Correlation between Collagen Type I/III Ratio and Scar Formation in Patients Undergoing Immediate Reconstruction with the Round Block Technique after Breast-Conserving Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Research Methods
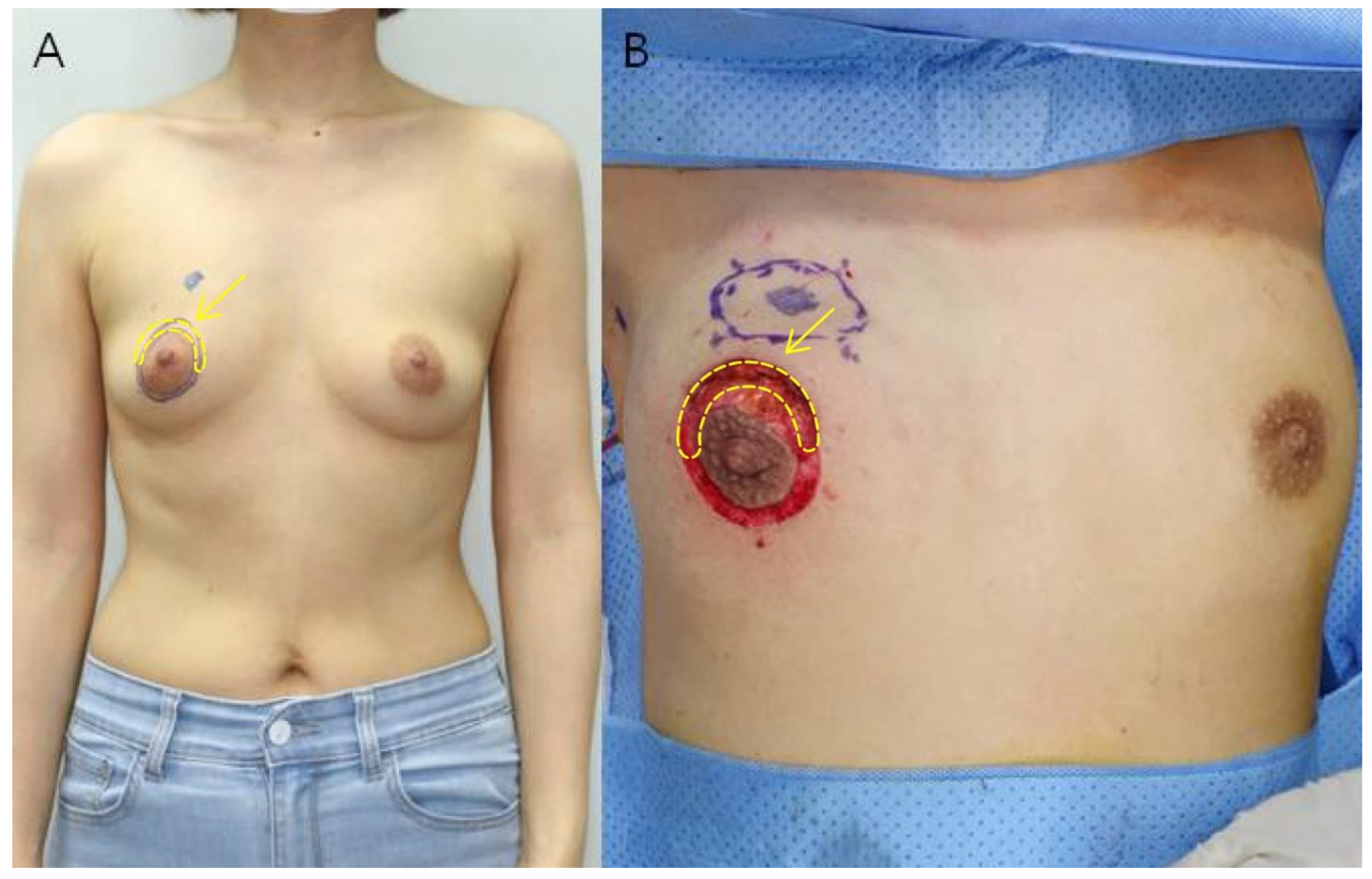
2.2.1. Tissue Sampling
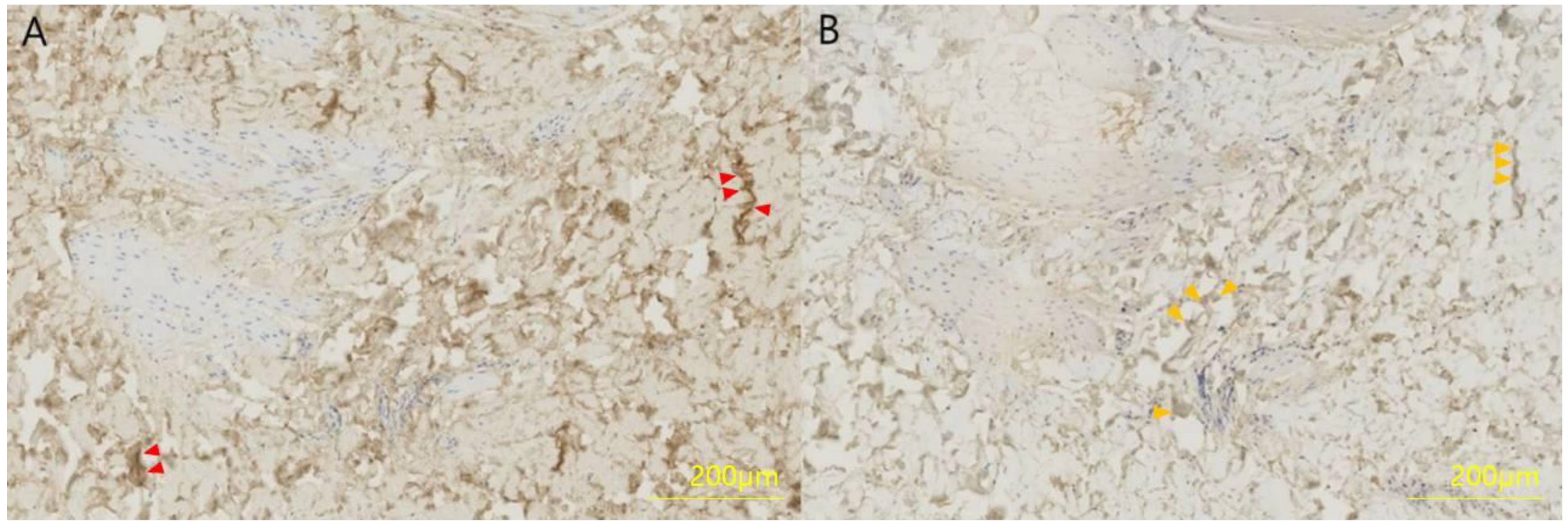
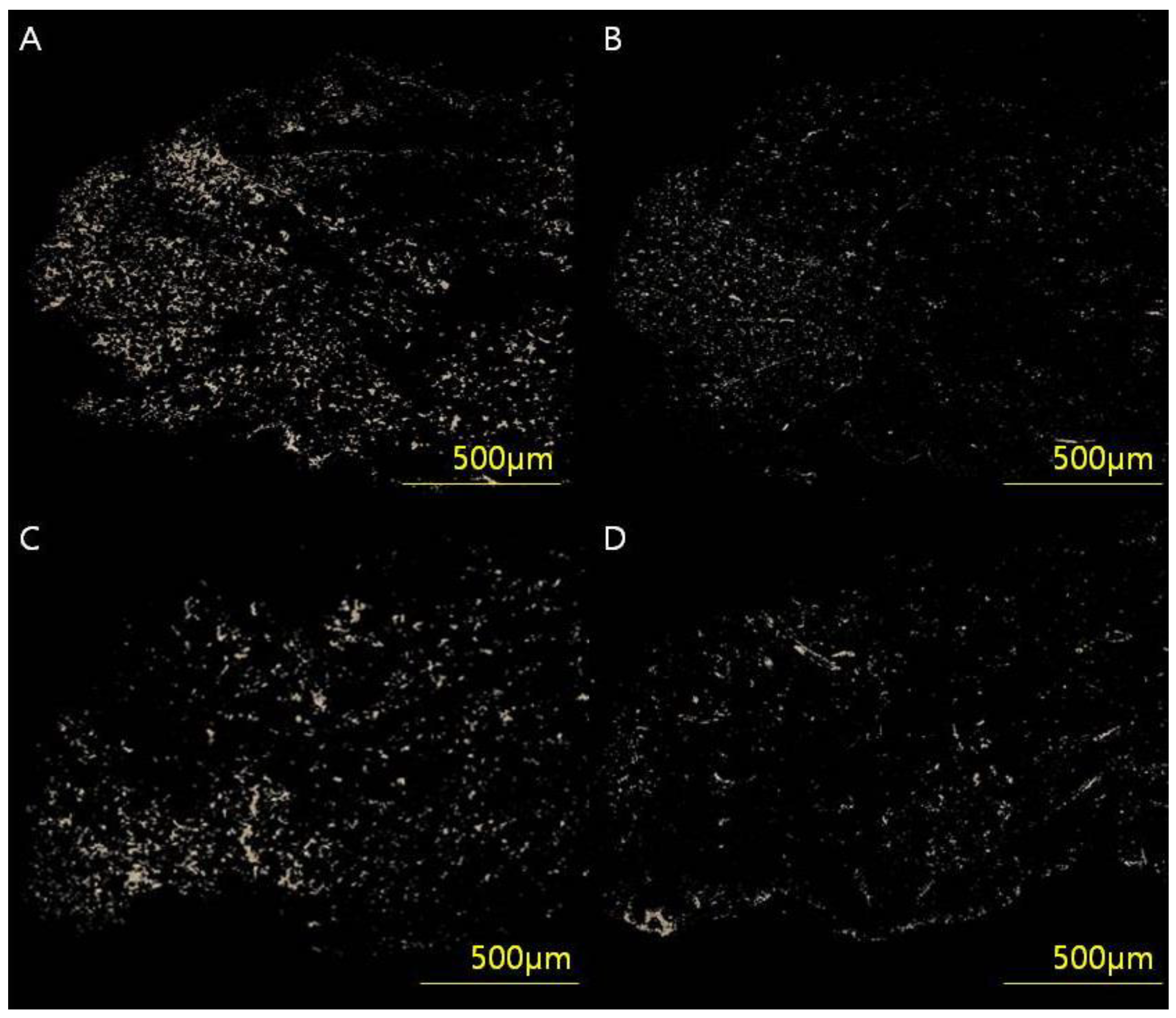
2.2.2. Immunohistochemistry
2.2.3. Measurement of Collagen Type I/III Ratio
2.2.4. Post-Operative Management
3. Evaluation Items
3.1. Baseline Characteristics
3.2. Postoperative Scar Assessment
4. Statistical Analysis
- Frequency analysis was performed on the baseline characteristics of study participants. Descriptive statistics are presented as medians with interquartile ranges or as numbers and percentages.
- Reliability analysis was performed to determine whether postoperative scan measurements were significantly consistent between two plastic surgeons.
- Pearson correlation analysis was performed to understand the relationship between baseline patient characteristics (age, BMI, weight of resected breast tissue, pathology, location, previous treatment history), collagen type I, III content and collagen type I/III ratio.
- Multiple regression analysis was performed with VSS as the dependent variable to verify the effect of items with significant results on scar formation.
