The DASciS Software for BSI Calculation as a Valuable Prognostic Tool in mCRPC Treated with 223RaCl2: A Multicenter Italian Study
Abstract
1. Introduction
1.1. Radium-223 Dichloride (223RaCl2)
1.2. Bone Scan Index (BSI)
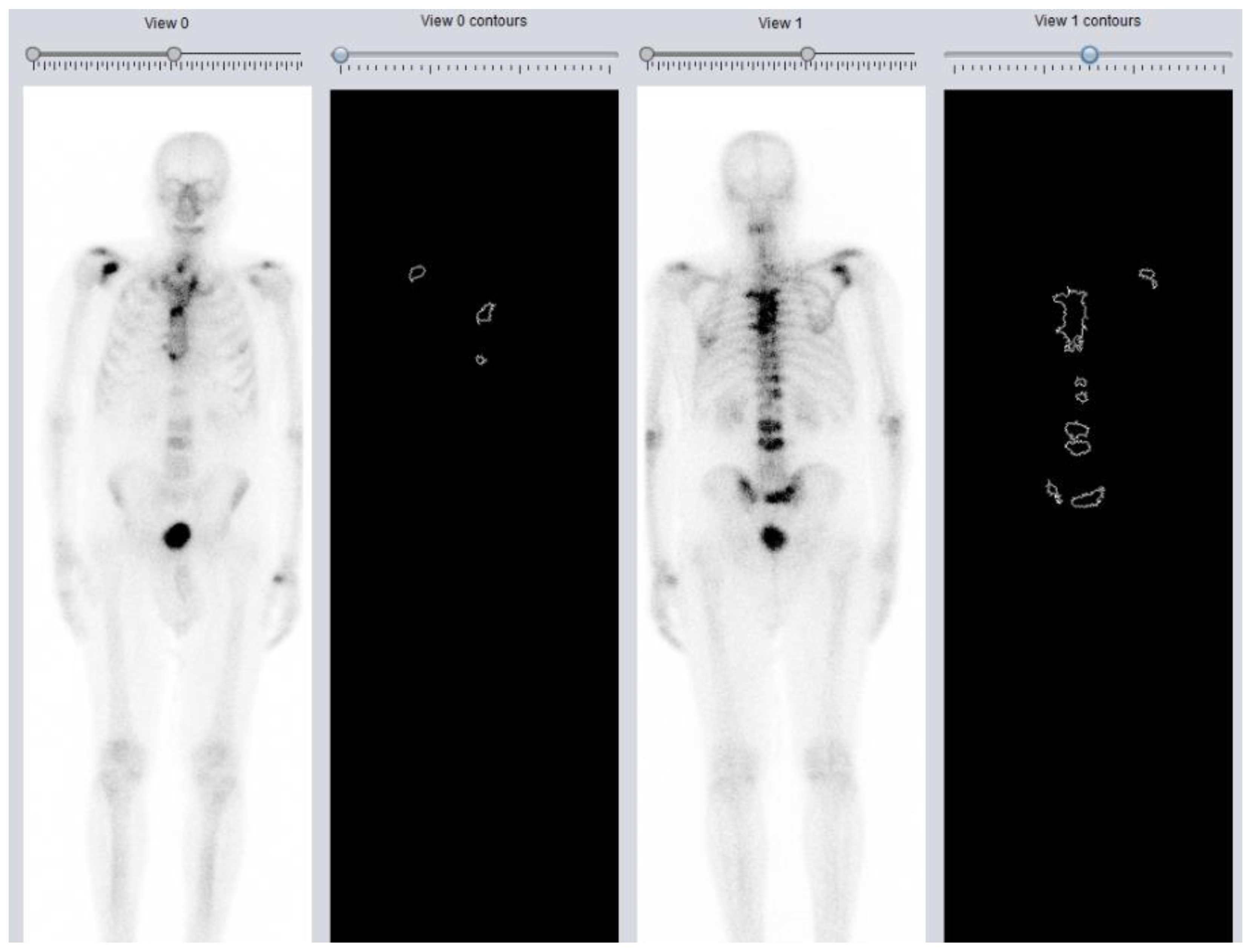
1.3. DASciS Software for BSI Calculation
1.4. Aim of the Study
2. Materials and Methods
2.1. DASciS Software
2.2. Statistical Analysis
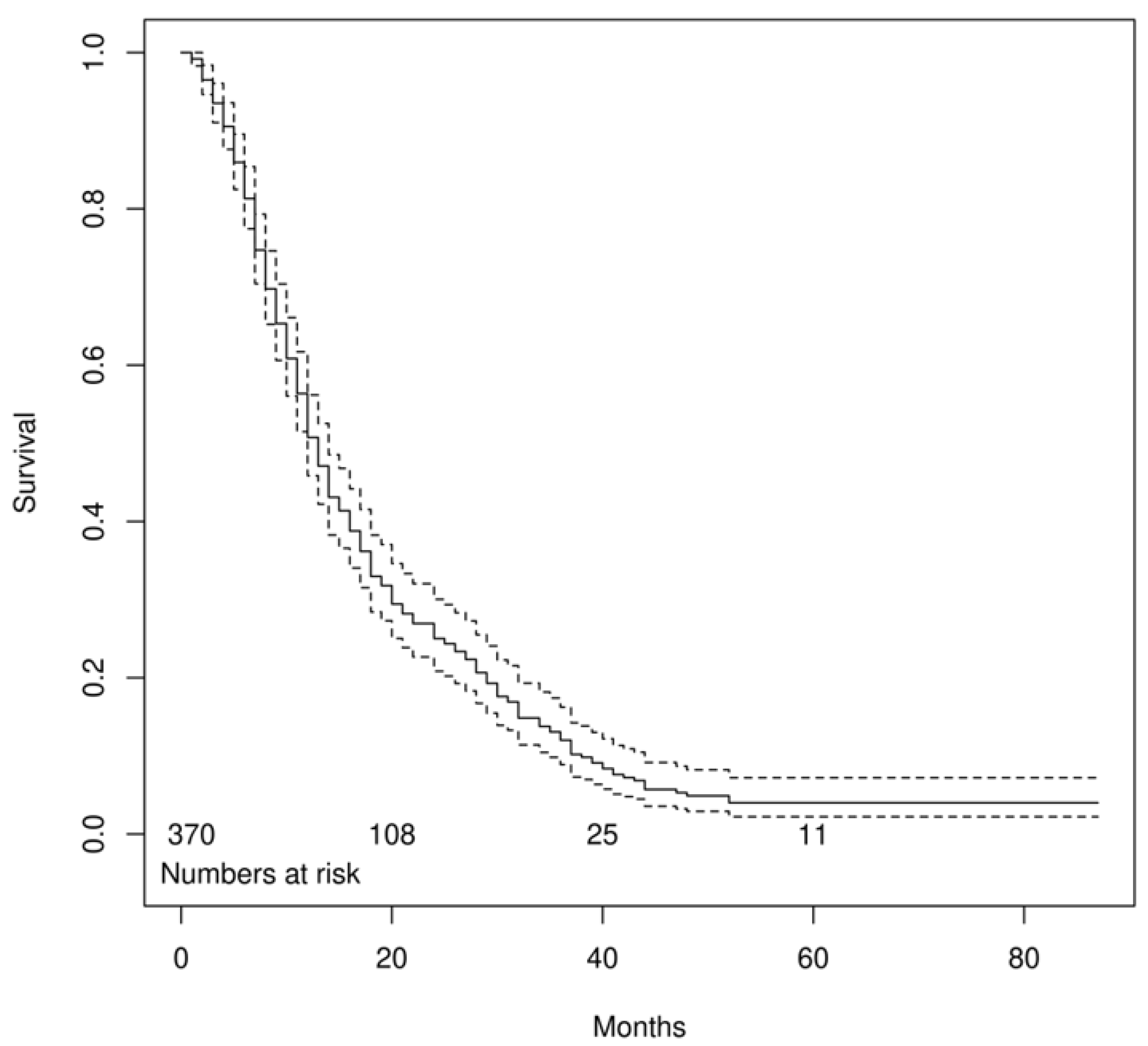
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Badia, X.; Chow, E.; Lipton, A.; Wardley, A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2008, 16, 879–889. [Google Scholar] [CrossRef]
- Keller, E.T.; Brown, J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J. Cell. Biochem. 2004, 91, 718–729. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014, 15, 738–746. [Google Scholar] [CrossRef]
- Kerr, C. 223Ra targets skeletal metastases and spares normal tissue. Lancet Oncol. 2002, 3, 453. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, G.; Follacchio, G.A.; Frantellizzi, V.; Prelaj, A.; Farcomeni, A.; Giuli, A.; Bianco, V.; Tomao, S. 223Ra-dichloride therapy in an elderly bone metastatic castration-resistant prostate cancer patient: A case report presentation and comparison with existing literature. Aging Clin. Exp. Res. 2018, 30, 677–680. [Google Scholar] [CrossRef]
- Harrison, M.R.; Wong, T.Z.; Armstrong, A.J.; George, D.J. Radium-223 chloride: A potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag. Res. 2013, 5, 1–14. [Google Scholar] [CrossRef]
- Kluetz, P.G.; Pierce, W.; Maher, V.E.; Zhang, H.; Tang, S.; Song, P.; Liu, Q.; Haber, M.T.; Leutzinger, E.E.; Al-Hakim, A.; et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2014, 20, 9–14. [Google Scholar] [CrossRef]
