Ischemia Impaired Wound Healing Model in the Rat—Demonstrating Its Ability to Test Proangiogenic Factors
Abstract
1. Introduction
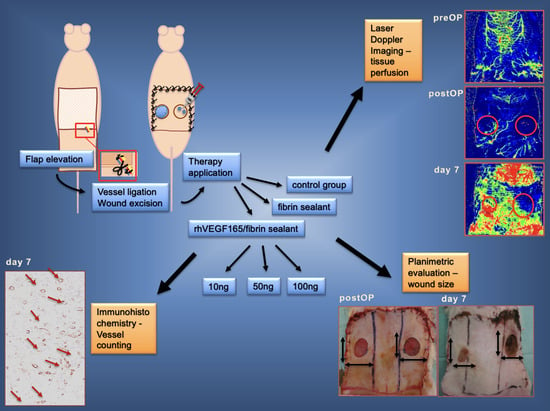
2. Materials and Methods
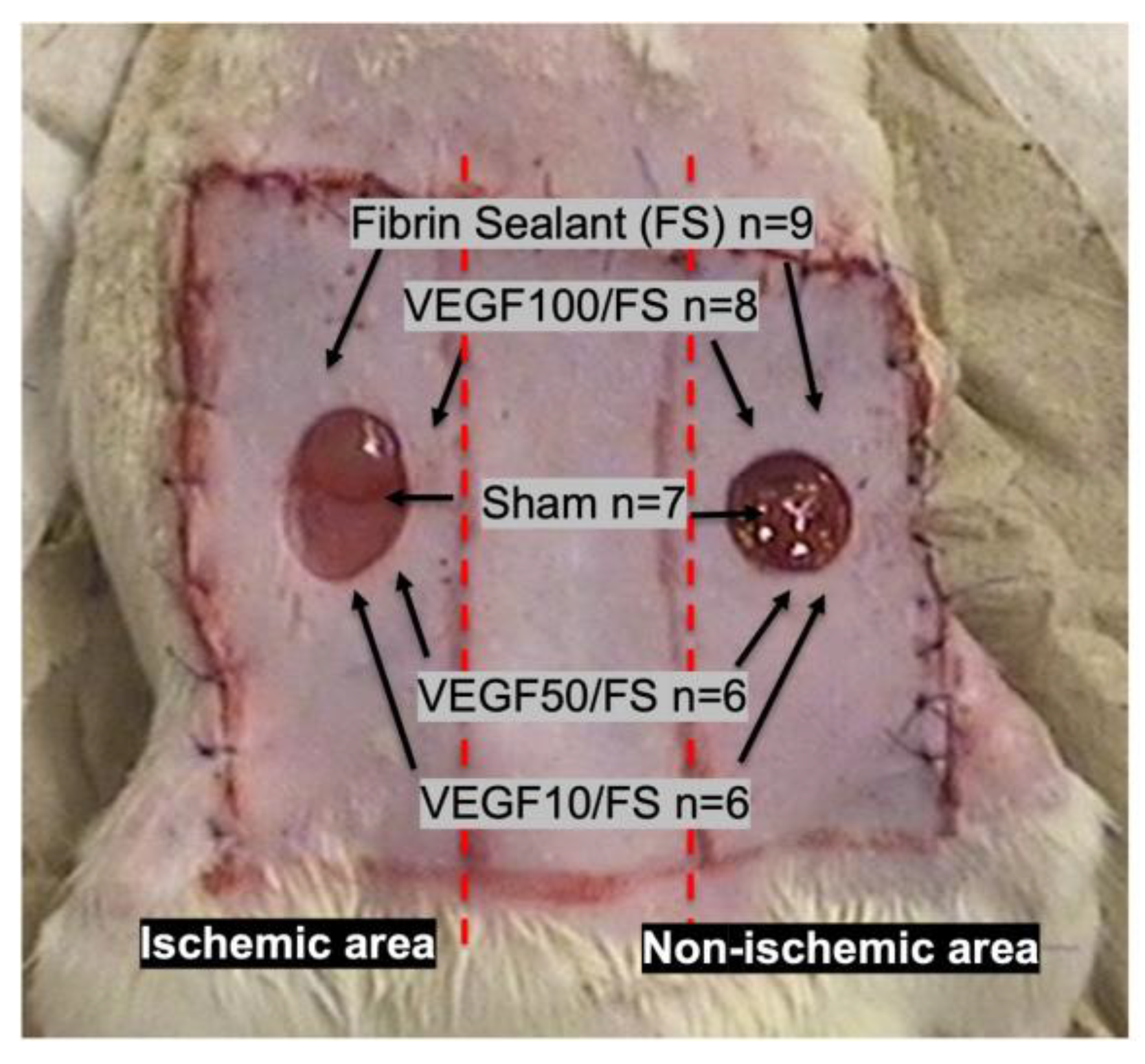
2.1. Animal Model
2.2. Study Groups
2.2.1. Fibrin Biomatrix Group
2.2.2. rhVEGF165 Group
2.2.3. Control Group
2.3. Analyses
2.3.1. Laser Doppler Imaging
2.3.2. Planimetric Analysis
2.3.3. Histological Analysis
2.3.4. Immunohistochemical Analysis
2.3.5. Statistical Analysis
3. Results
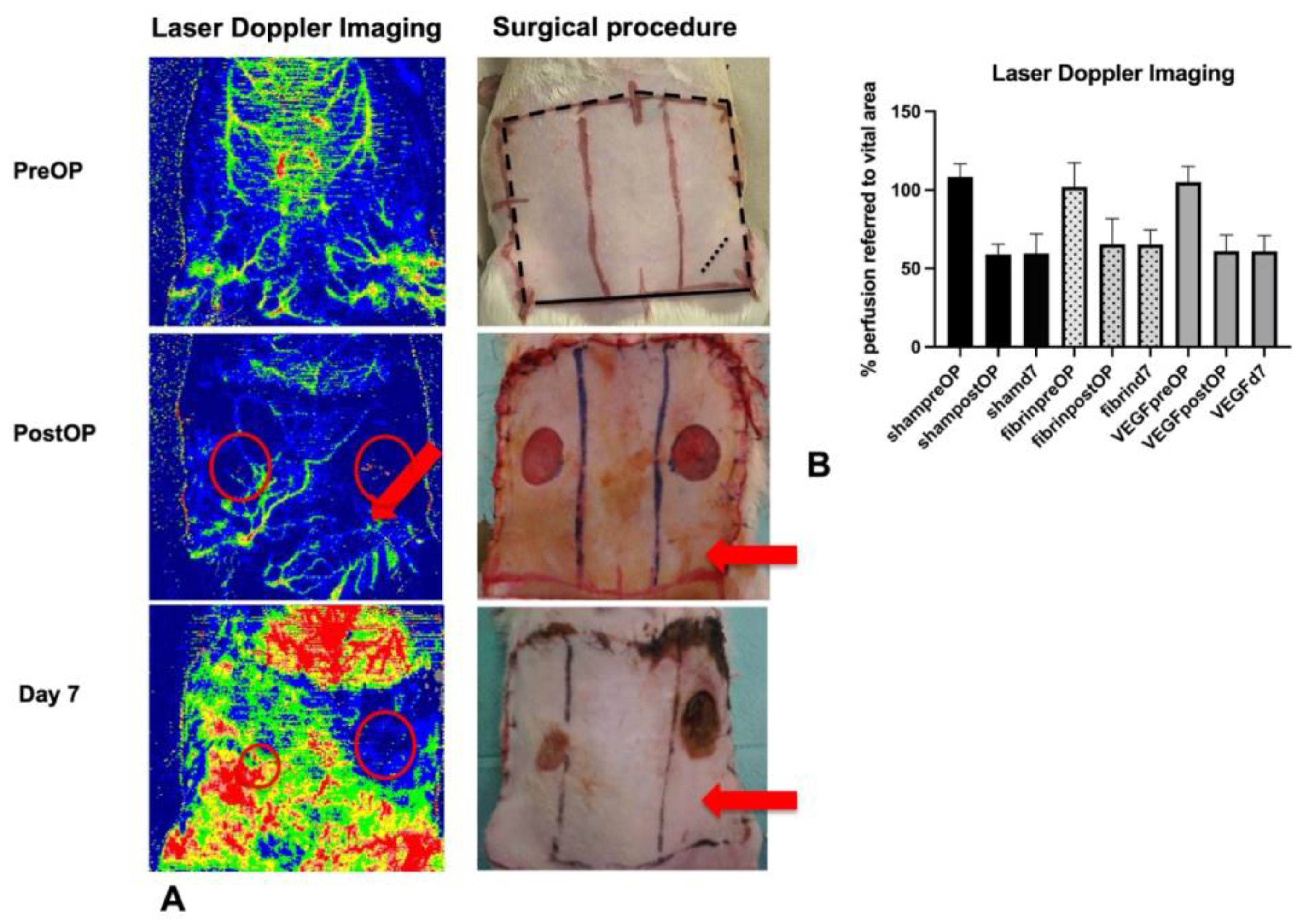
3.1. A Decrease in Flap Perfusion in the Ischemic Flap Area Was Assessed by Laser Doppler Imaging
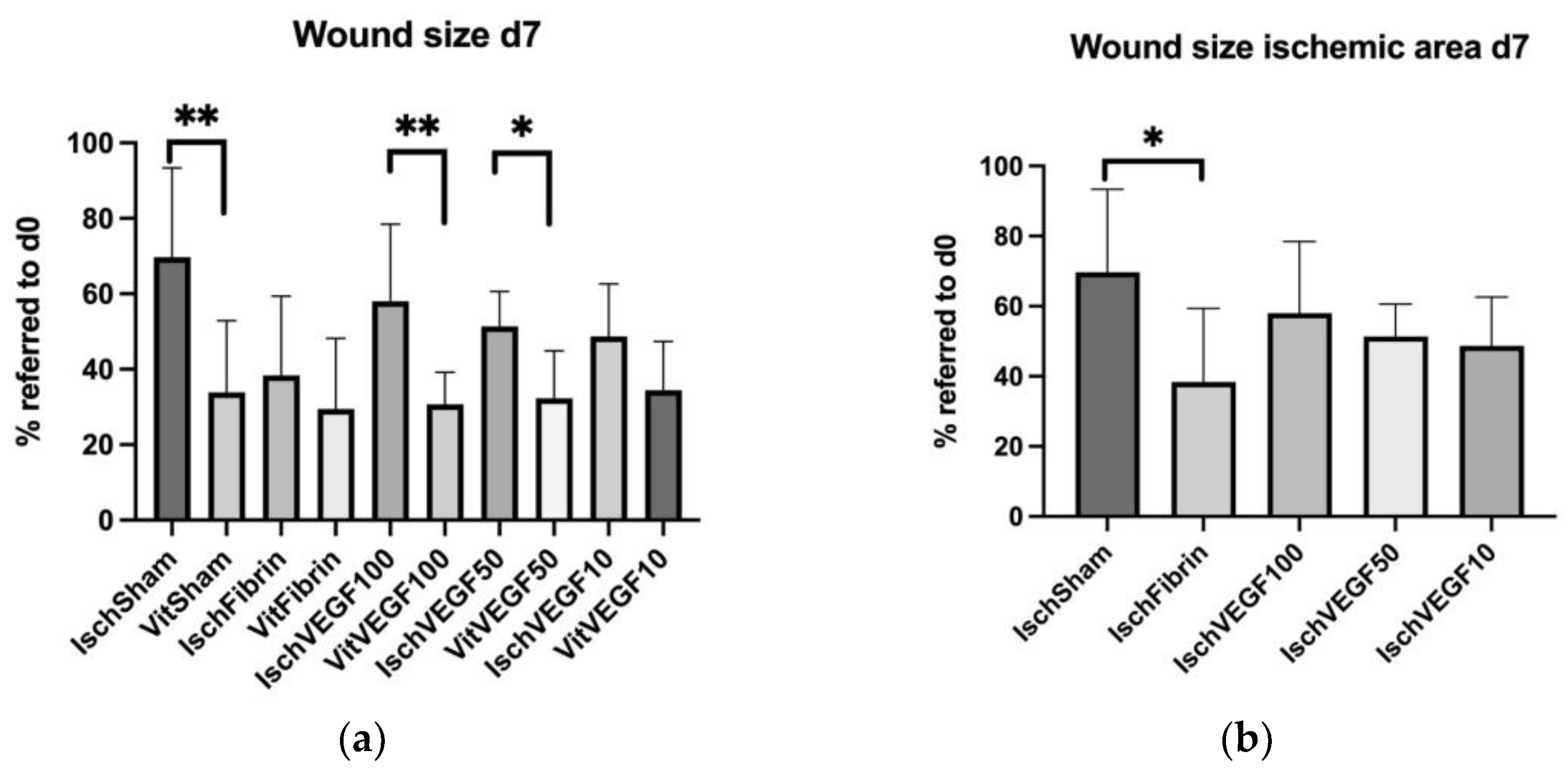
3.2. Planimetrical Analysis Showed Differently Disturbed Wound Healing in the Treated Ischemic Areas
3.3. H&E Staining Shows Differences in the Constitution of Newly Formed Granulation Tissue
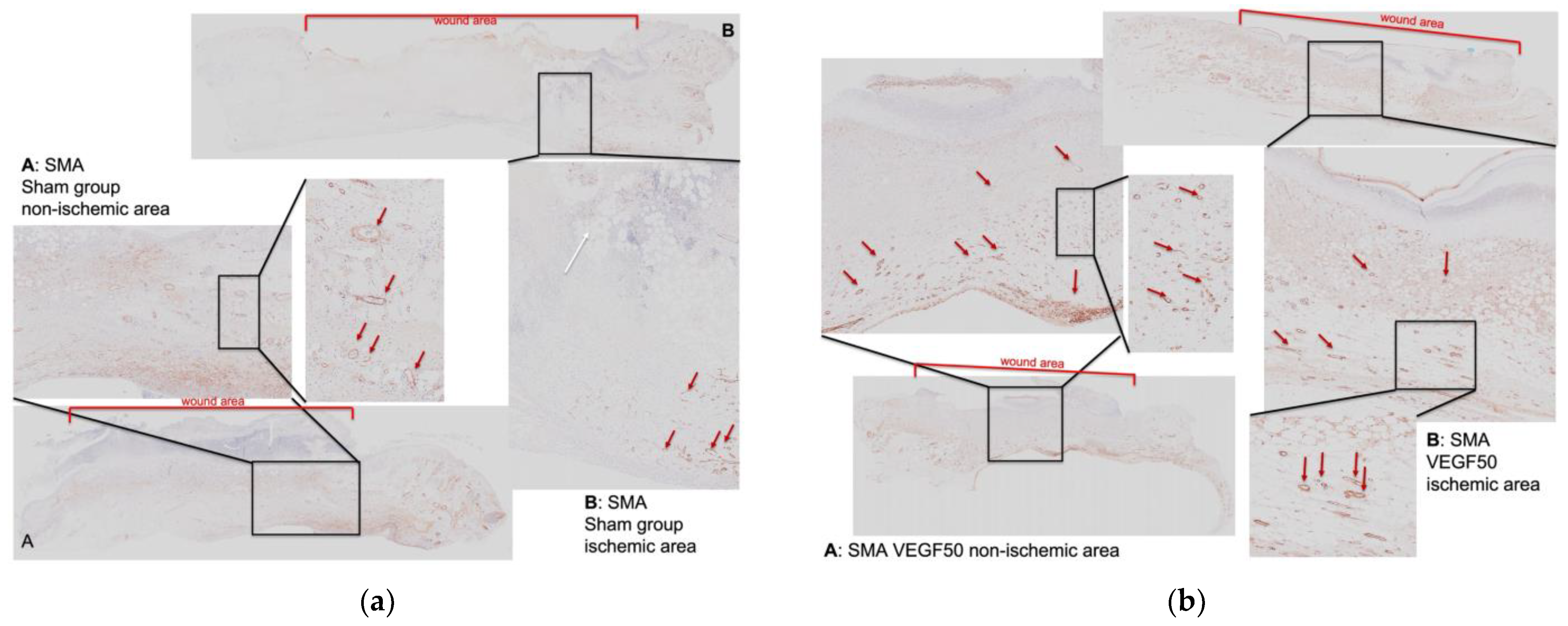
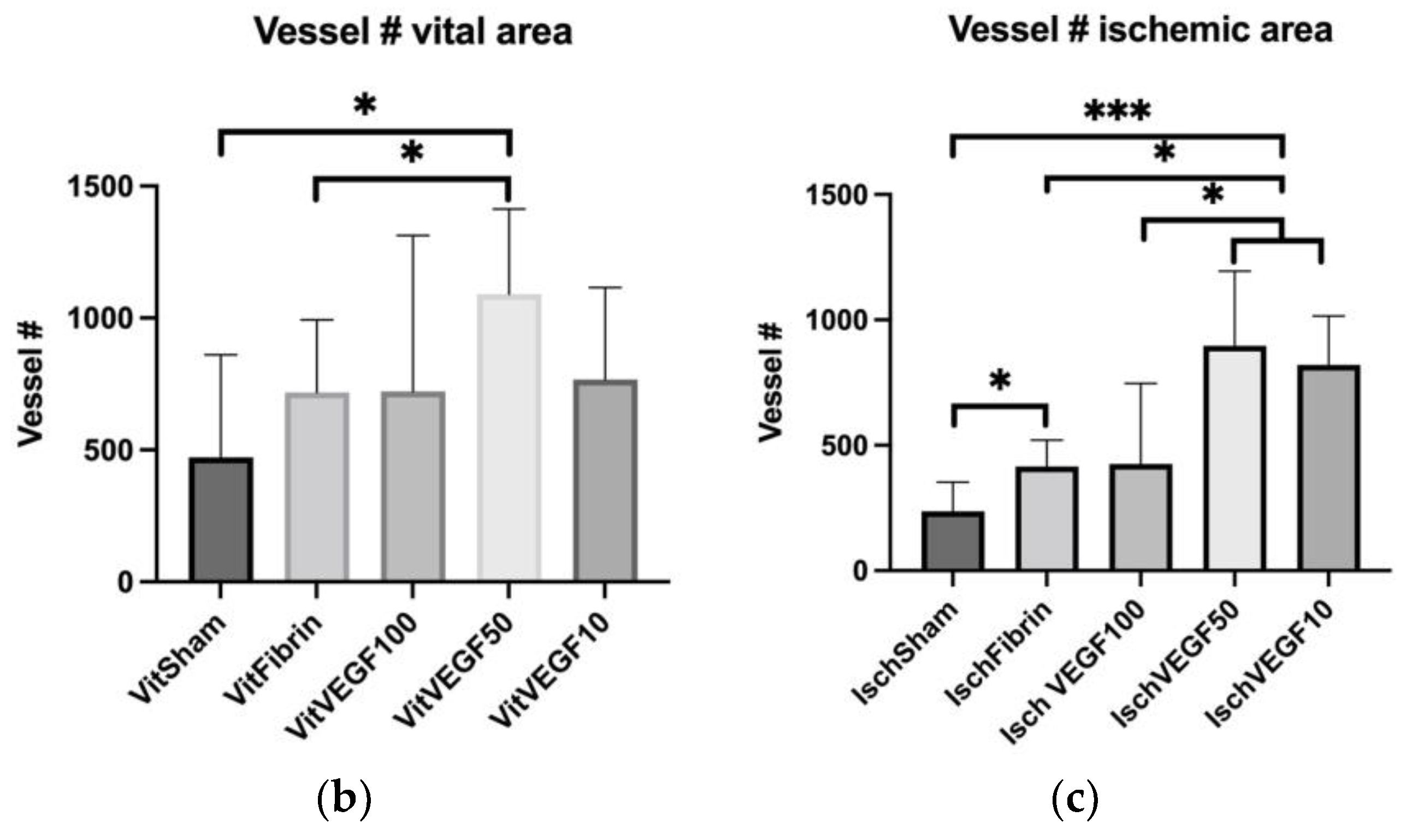
3.4. Detection of Neovascularization in the Wounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Rüttermann, M.; Maier-Hasselmann, A.; Nink-Grebe, B.; Burckhardt, M. Local treatment of chronic wounds: In patients with peripheral vascular disease, chronic venous insufficiency, and diabetes. Dtsch. Arztebl. Int. 2013, 110, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Buote, N.J. Updates in Wound Management and Dressings. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 289–315. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Lefering, R.; Storck, M.; Lawall, H.; Wozniak, G.; Mauckner, P.; Hochlenert, D.; Wetzel-Roth, W.; Sondern, K.; Hahn, M.; et al. NPWT resource use compared with standard moist wound care in diabetic foot wounds: DiaFu randomized clinical trial results. J. Foot Ankle Res. 2022, 15, 72. [Google Scholar] [CrossRef]
- Mittermayr, R.; Antonic, V.; Hartinger, J.; Kaufmann, H.; Redl, H.; Téot, L.; Stojadinovic, A.; Schaden, W. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012, 20, 456–465. [Google Scholar] [CrossRef]
- Robson, M.C.; Phillips, L.G.; Lawrence, W.T.; Bishop, J.B.; Youngerman, J.S.; Hayward, P.G.; Broemeling, L.D.; Heggers, J.P. The Safety and Effect of Topically Applied Recombinant Basic Fibroblast Growth Factor on the Healing of Chronic Pressure Sores. Ann. Surg. 1992, 216, 401–406. [Google Scholar] [CrossRef]
- Mittermayr, R.; Morton, T.; Hofmann, M.; Helgerson, S.; van Griensven, M.; Redl, H. Sustained (rh)VEGF(165) release from a sprayed fibrin biomatrix induces angiogenesis, up-regulation of endogenous VEGF-R2, and reduces ischemic flap necrosis. Wound Repair Regen. 2008, 16, 542–550. [Google Scholar] [CrossRef]
- Mittermayr, R.; Branski, L.; Moritz, M.; Jeschke, M.G.; Herndon, D.N.; Traber, D.; Schense, J.; Gampfer, J.; Goppelt, A.; Redl, H. Fibrin biomatrix-conjugated platelet-derived growth factor AB accelerates wound healing in severe thermal injury. J. Tissue Eng. Regen. Med. 2013, 10, E275–E285. [Google Scholar] [CrossRef]
