Porta, A.; Bassani, T.; Bari, V.; Pinna, G.D.; Maestri, R.; Guzzetti, S. Accounting for respiration is necessary to reliably infer Granger causality from cardiovascular variability series. IEEE Trans. Biomed. Eng. 2012, 59, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Mrowka, R.; Cimponeriu, L.; Patzak, A.; Rosenblum, M.G. Directionality of coupling of physiological subsystems: Age-related changes of cardiorespiratory interaction during different sleep stages in babies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1395–R1401. [Google Scholar] [CrossRef] [PubMed]
- Shiogai, Y.; Stefanovska, A.; McClintock, P.V.E. Nonlinear dynamics of cardiovascular ageing. Phys. Rep. 2010, 488, 51–110. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Pereda, E.; Petsche, H. Effective detection of coupling in short and noisy bivariate data. IEEE Trans. Syst. Man. Cybern. B Cybern. 2003, 33, 85–95. [Google Scholar] [CrossRef]
- Borovkova, E.I.; Prokhorov, M.D.; Kiselev, A.R.; Hramkov, A.N.; Mironov, S.A.; Agaltsov, M.V.; Ponomarenko, V.I.; Karavaev, A.S.; Drapkina, O.M.; Penzel, T. Directional couplings between the respiration and parasympathetic control of the heart rate during sleep and wakefulness in healthy subjects at different ages. Front. Netw. Physiol. 2022, 2, 942700. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 1969, 37, 424. [Google Scholar] [CrossRef]
- Lachert, P.; Zygierewicz, J.; Janusek, D.; Pulawski, P.; Sawosz, P.; Kacprzak, M.; Liebert, A.; Blinowska, K.J. Causal Coupling Between Electrophysiological Signals, Cerebral Hemodynamics and Systemic Blood Supply Oscillations in Mayer Wave Frequency Range. Int. J. Neural Syst. 2019, 29, 1850033. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Blinowska, K.J.; Kaminski, M.; Rassler, B.; Klimesch, W. Processing of fMRI-related anxiety and information flow between brain and body revealed a preponderance of oscillations at 0.15/0.16 Hz. Sci. Rep. 2022, 12, 9117. [Google Scholar] [CrossRef]
- Laux, L.; Hock, M.; Bergner-Koether, R.; Hodapp, V.; Renner, K.H.; Merzbacher, G. Das State-Trait-Angst-Depressions-Inventar [The State-Trait Anxiety-Depression Inventory]; Hogrefe: Goettingen, Germany, 2013. [Google Scholar]
- Spielberger, C.D.; Gorssuch, R.L.; Lushene, P.R.; Vagg, P.R.; Jacobs, G. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press Inc.: Palo Alto, CA, USA, 2009. [Google Scholar]
- Niazy, R.K.; Beckmann, C.F.; Iannetti, G.D.; Brady, J.M.; Smith, S.M. Removal of fMRI environment artifacts from EEG data using optimal basis sets. Neuroimage 2005, 28, 720–737. [Google Scholar] [CrossRef]
- Kugel, H.; Bremer, C.; Peschel, M.; Fischbach, R.; Lenzen, H.; Tombach, B.; Van Aken, H.; Heindel, W. Hazardous situation in the MR bore: Induction in ECG leads causes fire. Eur. Radiol. 2003, 13, 690–694. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Obrig, H.; Neufang, M.; Wenzel, R.; Kohl, M.; Steinbrink, J.; Einhäupl, K.; Villringer, A. Spontaneous low frequency oscillations of ccerebral hemodynamics and metabolism in human adults. Neuroimage 2000, 12, 623–639. [Google Scholar] [CrossRef]
- Buxton, R.B.; Uludag, K.; Dubowitz, D.J.; Liu, T.T. Modeling the hemodynamic response to brain activation. Neuroimage 2004, 23, 220–233. [Google Scholar] [CrossRef]