- Parikh, S.; Murray, L.; Kenning, L.; Bottomley, D.; Din, O.; Dixit, S.; Ferguson, C.; Handforth, C.; Joseph, L.; Mokhtar, D.; et al. Real-world Outcomes and Factors Predicting Survival and Completion of Radium 223 in Metastatic Castrate-resistant Prostate Cancer. Clin. Oncol. 2018, 30, 548–555. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Wong, W.W.; Anderson, E.M.; Mohammadi, H.; Daniels, T.B.; Schild, S.E.; Keole, S.R.; Choo, C.R.; Tzou, K.S.; Bryce, A.H.; Ho, T.H.; et al. Factors Associated With Survival Following Radium-223 Treatment for Metastatic Castration-resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, e969–e975. [Google Scholar] [CrossRef] [PubMed]
- Van den Wyngaert, T.; Tombal, B. The changing role of radium-223 in metastatic castrate-resistant prostate cancer: Has the EMA missed the mark with revising the label? Q. J. Nucl. Med. Mol. Imaging 2019, 63, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Love, C.; Din, A.S.; Tomas, M.B.; Kalapparambath, T.P.; Palestro, C.J. Radionuclide bone imaging: An illustrative review. Radiographics 2003, 23, 341–358. [Google Scholar] [CrossRef]
- Imbriaco, M.; Larson, S.M.; Yeung, H.W.; Mawlawi, O.R.; Erdi, Y.; Venkatraman, E.S.; Scher, H.I. A new parameter for measuring metastatic bone involvement by prostate cancer: The Bone Scan Index. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 1998, 4, 1765–1772. [Google Scholar]
- Takahashi, Y.; Yoshimura, M.; Suzuki, K.; Hashimoto, T.; Hirose, H.; Uchida, K.; Inoue, S.; Koizumi, K.; Tokuuye, K. Assessment of bone scans in advanced prostate carcinoma using fully automated and semi-automated bone scan index methods. Ann. Nucl. Med. 2012, 26, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Kaboteh, R.; Carducci, M.A.; Damber, J.E.; Stadler, W.M.; Hansen, M.; Edenbrandt, L.; Forsberg, G.; Nordle, Ö.; Pili, R.; et al. Assessment of the bone scan index in a randomized placebo-controlled trial of tasquinimod in men with metastatic castration-resistant prostate cancer (mCRPC). Urol. Oncol. 