Inflammation and Syndecan-4 Shedding from Cardiac Cells in Ischemic and Non-Ischemic Heart Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples from Patients
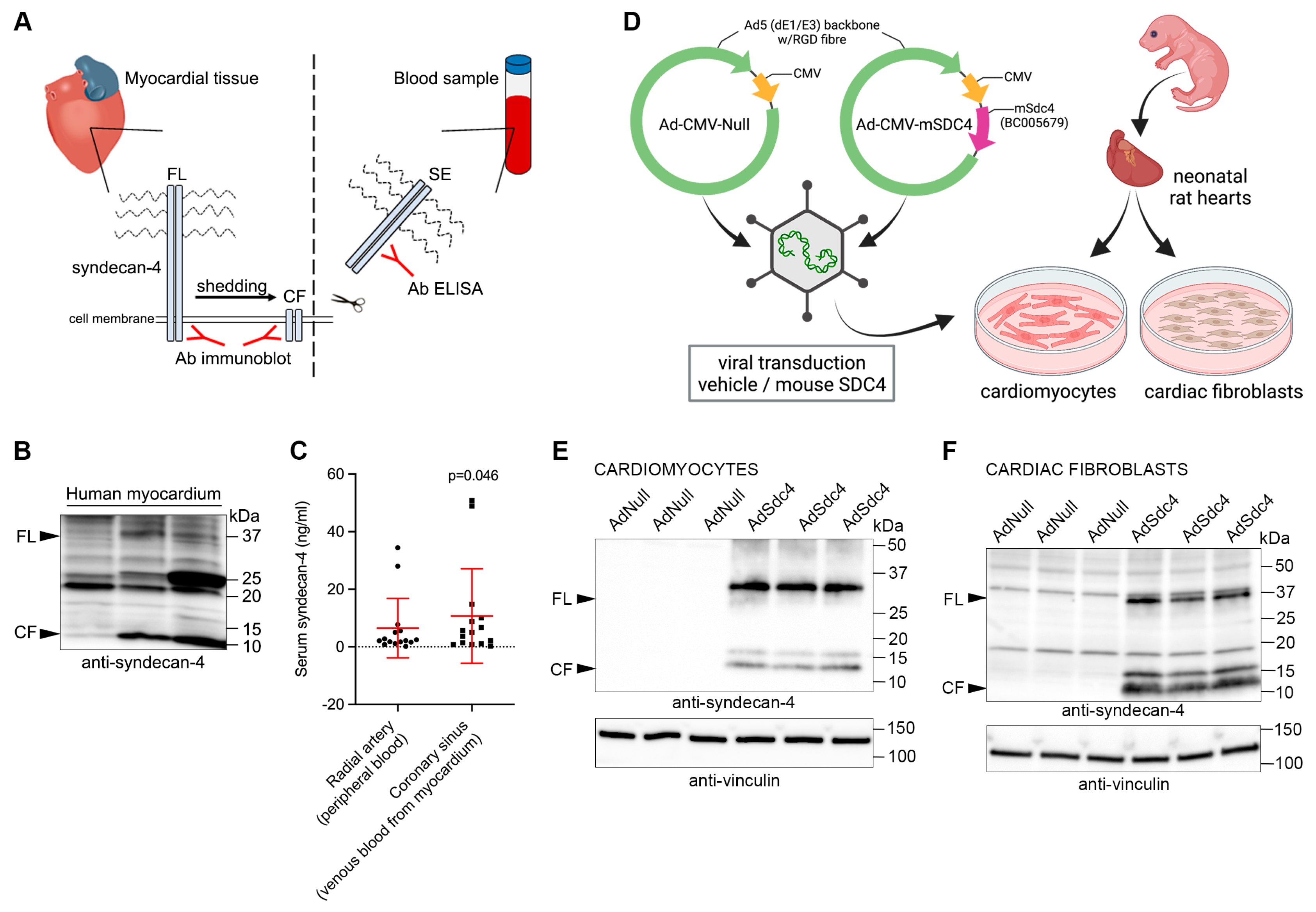
2.2. Myocardial Tissue Samples from Patients
2.3. Cardiac Myocyte and Fibroblast Cultures from Neonatal Rats
2.4. ELISA Analyses of Serum Samples
2.5. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.6. Protein Isolation and Immunoblotting
2.7. Statistics
3. Results
3.1. Syndecan-4 Is Shed in and from the Human Heart
3.2. Serum Syndecan-4 Levels Do Not Reflect the Inflammatory Status of the Heart in Patients with Heart Disease
3.3. Interleukin 1β-Increased Syndecan-4 Levels Is Attenuated by Immunomodulatory Therapy in Cultured Cardiomyocytes
3.4. Interleukin 1β-Increased Syndecan-4 Levels and Shedding Is Attenuated by Immunomodulatory Therapy in Cultured Cardiac Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarhene, M.; Wang, Y.; Wei, J.; Huang, Y.; Li, M.; Li, L.; Acheampong, E.; Zhengcan, Z.; Xiaoyan, Q.; Yunsheng, X.; et al. Biomarkers in heart failure: The past, current and future. Heart Fail. Rev. 2019, 24, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Reina-Couto, M.; Pereira-Terra, P.; Quelhas-Santos, J.; Silva-Pereira, C.; Albino-Teixeira, A.; Sousa, T. Inflammation in Human Heart Failure: Major Mediators and Therapeutic Targets. Front. Physiol. 2021, 12, 746494. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Gerull, B.; Klaassen, S.; Brodehl, A. The Genetic Landscape of Cardiomyopathies. In Genetic Causes of Cardiac Disease; Erdmann, J., Moretti, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 45–91. [Google Scholar] [CrossRef]
- Brodehl, A.; Belke, D.D.; Garnett, L.; Martens, K.; Abdelfatah, N.; Rodriguez, M.; Diao, C.; Chen, Y.X.; Gordon, P.M.; Nygren, A.; et al. Transgenic mice overexpressing desmocollin-2 (DSC2) develop cardiomyopathy associated with myocardial inflammation and fibrotic remodeling. PLoS ONE 2017, 12, e0174019. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Bajaj, N.S.; Gupta, K.; Gharpure, N.; Pate, M.; Chopra, L.; Kalra, R.; Prabhu, S.D. Effect of immunomodulation on cardiac remodelling and outcomes in heart failure: A quantitative synthesis of the literature. ESC Heart Fail. 2020, 7, 1319–1330. [Google Scholar] [CrossRef]
- Couchman, J.R. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010, 26, 89–114. [Google Scholar] [CrossRef]
- Choi, Y.; Chung, H.; Jung, H.; Couchman, J.R.; Oh, E.S. Syndecans as cell surface receptors: Unique structure equates with functional diversity. Matrix Biol. 2011, 30, 93–99. [Google Scholar] [CrossRef]