- Verberne, A.J.M.; Owens, N.C. Cortical modulation of the cardiovascular system. Progr. Neurobiol. 1998, 54, 149–168. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.; Yacoub, E.; Olman, C.A.; Auerbach, E.; Strupp, J.; Harel, N.; Uğurbil, K. Multiband multislice GE-EPI at 7 Tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010, 63, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Blinowska, K.J.; Kaminski, M.; Schwerdtfeger, A.R.; Rassler, B.; Schwarz, G.; Klimesch, W. Processing of fMRI-related anxiety and bi-directional information flow between prefrontal cortex and brainstem. Sci. Rep. 2021, 11, 22348. [Google Scholar] [CrossRef] [PubMed]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Lachaux, J.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 208, 194–208. [Google Scholar] [CrossRef]
- Hurtado, J.M.; Rubchinsky, L.L.; Sigvardt, K.A. Statistical method for detection of phase-locking episodes in neural oscillations. J. Neurophysiol. 2004, 91, 1883–1898. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Schwerdtfeger, A.; Seither-Preisler, A.; Brunner, C.; Aigner, C.S.; Brito, J.; Carmo, M.P.; Andrade, A. Brain-heart communication: Evidence for “central pacemaker” oscillations with a dominant frequency at 0.1 Hz in the cingulum. Clin. Neurophysiol. 2017, 128, 183–193. [Google Scholar] [CrossRef]
- Kaminski, M.J.; Blinowska, K.J. A new method of the description of the information flow in the brain structures. Biol. Cybern. 1991, 65, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Blinowska, K.J.; Kuś, R.; Kamiński, M. Granger causality and information flow in multivariate processes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2004, 70, 050902. [Google Scholar] [CrossRef] [PubMed]
- Geweke, J. Measurement of Linear Dependence and Feedback between Multiple Time Series. J. Am. Stat. Assoc. 1982, 77, 304–313. [Google Scholar] [CrossRef]
- Schulz, S.; Adochiei, F.C.; Edu, I.R.; Schroeder, R.; Costin, H.; Bär, K.J.; Voss, A. Cardiovascular and cardiorespiratory coupling analyses: A review. Philos. Trans. R. Soc. A 2013, 37, 20120191. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. The frequency architecture of brain and body oscillations: An analysis. Eur. J. Neurosci. 2018, 48, 2431–2453. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Rassler, B.; Schwerdtfeger, A.; Klimesch, W.; Andrade, A.; Schwarz, G.; Thayer, J.F. “Switch-off” of respiratory sinus arrhythmia may be associated with the activation of an oscillatory source (pacemaker) in the brain stem. Front. Physiol. 2019, 10, 939. [Google Scholar] [CrossRef]
- Del Negro, C.A.; Funk, G.D.; Feldman, J.L. Breathing matters. Nat. Rev. Neurosci. 2018, 19, 351–367. [Google Scholar] [CrossRef]
- Yang, C.F.; Feldman, J.L. Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J. Comp. Neurol. 2018, 526, 1389–1402. [Google Scholar] [CrossRef]
- Kluger, D.S.; Gross, J. Respiration modulates oscillatory neural network activity at rest. PLoS Biol. 2021, 19, e3001457. [Google Scholar] [CrossRef] [PubMed]
- Kluger, D.S.; Balestrieri, E.; Busch, N.A.; Gross, J. Respiration aligns perception with neural excitability. eLife 2021, 10, e70907. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Itagaki, A.; Hashizume, A.; Takahashi, A.; Ishizaka, R.; Ozaki, I. Observation of respiration-entrained brain oscillations with scalp EEG. Neurosci. Lett. 2023, 797, 137079. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Rassler, B.; Hefter, H. Coordination between breathing and forearm movements during sinusoidal tracking. Eur. J. Appl. Physiol. 2000, 81, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, E.N. Perception and the Conditioned Reflex; Pergamon: Oxford, UK, 1963. [Google Scholar]
