Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis
Abstract
1. Introduction
2. Materials and Methods
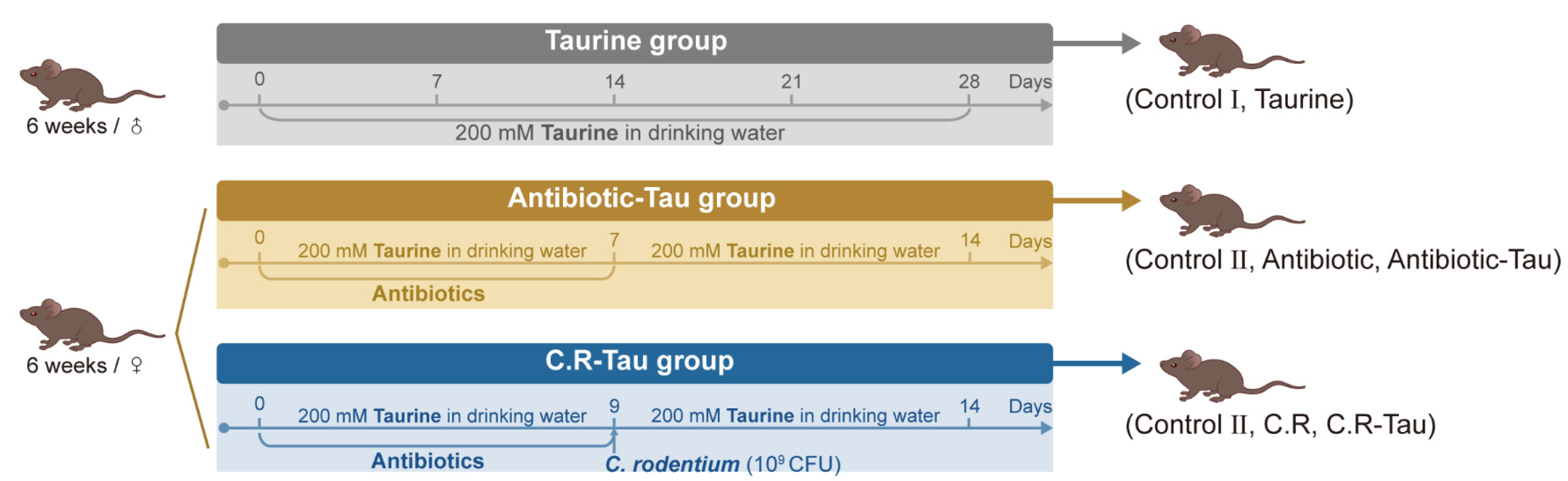
2.1. Animals and Treatments
2.2. Microbiome Analysis
2.3. GroEL Gene-Based Analysis of Lactobacillus Species
2.4. Bile Acid Measurements
2.5. Quantification of Cytokines in Colon
2.6. Determination of CFU
2.7. Statistical Analysis
3. Results
3.1. Taurine Supplementation Regulated Gut Microbiota Composition in Healthy Mice
3.2. Taurine Supplementation Affected Bile Acid Concentration and Composition in Healthy Mice
3.3. Taurine Supplementation Regulated Gut Microbiota Composition and Recovery of Lactobacillus in Antibiotic-Treated Mice
3.4. Taurine Supplementation Boosted Immunity in Antibiotic-Treated Mice
3.5. Taurine Supplementation Enhanced Colonization Resistance in Mice Infected with C. rodentium
3.6. Taurine Supplementation Regulated the Diversity and Composition of Gut Microbiota in Mice Infected with C. rodentium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huxtable, R.J. Physiological Actions of Taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- Ripps, H.; Shen, W. Review: Taurine: A “Very Essential” Amino Acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar] [PubMed]
- Duszka, K. Versatile Triad Alliance: Bile Acid, Taurine and Microbiota. Cells 2022, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Bertolone, L.; Roy, M.K.; Hay, A.M.; Morrison, E.J.; Stefanoni, D.; Fu, X.; Kanias, T.; Kleinman, S.; Dumont, L.J.; Stone, M.; et al. Impact of Taurine on Red Blood Cell Metabolism and Implications for Blood Storage. Transfusion 2020, 60, 1212–1226. [Google Scholar] [CrossRef]
- De Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Cook, A.M.; Denger, K. Metabolism of Taurine in Microorganisms: A Primer in Molecular Biodiversity? Adv. Exp. Med. Biol. 2006, 583, 3–13. [Google Scholar] [CrossRef]
- Du, G.; Liu, Z.; Yu, Z.; Zhuo, Z.; Zhu, Y.; Zhou, J.; Li, Y.; Chen, H. Taurine Represses Age-associated Gut Hyperplasia in Drosophila via Counteracting Endoplasmic Reticulum Stress. Aging Cell 2021, 20, e13319. [Google Scholar] [CrossRef]
- Zhao, Z.; Satsu, H.; Fujisawa, M.; Hori, M.; Ishimoto, Y.; Totsuka, M.; Nambu, A.; Kakuta, S.; Ozaki, H.; Shimizu, M. Attenuation by Dietary Taurine of Dextran Sulfate Sodium-Induced Colitis in Mice and of THP-1-Induced Damage to Intestinal Caco-2 Cell Monolayers. Amino Acids 2008, 35, 217–224. [Google Scholar] [CrossRef]
- Son, M.W.; Ko, J.I.; Doh, H.M.; Kim, W.B.; Park, T.S.; Shim, M.J.; Kim, B.K. Protective Effect of Taurine on TNBS-Induced Inflammatory Bowel Disease in Rats. Arch. Pharm. Res. 1998, 21, 531–536. [Google Scholar] [CrossRef]
- Grosheva, I.; Zheng, D.; Levy, M.; Polansky, O.; Lichtenstein, A.; Golani, O.; Dori-Bachash, M.; Moresi, C.; Shapiro, H.; Del Mare-Roumani, S.; et al. High-Throughput Screen Identifies Host and Microbiota Regulators of Intestinal Barrier Function. Gastroenterology 2020, 159, 1807–1823. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Z.; Shen, S.; Shan, W. Effects of Taurine on Gut Microbiota and Metabolism in Mice. Amino Acids 2016, 48, 1601–1617. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qin, Y.; Chen, M.; Zhang, Y.; Wang, X.; Dong, T.; Chen, G.; Sun, X.; Lu, T.; White, R.A.; et al. Gestational Diabetes Mellitus Is Associated with the Neonatal Gut Microbiota and Metabolome. BMC Med. 2021, 19, 120. [Google Scholar] [CrossRef]