- Feichtinger, G.A.; Hofmann, A.T.; Slezak, P.; Schuetzenberger, S.; Kaipel, M.; Schwartz, E.; Neef, A.; Nomikou, N.; Nau, T.; van Griensven, M.; et al. Sonoporation Increases Therapeutic Efficacy of Inducible and Constitutive BMP2/7 In Vivo Gene Delivery. Hum. Gene. Methods 2014, 25, 57–71. [Google Scholar] [CrossRef]
- Wolbank, S.; Peterbauer, A.; Wassermann, E.; Hennerbichler, S.; Voglauer, R.; Van Griensven, M.; Duba, H.-C.; Gabriel, C.; Redl, H.; Grillari, R. Labelling of human adipose-derived stem cells for non-invasive in vivo cell tracking. Cell Tissue Bank. 2006, 8, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Kraskiewicz, H.; Paprocka, M.; Bielawska-Pohl, A.; Krawczenko, A.; Panek, K.; Kaczyńska, J.; Szyposzyńska, A.; Psurski, M.; Kuropka, P.; Klimczak, A. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Res. Ther. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Nunan, R.; Harding, K.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model. Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095.e1–2105.e1. [Google Scholar] [CrossRef]
- Peirce, S.M.; Skalak, T.C.; Rodeheaver, G.T. Ischemia-reperfusion injury in chronic pressure ulcer formation: A skin model in the rat. Wound Repair Regen. 2000, 8, 68–76. [Google Scholar] [CrossRef]