- Thota, L.N.R.; Chignalia, A.Z. The role of the glypican and syndecan families of heparan sulfate proteoglycans in cardiovascular function and disease. Am. J. Physiol. Cell Physiol. 2022, 323, C1052–c1060. [Google Scholar] [CrossRef]
- Bishop, J.R.; Schuksz, M.; Esko, J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007, 446, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Manon-Jensen, T.; Itoh, Y.; Couchman, J.R. Proteoglycans in health and disease: The multiple roles of syndecan shedding. FEBS J. 2010, 277, 3876–3889. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Ikesue, M.; Danzaki, K.; Morimoto, J.; Sato, M.; Tanaka, S.; Kojima, T.; Tsutsui, H.; Uede, T. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circ. Res. 2011, 108, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Finsen, A.V.; Woldbaek, P.R.; Li, J.; Wu, J.; Lyberg, T.; Tønnessen, T.; Christensen, G. Increased syndecan expression following myocardial infarction indicates a role in cardiac remodeling. Physiol. Genom. 2004, 16, 301–308. [Google Scholar] [CrossRef]
- Finsen, A.V.; Lunde, I.G.; Sjaastad, I.; Østli, E.K.; Lyngra, M.; Jarstadmarken, H.O.; Hasic, A.; Nygård, S.; Wilcox-Adelman, S.A.; Goetinck, P.F.; et al. Syndecan-4 is essential for development of concentric myocardial hypertrophy via stretch-induced activation of the calcineurin-NFAT pathway. PLoS ONE 2011, 6, e28302. [Google Scholar] [CrossRef]
- Herum, K.M.; Lunde, I.G.; Skrbic, B.; Florholmen, G.; Behmen, D.; Sjaastad, I.; Carlson, C.R.; Gomez, M.F.; Christensen, G. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J. Mol. Cell. Cardiol. 2013, 54, 73–81. [Google Scholar] [CrossRef]
- Strand, M.E.; Aronsen, J.M.; Braathen, B.; Sjaastad, I.; Kvaløy, H.; Tønnessen, T.; Christensen, G.; Lunde, I.G. Shedding of syndecan-4 promotes immune cell recruitment and mitigates cardiac dysfunction after lipopolysaccharide challenge in mice. J. Mol. Cell. Cardiol. 2015, 88, 133–144. [Google Scholar] [CrossRef]
- Lunde, I.G.; Aronsen, J.M.; Melleby, A.O.; Strand, M.E.; Skogestad, J.; Bendiksen, B.A.; Ahmed, M.S.; Sjaastad, I.; Attramadal, H.; Carlson, C.R.; et al. Cardiomyocyte-specific overexpression of syndecan-4 in mice results in activation of calcineurin-NFAT signalling and exacerbated cardiac hypertrophy. Mol. Biol. Rep. 2022, 49, 11795–11809. [Google Scholar] [CrossRef]
- Strand, M.E.; Herum, K.M.; Rana, Z.A.; Skrbic, B.; Askevold, E.T.; Dahl, C.P.; Vistnes, M.; Hasic, A.; Kvaløy, H.; Sjaastad, I.; et al. Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart. FEBS J. 2013, 280, 2228–2247. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Fitzgerald, M.L.; Bernfield, M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J. Biol. Chem. 1997, 272, 14713–14720. [Google Scholar] [CrossRef]