- Fang, H.; Meng, F.; Piao, F.; Jin, B.; Li, M.; Li, W. Effect of Taurine on Intestinal Microbiota and Immune Cells in Peyer’s Patches of Immunosuppressive Mice. Adv. Exp. Med. Biol. 2019, 1155, 13–24. [Google Scholar] [CrossRef]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection Trains the Host for Microbiota-Enhanced Resistance to Pathogens. Cell 2021, 184, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.M.; Hu, Z.; Klimko, C.; Narayanan, S.; Deberardinis, R.; Sperandio, V. The Gut Commensal Bacteroides Thetaiotaomicron Exacerbates Enteric Infection through Modification of the Metabolic Landscape. Cell Host Microbe 2014, 16, 759–769. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Mao, B.; Yang, Q.; Zhao, J.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Microbial Biogeography and Core Microbiota of the Rat Digestive Tract. Sci. Rep. 2017, 7, 45840. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the Analysis of High-Throughput Sequencing Datasets: Characterizing RNA-Seq, 16S RRNA Gene Sequencing and Selective Growth Experiments by Compositional Data Analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef]
- Xie, M.; Pan, M.; Jiang, Y.; Liu, X.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. GroEL Gene-Based Phylogenetic Analysis of Lactobacillus Species by High-Throughput Sequencing. Genes 2019, 10, 530. [Google Scholar] [CrossRef]
- John, C.; Werner, P.; Worthmann, A.; Wegner, K.; Tödter, K.; Scheja, L.; Rohn, S.; Heeren, J.; Fischer, M. A Liquid Chromatography-Tandem Mass Spectrometry-Based Method for the Simultaneous Determination of Hydroxy Sterols and Bile Acids. J. Chromatogr. A 2014, 1371, 184–195. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, W.; Yu, L.; Tian, F.; Wang, G.; Lu, W.; Narbad, A.; Chen, W.; Zhai, Q. Evidence from Comparative Genomic Analyses Indicating That Lactobacillus-Mediated Irritable Bowel Syndrome Alleviation is Mediated by Conjugated Linoleic Acid Synthesis. Food Funct. 2021, 12, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Blachier, F. Amino Acids in Intestinal Physiology and Health. Adv. Exp. Med. Biol. 2020, 1265, 1–20. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, S.; Zou, D.; Dong, D.; He, X.; Liu, N.; Liu, W.; Huang, L. Metabolic Shifts and Structural Changes in the Gut Microbiota upon Branched-Chain Amino Acid Supplementation in Middle-Aged Mice. Amino Acids 2016, 48, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Sjövall, J. Dietary Glycine and Taurine on Bile Acid Conjugation in Man. Bile Acids and Steroids 75. Proc. Soc. Exp. Biol. Med. 1959, 100, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Hardison, W.G.M. Hepatic Taurine Concentration and Dietary Taurine as Regulators of Bile Acid Conjugation with Taurine. Gastroenterology 1978, 75, 71–75. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Tanaka, H.; Hashiba, H.; Kok, J.; Mierau, I. Bile Salt Hydrolase of Bifidobacterium Longum—Biochemical and Genetic Characterization. Appl. Environ. Microbiol. 2000, 66, 2502–2512. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Yin, J.; Lin, L.; Zhou, Y.; Hua, G.; Cao, P.; Ang, E.L.; Zhao, H.; Yuchi, Z.; et al. Biochemical and Structural Investigation of Taurine:2-Oxoglutarate Aminotransferase from Bifidobacterium Kashiwanohense. Biochem. J. 2019, 476, 1605–1619. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Antibiotic-Induced Gut Dysbiosis and Barrier Disruption and the Potential Protective Strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 1427–1452. [Google Scholar] [CrossRef]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.-C.; Armougom, F.; Robert, C.; Audoly, G.; Papazian, L.; Raoult, D. High-Level Colonisation of the Human Gut by Verrucomicrobia Following Broad-Spectrum Antibiotic Treatment. Int. J. Antimicrob. Agents 2013, 41, 149–155. [Google Scholar] [CrossRef]
- Hansen, C.H.F.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early Life Treatment with Vancomycin Propagates Akkermansia Muciniphila and Reduces Diabetes Incidence in the NOD Mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef]
- Hernández, E.; Bargiela, R.; Diez, M.S.; Friedrichs, A.; Pérez-Cobas, A.E.; Gosalbes, M.J.; Knecht, H.; Martínez-Martínez, M.; Seifert, J.; von Bergen, M.; et al. Functional Consequences of Microbial Shifts in the Human Gastrointestinal Tract Linked to Antibiotic Treatment and Obesity. Gut Microbes 2013, 4, 306–315. [Google Scholar] [CrossRef]
- Tsuchioka, T.; Fujiwara, T.; Sunagawa, M. Effects of Glutamic Acid and Taurine on Total Parenteral Nutrition. J. Pediatr. Surg. 2006, 41, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-R.; Liu, X.-C.; Zhang, J.-S.; Ji, C.-Y.; Qi, Y.-F. Taurine Drinking Attenuates the Burden of Intestinal Adult Worms and Muscle Larvae in Mice with Trichinella Spiralis Infection. Parasitol. Res. 2013, 112, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Mi, Y.; Liu, L.; Lv, C.; Zeng, W.; Zhang, C.; Li, J. Taurine Regulates Mucosal Barrier Function to Alleviate Lipopolysaccharide-Induced Duodenal Inflammation in Chicken. Amino Acids 2018, 50, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kellingray, L.; Le Gall, G.; Zhao, J.; Zhang, H.; Narbad, A.; Zhai, Q.; Chen, W. The Divergent Restoration Effects of Lactobacillus Strains in Antibiotic-Induced Dysbiosis. J. Funct. Foods 2018, 51, 142–152. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef]
- Deshmukh, H.S.; Liu, Y.; Menkiti, O.R.; Mei, J.; Dai, N.; O’Leary, C.E.; Oliver, P.M.; Kolls, J.K.; Weiser, J.N.; Worthen, G.S. The Microbiota Regulates Neutrophil Homeostasis and Host Resistance to Escherichia Coli K1 Sepsis in Neonatal Mice. Nat. Med. 2014, 20, 524–530. [Google Scholar] [CrossRef]