- Wassermann, E.; van Griensven, M.; Gstaltner, K.; Oehlinger, W.; Schrei, K.; Redl, H. A chronic pressure ulcer model in the nude mouse. Wound Repair Regen. 2009, 17, 480–484. [Google Scholar] [CrossRef]
- Stadler, I.; Zhang, R.-Y.; Oskoui, P.; Whittaker, M.B.S.; Lanzafame, R.J. Development of a Simple, Noninvasive, Clinically Relevant Model of Pressure Ulcers in the Mouse. J. Investig. Surg. 2004, 17, 221–227. [Google Scholar] [CrossRef]
- Salcido, R.; Popescu, A.; Ahn, C. Animal Models in Pressure Ulcer Research. J. Spinal. Cord Med. 2007, 30, 107–116. [Google Scholar] [CrossRef]
- Broadley, K.N.; Aquino, A.M.; Hicks, B.; Ditesheim, J.A.; McGee, G.S.; Demetriou, A.A.; Woodward, S.C.; Davidson, J.M. The diabetic rat as an impaired wound healing model: Stimulatory effects of transforming growth factor-beta and basic fibroblast growth factor. Biotechnol. Ther. 1989, 1, 55–68. [Google Scholar]
- Cromack, D.T.; Porras-Reyes, B.; Purdy, J.A.; Pierce, G.F.; Mustoe, T.A. Acceleration of tissue repair by transforming growth factor beta 1: Identification of in vivo mechanism of action with radiotherapy-induced specific healing deficits. Surgery 1993, 113, 36–42. [Google Scholar] [PubMed]
- Curtsinger, L.J.; Pietsch, J.D.; Brown, G.L.; Von Fraunhofer, A.; Ackerman, D.; Polk, H.C.; Schultz, G.S. Reversal of Adriamycin-impaired wound healing by transforming growth factor-beta. Surg. Gynecol. Obstet. 1989, 168, 517–522. [Google Scholar]