- Langeland, H.; Damås, J.K.; Mollnes, T.E.; Ludviksen, J.K.; Ueland, T.; Michelsen, A.E.; Løberg, M.; Bergum, D.; Nordseth, T.; Skjærvold, N.K.; et al. The inflammatory response is related to circulatory failure after out-of-hospital cardiac arrest: A prospective cohort study. Resuscitation 2022, 170, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Becker, L.B.; Choudhary, R.C.; Takegawa, R.; Shoaib, M.; Shinozaki, K.; Endo, Y.; Homma, K.; Rolston, D.M.; Eguchi, S.; et al. Hydrogen gas with extracorporeal cardiopulmonary resuscitation improves survival after prolonged cardiac arrest in rats. J. Transl. Med. 2021, 19, 462. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, D.; Mitic, V.; Stojanovic, M.; Petrovic, D.; Ignjatovic, A.; Milojkovic, M.; Dunjic, O.; Milenkovic, J.; Bojanic, V.; Deljanin Ilic, M. The Discriminatory Ability of Renalase and Biomarkers of Cardiac Remodeling for the Prediction of Ischemia in Chronic Heart Failure Patients With the Regard to the Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 691513. [Google Scholar] [CrossRef]
- Wernly, B.; Fuernau, G.; Masyuk, M.; Muessig, J.M.; Pfeiler, S.; Bruno, R.R.; Desch, S.; Muench, P.; Lichtenauer, M.; Kelm, M.; et al. Syndecan-1 Predicts Outcome in Patients with ST-Segment Elevation Infarction Independent from Infarct-related Myocardial Injury. Sci. Rep. 2019, 9, 18367. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Zheng, J.; Song, D.; Zheng, S.; Ren, L.; Wang, Y.; Yao, Y.; Wang, Y.; Liu, Y.; et al. Syndecan-1 as an independent risk factor for the incidence of adverse cardiovascular events in patients having stage C and D heart failure with non-ischemic dilated cardiomyopathy. Clin. Chim. Acta 2019, 490, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.M.; Meneses, G.C.; Sousa, N.E.; Menezes, R.R.; Parahyba, M.C.; Martins, A.M.; Libório, A.B. Syndecan-1 in Acute Decompensated Heart Failure--Association With Renal Function and Mortality. Circ. J. 2015, 79, 1511–1519. [Google Scholar] [CrossRef]
- Tromp, J.; van der Pol, A.; Klip, I.T.; de Boer, R.A.; Jaarsma, T.; van Gilst, W.H.; Voors, A.A.; van Veldhuisen, D.J.; van der Meer, P. Fibrosis marker syndecan-1 and outcome in patients with heart failure with reduced and preserved ejection fraction. Circ. Heart Fail. 2014, 7, 457–462. [Google Scholar] [CrossRef]
- Kojima, T.; Takagi, A.; Maeda, M.; Segawa, T.; Shimizu, A.; Yamamoto, K.; Matsushita, T.; Saito, H. Plasma levels of syndecan-4 (ryudocan) are elevated in patients with acute myocardial infarction. Thromb. Haemost. 2001, 85, 793–799. [Google Scholar] [CrossRef]