- Ganal, S.C.; Sanos, S.L.; Kallfass, C.; Oberle, K.; Johner, C.; Kirschning, C.; Lienenklaus, S.; Weiss, S.; Staeheli, P.; Aichele, P.; et al. Priming of Natural Killer Cells by Nonmucosal Mononuclear Phagocytes Requires Instructive Signals from Commensal Microbiota. Immunity 2012, 37, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota Regulates Immune Defense against Respiratory Tract Influenza A Virus Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Schuller-Levis, G.B.; Park, E. Taurine and Its Chloramine: Modulators of Immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, N.; Zhang, F.; Yue, W.; Liang, M. Effect of Taurine on Leucocyte Function. Eur. J. Pharmacol. 2009, 616, 275–280. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef]
- Molloy, M.J.; Grainger, J.R.; Bouladoux, N.; Hand, T.W.; Koo, L.Y.; Naik, S.; Quinones, M.; Dzutsev, A.K.; Gao, J.-L.; Trinchieri, G.; et al. Intraluminal Containment of Commensal Outgrowth in the Gut during Infection-Induced Dysbiosis. Cell Host Microbe 2013, 14, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Behnsen, J.; Jellbauer, S.; Wong, C.P.; Edwards, R.A.; George, M.D.; Ouyang, W.; Raffatellu, M. The Cytokine IL-22 Promotes Pathogen Colonization by Suppressing Related Commensal Bacteria. Immunity 2014, 40, 262–273. [Google Scholar] [CrossRef]
- Forte, E.; Borisov, V.B.; Falabella, M.; Colaço, H.G.; Tinajero-Trejo, M.; Poole, R.K.; Vicente, J.B.; Sarti, P.; Giuffrè, A. The Terminal Oxidase Cytochrome Bd Promotes Sulfide-Resistant Bacterial Respiration and Growth. Sci. Rep. 2016, 6, 23788. [Google Scholar] [CrossRef]







| Bile Acids | Molecular Formula | Control Ⅰ (pg/mg) | Taurine (pg/mg) | p-Value |
|---|---|---|---|---|
| TLCA | C26H45NO5S | 0.84 ± 0.24 | 3.75 ± 0.51 | 0.0003 *** |
| TDCA | C26H45NO6S | 6.78 ± 1.58 | 36.37 ± 4.69 | <0.0001 **** |
| THDCA | C26H45NO6S | 1.59 ± 0.39 | 7.22 ± 0.95 | <0.0001 **** |
| TUDCA | C26H45NO6S | 0.64 ± 0.08 | 1.42 ± 0.28 | 0.0218 * |
| T-β-MCA | C26H45NO7S | 23.27 ± 4.09 | 99.09 ± 10.84 | <0.0001 **** |
| TCA | C26H45NO7S | 9.13 ± 1.16 | 23.60 ± 4.67 | 0.0148 * |
| TCDCA | C26H45NO6S | 3.53 ± 0.88 | 3.85 ± 1.20 | 0.8402 |
| LCA | C24H40O3 | 3187.10 ± 579.74 | 1687.12 ± 232.76 | 0.0351 * |
| CDCA | C24H40O4 | 218.27 ± 48.82 | 241.55 ± 68.35 | 0.8002 |
| DCA | C24H40O4 | 58,850.10 ± 9636.26 | 45,730.93 ± 4574.82 | 0.2585 |
| UDCA | C24H40O4 | 552.15 ± 144.52 | 555.15 ± 139.47 | 0.9889 |
| HDCA | C24H40O4 | 3801.19 ± 837.05 | 1902.31 ± 203.96 | 0.0509 |
| CA | C24H40O5 | 7746.34 ± 2424.70 | 10,806.87 ± 2805.21 | 0.4503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Li, M.; Yu, L.; Tian, F.; Zhao, J.; Zhai, Q. Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis. Biomedicines 2023, 11, 1048. https://doi.org/10.3390/biomedicines11041048
Qian W, Li M, Yu L, Tian F, Zhao J, Zhai Q. Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis. Biomedicines. 2023; 11(4):1048. https://doi.org/10.3390/biomedicines11041048
Chicago/Turabian StyleQian, Weike, Mingyang Li, Leilei Yu, Fengwei Tian, Jianxin Zhao, and Qixiao Zhai. 2023. "Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis" Biomedicines 11, no. 4: 1048. https://doi.org/10.3390/biomedicines11041048
APA StyleQian, W., Li, M., Yu, L., Tian, F., Zhao, J., & Zhai, Q. (2023). Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis. Biomedicines, 11(4), 1048. https://doi.org/10.3390/biomedicines11041048