- Trujillo, A.N.; Kesl, S.L.; Sherwood, J.; Wu, M.G.L. Demonstration of the rat ischemic skin wound model. J. Vis. Exp. 2015, 6, 2–8. [Google Scholar]
- Chen, C.; Schultz, G.S.; Bloch, M.; Edwards, P.D.; Tebes, S.; Mast, B.A. Molecular and mechanistic validation of delayed healing rat wounds as a model for human chronic wounds. Wound Repair Regen. 1999, 7, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xia, Y.; Roth, S.I.; Gruskin, E.; Mustoe, T.A. Transforming Growth Factor-beta1 Fails to Stimulate Wound Healing and Impairs Its Signal Transduction in an Aged Ischemic Ulcer Model Importance of Oxygen and Age. Am. J. Pathol. 1999, 154, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Martin, J.R.; Sarett, S.M.; Pollins, A.C.; Cardwell, N.L.; Davidson, J.M.; Guelcher, S.A.; Nanney, L.B.; Duvall, C.L. Porcine Ischemic Wound-Healing Model for Preclinical Testing of Degradable Biomaterials. Tissue Eng. Part C Methods 2017, 23, 754–762. [Google Scholar] [CrossRef]
- Leong-Poi, H.; Kuliszewski, M.A.; Lekas, M.; Sibbald, M.; Teichert-Kuliszewska, K.; Klibanov, A.L.; Stewart, D.J.; Lindner, J.R. Therapeutic Arteriogenesis by Ultrasound-Mediated VEGF165 Plasmid Gene Delivery to Chronically Ischemic Skeletal Muscle. Circ. Res. 2007, 101, 295–303. [Google Scholar] [CrossRef]
- Hofmann, A.T.; Neumann, S.; Ferguson, J.; Redl, H.; Mittermayr, R. A Rodent Excision Model for Ischemia-Impaired Wound Healing. Tissue Eng. Part C Methods 2017, 23, 995–1002. [Google Scholar] [CrossRef]