- Barry, R.J.; Rushby, J.A. An orienting reflex perspective on anteriorisation of the P3 of the event-related potential. Exp. Brain Res. 2006, 173, 539–545. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Solis-Escalante, T.; Barry, R.J.; Klobassa, D.S.; Neuper, C.; Müller-Putz, G.R. Brisk heart rate and EEG changes during execution and withholding of cue-paced foot motor imagery. Front. Hum. Neurosci. 2013, 7, 379. [Google Scholar] [CrossRef]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef]
- Rassler, B.; Kohl, J. Coordination-related changes in the rhythms of breathing and walking in humans. Eur. J. Appl. Physiol. 2000, 82, 280–288. [Google Scholar] [CrossRef]
- McDermott, W.J.; Van Emmerik, R.E.; Hamill, J. Running training and adaptive strategies of locomotor-respiratory coordination. Eur. J. Appl. Physiol. 2003, 89, 435–444. [Google Scholar] [CrossRef]
- O’Halloran, J.; Hamill, J.; McDermott, W.J.; Remelius, J.G.; Van Emmerik, R.E. Locomotor-respiratory coupling patterns and oxygen consumption during walking above and below preferred stride frequency. Eur. J. Appl. Physiol. 2012, 112, 929–940. [Google Scholar] [CrossRef]
- Hoffmann, C.P.; Torregrosa, G.; Bardy, B.G. Sound stabilizes locomotor-respiratory coupling and reduces energy cost. PLoS ONE 2012, 7, e45206. [Google Scholar] [CrossRef]
- Rassler, B.; Waurick, S.; Ebert, D. Einfluss zentralnervoser ¨ Koordination im Sinne v. HOLSTs auf die Steuerung von Atem- und Extremit¨ atenmotorik des Menschen [Effect of central coordination in the sense of v. Holst on the control of breathing and limb movements in humans]. Biol. Cybern. 1990, 63, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, P.; Kohl, J. Analysis of co-ordination between breathing and exercise rhythms in man. J. Physiol. 1993, 471, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Perlitz, V.; Lambertz, M.; Cotuk, B.; Grebe, R.; Vandenhouten, R.; Flatten, G.; Petzold, E.R.; Schmid-Schönbein, H.; Langhorst, P. Cardiovascular rhythms in the 0.15-Hz band: Common origin of identical phenomena in man and dog in the reticular formation of the brain stem? Eur. J. Physiol. 2004, 448, 579–591. [Google Scholar] [CrossRef]
- Porta, A.; Castiglioni, P.; Di Rienzo, M.; Bassani, T.; Bari, V.; Faes, L.; Nollo, G.; Cividjan, A.; Quintin, L. Cardiovascular control and time domain Granger causality: Insights from selective autonomic blockade. Phil. Trans. R. Soc. A 2013, 371, 20120161. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Raabe, J. Co-ordination of breathing with rhythmic head and eye movements and with passive turnings of the body. Eur. J. Appl. Physiol. 2003, 90, 125–130. [Google Scholar] [CrossRef]
- Heck, D.H.; McAfee, S.S.; Liu, Y.; Babajani-Feremi, A.; Rezaie, R.; Freeman, W.J.; Wheless, J.W.; Papanicolaou, A.C.; Ruszinkó, M.; Sokolov, Y.; et al. Breathing as a Fundamental Rhythm of Brain Function. Front. Neural Circuits 2017, 10, 115. [Google Scholar] [CrossRef]
- Tort, A.B.L.; Brankačk, J.; Draguhn, A. Respiration-Entrained Brain Rhythms Are Global but Often Overlooked. Trends Neurosci. 2018, 41, 186–197. [Google Scholar] [CrossRef]
- Jung, F.; Yanovsky, Y.; Brankačk, J.; Tort, A.B.L.; Draguhn, A. Respiratory entrainment of units in the mouse parietal cortex depends on vigilance state. Pflugers Arch. 2023, 475, 65–76. [Google Scholar] [CrossRef]