5. Results
5.1. Demographic and Clinical Characteristics
5.2. Scar Evaluation
5.3. Analysis of Correlations between Variables
5.4. Factors Influencing Mean VSS
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, B.; McKenna, S.; Siddhi, K.; McGrouther, D.; Bayat, A. The hidden cost of skin scars: Quality of life after skin scarring. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Oswaldo, J.I.E.; Tripodi, D.; Al-shaqsi, Y.; Woods, O.E.F.; Valero, R. Effects of mechanical forces on the formation of cutaneous wounds during skin expansion and emerging therapies for wound healing and scar prevention. Saudi Med. J. 2023, 44, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T.A.; Cooter, R.D.; Gold, M.H.; Hobbs, F.D.R.; Ramelet, A.-A.; Shakespeare, P.G.; Stella, M.; Téot, L.; Wood, F.M.; Ziegler, U.E. International Clinical Recommendations on Scar Management. Plast. Reconstr. Surg. 2002, 110, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.A.; Marshall, C.D.; Leavitt, T.; Hu, M.S.; Moore, A.L.; Gonzalez, J.G.; Longaker, M.T.; Gurtner, G.C. Mechanical Forces in Cutaneous Wound Healing: Emerging Therapies to Minimize Scar Formation. Adv. Wound Care 2018, 7, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Guastafierro, A.; Avvedimento, S.; Pieretti, G.; Gulotta, E.; Izzo, S.; Nicoletti, G.F.; Ciccarelli, F. Steri-Strips™ vs. intracuticular skin suture in endoscopic release of carpal tunnel: A retrospective study and review of the literature. Eur. J. Plast. Surg. 2020, 43, 415–420. [Google Scholar] [CrossRef]
- Zurada, J.M.; Kriegel, D.; Davis, I.C. Topical treatments for hypertrophic scars. J. Am. Acad. Dermatol. 2006, 55, 1024–1031. [Google Scholar] [CrossRef]
- Gold, M.H.; Berman, B.; Clementoni, M.T.; Gauglitz, G.G.; Nahai, F.; Murcia, C. Updated international clinical recommendations on scar management: Part 1—Evaluating the evidence. Dermatol. Surg. 2014, 40, 817–824. [Google Scholar]
- Lee, Y.I.; Kim, J.; Yang, C.E.; Hong, J.W.; Lee, W.J.; Lee, J.H. Combined therapeutic strategies for keloid treatment. Dermatol. Surg. 2019, 45, 802–810. [Google Scholar] [CrossRef]