- Takahashi, R.; Negishi, K.; Watanabe, A.; Arai, M.; Naganuma, F.; Ohyama, Y.; Kurabayashi, M. Serum syndecan-4 is a novel biomarker for patients with chronic heart failure. J. Cardiol. 2011, 57, 325–332. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; von Haehling, S.; Aronow, W.S.; Ahmed, M.I.; Rysz, J.; Banach, M. Heart failure biomarkers in patients with dilated cardiomyopathy. Int. J. Cardiol. 2013, 168, 2404–2410. [Google Scholar] [CrossRef]
- Solbu, M.D.; Kolset, S.O.; Jenssen, T.G.; Wilsgaard, T.; Løchen, M.L.; Mathiesen, E.B.; Melsom, T.; Eriksen, B.O.; Reine, T.M. Gender differences in the association of syndecan-4 with myocardial infarction: The population-based Tromsø Study. Atherosclerosis 2018, 278, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.A.J.; Merlo, M.; Dominguez, F.; Wang, P.; Henkens, M.T.H.M.; Adriaens, M.E.; Hazebroek, M.R.; Masè, M.; Escobar, L.E.; Cobas-Paz, R.; et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur. Heart J. 2020, 42, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Merken, J.; Hazebroek, M.; Van Paassen, P.; Verdonschot, J.; Van Empel, V.; Knackstedt, C.; Abdul Hamid, M.; Seiler, M.; Kolb, J.; Hoermann, P.; et al. Immunosuppressive Therapy Improves Both Short- and Long-Term Prognosis in Patients With Virus-Negative Nonfulminant Inflammatory Cardiomyopathy. Circ. Heart Fail. 2018, 11, e004228. [Google Scholar] [CrossRef] [PubMed]
- Vanhaverbeke, M.; Vausort, M.; Veltman, D.; Zhang, L.; Wu, M.; Laenen, G.; Gillijns, H.; Moreau, Y.; Bartunek, J.; Van De Werf, F.; et al. Peripheral Blood RNA Levels of QSOX1 and PLBD1 Are New Independent Predictors of Left Ventricular Dysfunction After Acute Myocardial Infarction. Circ. Genom. Precis. Med. 2019, 12, e002656. [Google Scholar] [CrossRef] [PubMed]
- Braathen, B.; Tønnessen, T. Cold blood cardioplegia reduces the increase in cardiac enzyme levels compared with cold crystalloid cardioplegia in patients undergoing aortic valve replacement for isolated aortic stenosis. J. Thorac. Cardiovasc. Surg. 2010, 139, 874–880. [Google Scholar] [CrossRef]
- Almaas, V.M.; Haugaa, K.H.; Strøm, E.H.; Scott, H.; Dahl, C.P.; Leren, T.P.; Geiran, O.R.; Endresen, K.; Edvardsen, T.; Aakhus, S.; et al. Increased amount of interstitial fibrosis predicts ventricular arrhythmias, and is associated with reduced myocardial septal function in patients with obstructive hypertrophic cardiomyopathy. Europace 2013, 15, 1319–1327. [Google Scholar] [CrossRef]
- Rypdal, K.B.; Erusappan, P.M.; Melleby, A.O.; Seifert, D.E.; Palmero, S.; Strand, M.E.; Tønnessen, T.; Dahl, C.P.; Almaas, V.; Hubmacher, D.; et al. The extracellular matrix glycoprotein ADAMTSL2 is increased in heart failure and inhibits TGFβ signalling in cardiac fibroblasts. Sci. Rep. 2021, 11, 19757. [Google Scholar] [CrossRef]