- Weihs, A.M.; Fuchs, C.; Teuschl, A.H.; Hartinger, J.; Slezak, P.; Mittermayr, R.; Redl, H.; Junger, W.G.; Sitte, H.H.; Rünzler, D. Shock Wave Treatment Enhances Cell Proliferation and Improves Wound Healing by ATP Release-coupled Extracellular Signal-regulated Kinase (ERK) Activation. J. Biol. Chem. 2014, 289, 27090–27104. [Google Scholar] [CrossRef]
- Sacchi, V.; Mittermayr, R.; Hartinger, J.; Martino, M.M.; Lorentz, K.M.; Wolbank, S.; Hofmann, A.; Largo, R.A.; Marschall, J.S.; Groppa, E.; et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc. Natl. Acad. Sci. USA 2014, 111, 6952–6957. [Google Scholar] [CrossRef]
- Sahni, A.; Francis, C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000, 96, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T. Molecular Mechanisms of VEGF-A Action during Tissue Repair. J. Investig. Dermatol. Symp. Proc. 2006, 11, 79–86. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A.; Perez-Amodio, S.; Rubio, N.; Vila, O.F.; Navarro-Requena, C.; Castaño, O.; Sanchez-Ferrero, A.; Marti-Munoz, J.; Alsina-Giber, M.; et al. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Berger, A.C.; Feldman, A.L.; Gnant, M.F.; Kruger, E.A.; Sim, B.L.; Hewitt, S.; Figg, W.D.; Alexander, H.; Libutti, S.K. The Angiogenesis Inhibitor, Endostatin, Does Not Affect Murine Cutaneous Wound Healing. J. Surg. Res. 2000, 91, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, J.; Tam, B.Y.; Sundram, U.; von