- Herrero, J.L.; Khuvis, S.; Yeagle, E.; Cerf, M.; Mehta, A.D. Breathing above the brain stem: Volitional control and attentional modulation in humans. J. Neurophysiol. 2018, 119, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Zaccaro, A.; Piarulli, A.; Laurino, M.; Garbella, E.; Menicucci, D.; Neri, B.; Gemignani, A. How Breath-Control Can Change Your Life: A Systematic Review on Psycho-Physiological Correlates of Slow Breathing. Front. Hum. Neurosci. 2018, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Stancák, A., Jr.; Kuna, M. EEG changes during forced alternate nostril breathing. Int. J. Psychophysiol. 1994, 18, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Telles, S.; Verma, S.; Sharma, S.K.; Gupta, R.K.; Balkrishna, A. Alternate-Nostril Yoga Breathing Reduced Blood Pressure While Increasing Performance in a Vigilance Test. Med. Sci. Monit. Basic Res. 2017, 23, 392–398. [Google Scholar] [CrossRef] [PubMed]







| Subject | AS | RSA/nRSA in % of RRI Waves | Average Rate | BOLD Analysis | RRI | Respiration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.10 Hz Band | 0.16 Hz Band | 0.10 Hz Band | 0.16 Hz Band | 0.32 Hz Band | ||||||||||||||
| RSA% | nRSA% | RRI [Hz] | Resp. [Hz] | TD [s] | Sigbin% | ROI | PD [s] | n [%] | PD [s] | n [%] | PD [s] | n [%] | PD [s] | n [%] | PD [s] | n [%] | ||
| Group 1 | ||||||||||||||||||
| #18R1 | 28 | 12% | 63% | 0.12 | 0.16 | 2.2 | 36% | 103 | 9.8 | 47% | 6.9 | 40% | 10.0 | 22% | 6.5 | 39% | 3.2 | 26% |
| #3R1 | 26 | 13% | 29% | 0.21 | 0.22 | 1.7 | 24% | 93 | 10.5 | 4% | 6.5 | 13% | --- | 0% | 6.4 | 15% | 3.6 | 39% |
| #11R1 | 25 | 5% | 80% | 0.13 | 0.13 | 2.3 | 70% | 93 | 9.8 | 39% | 6.9 | 54% | 9.9 | 38% | 6.9 | 48% | --- | 0% |
| Mean | 26.3 | 10.0% | 57.3% | 0.15 | 0.17 | 2.1 | 43.3% | 10.0 | 30% | 6.8 | 35% | 9.9 | 20% | 6.6 | 34% | 3.4 | 22% | |
| SD | 1.5 | 4.4% | 26.0% | 0.05 | 0.04 | 0.3 | 23.9% | 0.4 | 22% | 0.2 | 21% | 0.1 | 19% | 0.3 | 17% | 0.3 | 20% | |
| Group 2 | ||||||||||||||||||
| #24R1 | 25 | 12% | 54% | 0.25 | 0.26 | 1.5 | 26% | 93 | --- | 0% | 6.8 | 2% | --- | 0% | 5.9 | 6% | 3.4 | 63% |
| #16R4 | 24 | 18% | 14% | 0.22 | 0.33 | 0.8 | 31% | 93 | 9.9 | 11% | 6.4 | 19% | --- | 0% | 5.9 | 1% | 3.0 | 97% |
| #9R1 | 23 | 58% | 33% | 0.14 | 0.33 | 1.9 | 36% | 93 | 9.9 | 38% | 7.0 | 33% | --- | 0% | --- | 0% | 3.0 | 78% |
| #13R2 | 22 | 47% | 41% | 0.10 | 0.35 | 2.0 | 34% | 103 | 11.7 | 61% | 6.9 | 33% | --- | 0% | --- | 0% | 2.8 | 87% |
| #14R4 | 22 | 6% | 58% | 0.21 | 0.31 | 1.4 | 27% | 93 | 9.9 | 20% | 6.5 | 10% | --- | 0% | --- | 0% | 3.2 | 88% |
| #20R1 | 22 | 27% | 47% | 0.14 | 0.27 | 2.4 | 47% | 93 | 9.6 | 42% | 6.7 | 24% | --- | 0% | 6.0 | 2% | 3.4 | 65% |
| Mean | 23.0 | 28.0% | 41.2% | 0.18 | 0.31 | 1.7 | 33.5% | 10.2 | 29% | 6.7 | 21% | --- | 0% | 5.9 | 1% | 3.1 | 80% | |
| SD | 1.3 | 20.5% | 16.0% | 0.06 | 0.04 | 0.6 | 7.7% | 0.8 | 22% | 0.3 | 13% | --- | 0% | 0.1 | 2% | 0.2 | 14% | |
| Group 3 | ||||||||||||||||||
| #6R1 | 29 | 11% | 20% | 0.17 | 0.34 | −0.5 | 23% | 97 | 10.3 | 29% | 6.7 | 14% | --- | 0% | --- | 0% | 3.0 | 93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rassler, B.; Blinowska, K.; Kaminski, M.; Pfurtscheller, G. Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines 2023, 11, 1028. https://doi.org/10.3390/biomedicines11041028
Rassler B, Blinowska K, Kaminski M, Pfurtscheller G. Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines. 2023; 11(4):1028. https://doi.org/10.3390/biomedicines11041028
Chicago/Turabian StyleRassler, Beate, Katarzyna Blinowska, Maciej Kaminski, and Gert Pfurtscheller. 2023. "Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety" Biomedicines 11, no. 4: 1028. https://doi.org/10.3390/biomedicines11041028
APA StyleRassler, B., Blinowska, K., Kaminski, M., & Pfurtscheller, G. (2023). Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines, 11(4), 1028. https://doi.org/10.3390/biomedicines11041028