- Lipphardt, M.; Dihazi, H.; Maas, J.-H.; Schäfer, A.-K.; Amlaz, S.I.; Ratliff, B.B.; Koziolek, M.J.; Wallbach, M. Syndecan-4 as a Marker of Endothelial Dysfunction in Patients with Resistant Hypertension. J. Clin. Med. 2020, 9, 3051. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, D.; Chen, Z.; Xu, B. Factors associated with microvascular occlusion in patients with ST elevation myocardial infarction after primary percutaneous coronary intervention. J. Int. Med. Res. 2021, 49, 3000605211024490. [Google Scholar] [CrossRef]
- Nikaido, T.; Tanino, Y.; Wang, X.; Sato, S.; Misa, K.; Fukuhara, N.; Sato, Y.; Fukuhara, A.; Uematsu, M.; Suzuki, Y.; et al. Serum syndecan-4 as a possible biomarker in patients with acute pneumonia. J. Infect. Dis. 2015, 212, 1500–1508. [Google Scholar] [CrossRef]
- Sato, Y.; Tanino, Y.; Wang, X.; Nikaido, T.; Sato, S.; Misa, K.; Togawa, R.; Frevert, C.W.; Munakata, M. Baseline serum syndecan-4 predicts prognosis after the onset of acute exacerbation of idiopathic interstitial pneumonia. PLoS ONE 2017, 12, e0176789. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Ning, P.; Zheng, Y.; Shang, Y.; Zhou, B.; Gao, Z. Serum suPAR and syndecan-4 levels predict severity of community-acquired pneumonia: A prospective, multi-centre study. Crit. Care 2018, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, M.; Pinno, K.; Ehnold, L.I.; Märtens, N.; Märtson, A.; Pap, T.; Stärke, C.; Lohmann, C.H.; Bertrand, J. MMP-9 mediated Syndecan-4 shedding correlates with osteoarthritis severity. Osteoarthr. Cartil. 2021, 29, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.J.; Tang, L.Z.; Li, W.H.; Xu, Z.S.; Zhang, L.L.; Cheng, F.G.; Chen, H.X.; Wang, Z.H.; Luo, Y.C.; Dai, A.N.; et al. Serum syndecan-4 is associated with nonalcoholic fatty liver disease. J. Dig. Dis. 2021, 22, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Herum, K.M.; Lunde, I.G.; Skrbic, B.; Louch, W.E.; Hasic, A.; Boye, S.; Unger, A.; Brorson, S.-H.; Sjaastad, I.; Tønnessen, T.; et al. Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart. Cardiovasc. Res. 2015, 106, 217–226. [Google Scholar] [CrossRef]
- Li, G.; Xie, J.; Chen, J.; Li, R.; Wu, H.; Zhang, X.; Chen, Q.; Gu, R.; Xu, B. Syndecan-4 deficiency accelerates the transition from compensated hypertrophy to heart failure following pressure overload. Cardiovasc. Pathol. 2017, 28, 74–79. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Li, R.; Dai, Q.; Yong, Y.; Zong, B.; Xu, Y.; Li, E.; Ferro, A.; Xu, B. Syndecan-4 over-expression preserves cardiac function in a rat model of myocardial infarction. J. Mol. Cell. Cardiol. 2012, 53, 250–258. [Google Scholar] [CrossRef]