Degenfeld, G.; Blau, H.M.; Kuo, C.J.; Cooke, J.P. Discordant effects of a soluble VEGF receptor on wound healing and angiogenesis. Gene Ther. 2004, 11, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Abbade, L.P.F.; Jr, R.S.F.; Dos Santos, L.D.; Barraviera, B. Chronic venous ulcers: A review on treatment with fibrin sealant and prognostic advances using proteomic strategies. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190101. [Google Scholar] [CrossRef]
- Clark, R.A.F. Fibrin and wound healing. Ann. N. Y. Acad. Sci. 2001, 936, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999, 77, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Keller, G.A.; Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 1993, 4, 1317–1326. [Google Scholar] [CrossRef]
- Ehrbar, M.; Djonov, V.G.; Schnell, C.; Tschanz, S.A.; Martiny-Baron, G.; Schenk, U.; Wood, J.; Burri, P.H.; Hubbell, J.A.; Zisch, A.H. Cell-Demanded Liberation of VEGF121 From Fibrin Implants Induces Local and Controlled Blood Vessel Growth. Circ. Res. 2004, 94, 1124–1132. [Google Scholar] [CrossRef]
- Michlits, W.; Mittermayr, R.; Schäfer, R.; Redl, H.; Aharinejad, S. Fibrin-embedded administration of VEGF plasmid enhances skin flap survival. Wound Repair Regen. 