- Ogawa, R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: A 2020 update of the algorithms published 10 years ago. Plast. Reconstr. Surg. 2022, 149, 79e–94e. [Google Scholar] [CrossRef]
- Butler, P.D.; Longaker, M.T.; Yang, G.P. Current progress in keloid research and treatment. J. Am. Coll. Surg. 2008, 206, 731–741. [Google Scholar] [CrossRef]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef]
- Kim, H.I.; Kwak, C.Y.; Kim, H.Y.; Yi, H.S.; Park, E.J.; Kim, J.H.; Park, J.H. Correlation between dermal thickness and scar formation in female patients after thyroidectomy. Arch Craniofac. Surg. 2018, 19, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Aziz, J.; Ahmad, M.F.; Rahman, M.T.; Yahya, N.A.; Czernuszka, J.; Radzi, Z. AFM analysis of collagen fibrils in expanded scalp tissue after anisotropic tissue expansion. Int. J. Biol. Macromol. 2018, 107, 1030–1038. [Google Scholar] [CrossRef]
- Meigel, W.N.; Gay, S.; Weber, L. Dermal architecture and collagen type distribution. Arch Dermatol. Res. 1977, 259, 1–10. [Google Scholar] [CrossRef]
- Whitby, D.J.; Ferguson, M.W. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 1991, 112, 651–668. [Google Scholar] [CrossRef]
- Pauschinger, M.; Knopf, D.; Petschauer, S.; Doerner, A.; Poller, W.; Schwimmbeck, P.L.; Kühl, U.; Schultheiss, H.P. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation 1999, 99, 2750–2756. [Google Scholar] [CrossRef] [Green Version]
- In, S.K.; Kim, Y.S.; Kim, H.S.; Park, J.H.; Kim, H.I.; Yi, H.S.; Park, J.C.; Jeon, C.W.; Choi, J.H.; Jung, S.U.; et al. Retrospective review of 108 breast reconstructions using the round block technique after breast-conserving surgery: Indications, complications, and outcomes. Arch. Plast. Surg. 2020, 47, 574–582. [Google Scholar] [CrossRef]
- Wang, C.; Rong, Y.; Ning, F.; Zhang, G. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Cameron, A.M.; Turner, C.T.; Adams, D.H.; Jackson, J.E.; Melville, E.; Arkell, R.M.; Cowin, A.J. Flightless I is a key regulator of the fibroproliferative process in hypertrophic scarring and a target for a novel antiscarring therapy. Br. J. Dermatol. 2016, 174, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.J.; Longaker, M.T.; Lorenz, H.P. Scarless Fetal Wound Healing: A Basic Science Review. Plast. Reconstr. Surg. 2010, 126, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics: Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med. Hypotheses 2008, 71, 493–500. [Google Scholar] [CrossRef]