- Hartman, M.H.T.; Groot, H.E.; Leach, I.M.; Karper, J.C.; van der Harst, P. Translational overview of cytokine inhibition in acute myocardial infarction and chronic heart failure. Trends Cardiovasc. Med. 2018, 28, 369–379. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Canada, J.; Carbone, S.; Trankle, C.; Buckley, L.; Oddi Erdle, C.; Abouzaki, N.A.; Dixon, D.; Kadariya, D.; Christopher, S.; et al. Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 2017, 10, e004373. [Google Scholar] [CrossRef]




| Human Syndecan-4 ELISA Kit—IBL 27188 | ||||||
|---|---|---|---|---|---|---|
| Healthy Controls | Patient Population | |||||
| N | Serum Syndecan-4 (ng/mL) | Disease | N | Serum Syndecan-4 (ng/mL) | p-Value vs. Healthy | Reference |
| 21 | 5.7 ± 3.3 | Chronic heart failure | 45 | 22.5 ± 12.3 | p < 0.01 | [30] |
| 11 | 15.1 ± 2.6 | Acute pneumonia | 30 | 24.7 ± 9.2 | p = 0.006 | [41] |
| 45 | 16.05 ± 0.77 | Idiopathic interstitial pneumonia | 62 | 25.22 ± 3.72 | [42] | |
| SD-IIP/AE-IIP | 56 | 10.65 ± 0.73 | p < 0.05 (SD-IIP) | |||
| 30 | 14.30 ± 5.34 | Community-acquired pneumonia | 103 | 9.54 ± 5.92 | [43] | |
| SCAP/non-SCAP | 149 | 10.15 ± 4.37 | p < 0.001 | |||
| 35 | 14.7 ± 0.6 | Resistant hypertension | 19 | 35.8 ± 5.3 | p < 0.001 | [39] |
| 16 | 18.99 ± 1.36 | Osteoarthritis | 29 | 19,23 ± 9.16 | NS | [44] |
| 376 | 4.34 ± 1.78 | Non-alcoholic fatty liver disease | 157 | 11.25 ± 5.15 | p < 0.001 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strand, M.E.; Vanhaverbeke, M.; Henkens, M.T.H.M.; Sikking, M.A.; Rypdal, K.B.; Braathen, B.; Almaas, V.M.; Tønnessen, T.; Christensen, G.; Heymans, S.; et al. Inflammation and Syndecan-4 Shedding from Cardiac Cells in Ischemic and Non-Ischemic Heart Disease. Biomedicines 2023, 11, 1066. https://doi.org/10.3390/biomedicines11041066
Strand ME, Vanhaverbeke M, Henkens MTHM, Sikking MA, Rypdal KB, Braathen B, Almaas VM, Tønnessen T, Christensen G, Heymans S, et al. Inflammation and Syndecan-4 Shedding from Cardiac Cells in Ischemic and Non-Ischemic Heart Disease. Biomedicines. 2023; 11(4):1066. https://doi.org/10.3390/biomedicines11041066
Chicago/Turabian StyleStrand, Mari E., Maarten Vanhaverbeke, Michiel T. H. M. Henkens, Maurits A. Sikking, Karoline B. Rypdal, Bjørn Braathen, Vibeke M. Almaas, Theis Tønnessen, Geir Christensen, Stephane Heymans, and et al. 2023. "Inflammation and Syndecan-4 Shedding from Cardiac Cells in Ischemic and Non-Ischemic Heart Disease" Biomedicines 11, no. 4: 1066. https://doi.org/10.3390/biomedicines11041066
APA StyleStrand, M. E., Vanhaverbeke, M., Henkens, M. T. H. M., Sikking, M. A., Rypdal, K. B., Braathen, B., Almaas, V. M., Tønnessen, T., Christensen, G., Heymans, S., & Lunde, I. G. (2023). Inflammation and Syndecan-4 Shedding from Cardiac Cells in Ischemic and Non-Ischemic Heart Disease. Biomedicines, 11(4), 1066. https://doi.org/10.3390/biomedicines11041066