2007, 15, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Distler, J.H.W.; Scheid, A.; Acker, T.; Hirth, A.; Rethage, J.; Michel, B.A.; Gay, R.E.; Müller-Ladner, U.; Matucci-Cerinic, M.; et al. Uncontrolled Expression of Vascular Endothelial Growth Factor and Its Receptors Leads to Insufficient Skin Angiogenesis in Patients With Systemic Sclerosis. Circ. Res. 2004, 95, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.; Angle, N.; et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 809–813. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, A.T.; Slezak, P.; Neumann, S.; Ferguson, J.; Redl, H.; Mittermayr, R. Ischemia Impaired Wound Healing Model in the Rat—Demonstrating Its Ability to Test Proangiogenic Factors. Biomedicines 2023, 11, 1043. https://doi.org/10.3390/biomedicines11041043
Hofmann AT, Slezak P, Neumann S, Ferguson J, Redl H, Mittermayr R. Ischemia Impaired Wound Healing Model in the Rat—Demonstrating Its Ability to Test Proangiogenic Factors. Biomedicines. 2023; 11(4):1043. https://doi.org/10.3390/biomedicines11041043
Chicago/Turabian StyleHofmann, Anna T., Paul Slezak, Sabine Neumann, James Ferguson, Heinz Redl, and Rainer Mittermayr. 2023. "Ischemia Impaired Wound Healing Model in the Rat—Demonstrating Its Ability to Test Proangiogenic Factors" Biomedicines 11, no. 4: 1043. https://doi.org/10.3390/biomedicines11041043
APA StyleHofmann, A. T., Slezak, P., Neumann, S., Ferguson, J., Redl, H., & Mittermayr, R. (2023). Ischemia Impaired Wound Healing Model in the Rat—Demonstrating Its Ability to Test Proangiogenic Factors. Biomedicines, 11(4), 1043. https://doi.org/10.3390/biomedicines11041043