- Wolfram, D.; Tzankov, A.; Pulzl, P.; Piza-Katzer, H. Hypertrophic scars and keloids—A review of their pathophysiology, risk factors, and therapeutic management. Dermatol. Surg. 2009, 35, 171–181. [Google Scholar] [CrossRef]
- Szabowski, A.; Maas Szabowski, N.; Andrecht, S.; Kolbus, A.; Schorpp Kistner, M.; Fusenig, N.E.; Angel, P. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal epidermal interaction in skin. Cell 2000, 103, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, G.S.; Dodsworth, J.; van Boxtel, E.; Tarnuzzer, R.W.; Horan, M.A.; Schultz, G.S.; Ferguson, M.W. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997, 3, 1209–1215. [Google Scholar] [CrossRef]
- Crowe, M.J.; Doetschman, T.; Greenhalgh, D.G. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J. Investig. Dermatol. 2000, 115, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H. Structure and function of the skin. In Shimizu’s Textbook of Dermatology; Shimizu, H., Ed.; Hokkaido University Press: Sapporo, Japan, 2007; pp. 12–16. [Google Scholar]
- Peeters, E.; De Hertogh, G.; Junge, K.; Klinge, U.; Miserez, M. Skin as marker for collagen type l/lll ratio in abdominal wall fascia. Springerlink 2014, 18, 519–525. [Google Scholar] [CrossRef]
- Langenbach, M.R.; Lisovets, R.; Varga-Szabo, D.; Bonicke, L. Decreased collagen ratio type l/lll in association with hemorrhoidal disease. J. Transl. Sci. 2018, 5, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Mahar, P.D.; Spinks, A.B.; Cleland, H.; Bekhor, P.; Waibel, J.S.; Lo, C.; Goodman, G. Improvement of burn scars treated with fractional ablative CO2 lasers—A systematic review and meta-analysis using the Vancouver Scar Scale. J. Burn. Care Res. 2021, 42, 200–206. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, J.; Hu, C.; Zhu, F. Effectiveness and safety of botulinum toxin type A injection for scar prevention: A systematic review and meta-analysis. Aesthetic Plast. Surg. 2019, 43, 1241–1249. [Google Scholar] [CrossRef]
- Ahn, K.H.; Park, E.S.; Nam, S.M. Usefulness of a 1064 nm microlens array-type, picosecond-dominant laser for pigmented scars with improvement of Vancouver Scar Scale. Med. Lasers 2019, 8, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sun, Z.; Chen, J.; Zhang, Y.; Shi, H.; Zhu, L. Genetic polymorphisms in collagen-related genes are associated with pelvic organ prolapse. Menopause 2019, 27, 223–229. [Google Scholar] [CrossRef]
- Ikeda, T.; Mabuchi, A.; Fukuda, A.; Kawakami, A.; Yamada, R.; Yamamoto, S.; Miyoshi, K.; Haga, N.; Hiraoka, H.; Takatori, Y.; et al. Association analysis of single nucleotide polymorphisms in cartilage-specific collagen genes with knee and hip osteoarthritis in the Japanese population. J. Bone Miner. Res. 2002, 17, 1290–1296. [Google Scholar] [CrossRef]
- Fajar, A.; Warsinggih; Syarifuddin, E.; Hendarto, J.; Labeda, I.; Lusikooy, R.E.; Mappincara; Dani, M.I.; Sampetoding, S.; Kusuma, M.I.; et al. The relationship between glycine levels in collagen in the anterior rectus sheath tissue and the onset of indirct inguinal hernia: A cross-sectional study. Ann. Med. Surg. 2021, 73, 103166. [Google Scholar] [CrossRef]
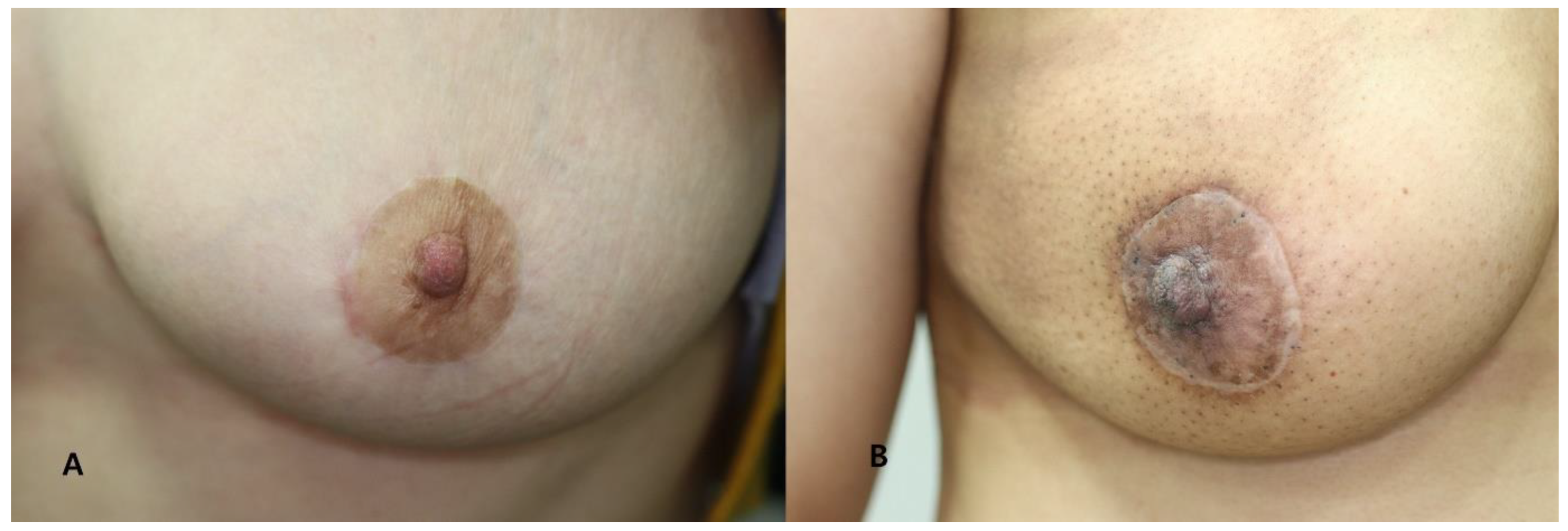
| Scar Characteristic | Score |
|---|---|
| Vascularity | |
| Normal | 0 |
| Pink | 1 |
| Red | 2 |
| Purple | 3 |
| Pigmentation Normal | 0 |
| Hypopigmentation | 1 |
| Hyperpigmentation | 2 |
| Pliability | |
| Normal | 0 |
| Supple | 1 |
| Yielding | 2 |
| Firm | 3 |
| Ropes | 4 |
| Contracture | 5 |
| Height (mm) | |
| Flat | 0 |
| <2 | 1 |
| 2~5 | 2 |
| >5 | 3 |
| Total score | 13 |
| Characteristic | Value (%) |
|---|---|
| Total no. of patients | 78 |
| Mean age (year) | 48.77 ± 9.40 |
| Mean BMI (kg/m2) | 23.76 ± 3.92 |
| Weight of resected breast tissue (g) | 25.79 ± 11.98 |
| Tumor type | |
| DCIS | 40 (51.3) |
| LCIS | 2 (2.6) |
| IDC | 36 (46.2) |
| ILC | 0 (0) |
| Tumor Location | |
| UL | 32 (41) |
| UM | 22 (28.2) |
| LL | 10 (12.8) |
| LM | 14 (17.9) |
| Previous treatment history | |
| Yes | 12 (15.3) |
| Preoperative chemotherapy | 8 (10.2) |
| Preoperative hormonal therapy | 4 (5.1) |
| No | 63 (80.7) |
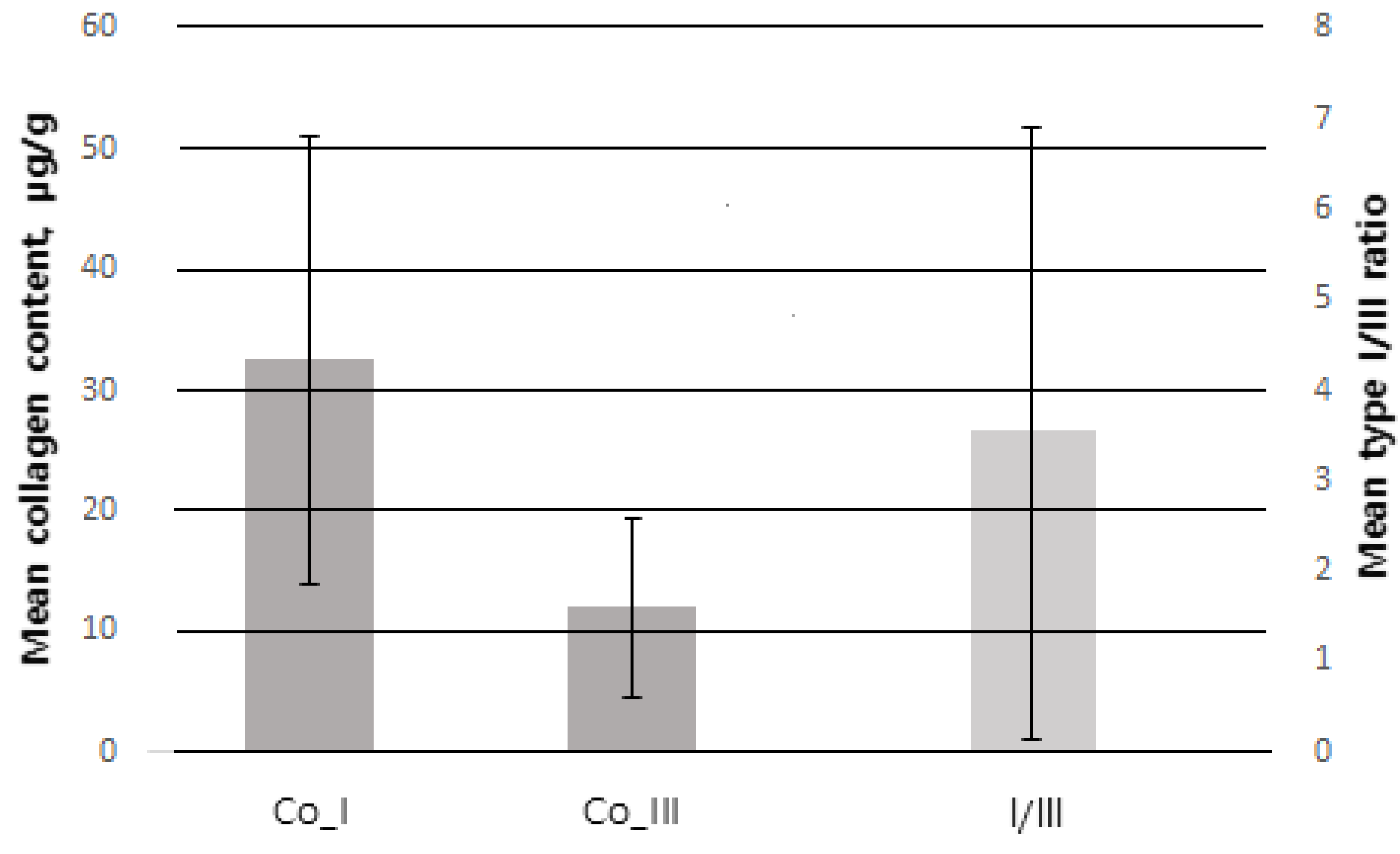
| Mean collagen content | |
| Collagen Type I (µg/g) | 31.63 ± 18.22 |
| Collagen Type III (µg/g) | 11.41 ± 6.27 |
| Collagen type I/III ratio | 3.85 ± 3.72 |
| Mean VSS | Interclass Correlation | p-Value | |
|---|---|---|---|
| Doctor A | 1.92 ± 2.01 | 0.920 | <0.001 * |
| Doctor B | 1.79 ± 1.89 |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Age | 1 | ||||||
| 2. BMI | 0.237 | 1 | |||||
| 3. WRBT | 0.009 | 0.112 | 1 | ||||
| 4. Co_I | 0.112 | −0.030 | −0.065 | 1 | |||
| 5. Co_III | 0.075 | −0.022 | −0.068 | −0.070 | 1 | ||
| 6. I/III | 0.172 | 0.236 | 0.139 | 0.455 ** | −0.340 * | 1 | |
| 7. VSS | −0.013 | 0.118 | −0.044 | 0.412 * | −0.326 * | 0.552 ** | 1 |
| Dependent Variable | Independent Variable | B | S.E. | β | t | p-Value | VIF |
|---|---|---|---|---|---|---|---|
| VSS | C | 1.594 | 1.191 | 1.338 | 0.191 | ||
| Co_I | 0.015 | 0.011 | 0.219 | 1.344 | 0.189 | 1.338 | |
| Co_III | −0.024 | 0.022 | −0.167 | −1.097 | 0.281 | 1.169 | |
| I/III | 0.086 | 0.037 | 0.415 | 2.312 | 0.028 | 1.625 | |
| F = 3.255 (p < 0.01), R2 = 0.387, adjR2 = 0.268, D-W = 1.361 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-y.; Im, H.-y.; Chang, H.-k.; Jeong, H.-d.; Park, J.-h.; Kim, H.-i.; Yi, H.-s.; Kim, Y.-s. Correlation between Collagen Type I/III Ratio and Scar Formation in Patients Undergoing Immediate Reconstruction with the Round Block Technique after Breast-Conserving Surgery. Biomedicines 2023, 11, 1089. https://doi.org/10.3390/biomedicines11041089
Kim H-y, Im H-y, Chang H-k, Jeong H-d, Park J-h, Kim H-i, Yi H-s, Kim Y-s. Correlation between Collagen Type I/III Ratio and Scar Formation in Patients Undergoing Immediate Reconstruction with the Round Block Technique after Breast-Conserving Surgery. Biomedicines. 2023; 11(4):1089. https://doi.org/10.3390/biomedicines11041089
Chicago/Turabian StyleKim, Hyo-young, Ho-young Im, Hee-kyung Chang, Hwan-do Jeong, Jin-hyung Park, Hong-il Kim, Hyung-suk Yi, and Yoon-soo Kim. 2023. "Correlation between Collagen Type I/III Ratio and Scar Formation in Patients Undergoing Immediate Reconstruction with the Round Block Technique after Breast-Conserving Surgery" Biomedicines 11, no. 4: 1089. https://doi.org/10.3390/biomedicines11041089