2014, 32, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Frantellizzi, V.; Pani, A.; Ippoliti, M.D.; Farcomeni, A.; Aloise, I.; Colosi, M.; Polito, C.; Pani, R.; Vincentis, G. Scintigraphic load of bone disease evaluated by DASciS software as a survival predictor in metastatic castration-resistant prostate cancer patients candidates to 223RaCl treatment. Radiol. Oncol. 2019, 54, 40–47. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T.; et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Bauckneht, M.; Rebuzzi, S.E.; Ponzano, M.; Borea, R.; Signori, A.; Frantellizzi, V.; Lodi Rizzini, E.; Mascia, M.; Lavelli, V.; Miceli, A.; et al. Prognostic Value of the BIO-Ra Score in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223 after the European Medicines Agency Restricted Use: Secondary Investigations of the Multicentric BIO-Ra Study. Cancers 2022, 14, 1744. [Google Scholar] [CrossRef]
- Bauckneht, M.; Rebuzzi, S.E.; Signori, A.; Donegani, M.I.; Murianni, V.; Miceli, A.; Borea, R.; Raffa, S.; Damassi, A.; Ponzano, M.; et al. The Prognostic Role of Baseline Metabolic Tumor Burden and Systemic Inflammation Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223: A Proof of Concept Study. Cancers 2020, 12, 3213. [Google Scholar] [CrossRef]
- Baldari, S.; Boni, G.; Bortolus, R.; Caffo, O.; Conti, G.; De Vincentis, G.; Monari, F.; Procopio, G.; Santini, D.; Seregni, E.; et al. Management of metastatic castration-resistant prostate cancer: A focus on radium-223: Opinions and suggestions from an expert multidisciplinary panel. Crit. Rev. Oncol. Hematol. 2017, 113, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Carrio, I.; De Vincentis, G.; Fanti, S.; Ilhan, H.; Mommsen, C.; Nitzsche, E.; Sundram, F.; Vogel, W.; Oyen, W.; et al. Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1671–1678. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Bradski, G. The OpenCV Library. Dr. Dobb’s J. Softw. Tools 2000, 120, 122–125. [Google Scholar]
- Suzuki, S.; Be, K. Topological structural analysis of digitized binary images by border following. Comput. Vis. Graph. Image Process. 1985, 30, 32–46. [Google Scholar] [CrossRef]
- Green, G. 10. An Essay on the Application of mathematical Analysis to the theories of Electricity and Magnetism. J. Fur Die Reine Und Angew. Math. 1854, 1854, 161–221. [Google Scholar] [CrossRef]
- Finlay, I.G.; Mason, M.D.; Shelley, M. Radioisotopes for the palliation of metastatic bone cancer: A systematic review. Lancet Oncol. 2005, 6, 392–400. [Google Scholar] [CrossRef]
- Frantellizzi, V.; De Feo, M.S.; Di Rocco, A.; Pontico, M.; Pani, A.; Farcomeni, A.; Cosma, L.; Lazri, J.; De Vincentis, G. Baseline quality of life predicts overall survival in patients with mCRPC treated with (223)Ra-dichloride. Hell. J. Nucl. Med. 2020, 23, 12–20. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Farcomeni, A.; Follacchio, G.A.; Pacilio, M.; Pellegrini, R.; Pani, R.; De Vincentis, G. A 3-variable prognostic score (3-PS) for overall survival prediction in metastatic castration-resistant prostate cancer treated with 223Radium-dichloride. Ann. Nucl. Med. 2018, 32, 142–148. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Monari, F.; Mascia, M.; Costa, R.; Rubini, G.; Spanu, A.; Di Rocco, A.; Lodi Rizzini, E.; Cindolo, L.; Licari, M.; et al. Validation of the 3-variable prognostic score (3-PS) in mCRPC patients treated with 223Radium-dichloride: A national multicenter study. Ann. Nucl. Med. 2020, 34, 772–780. [Google Scholar] [CrossRef]
- Etchebehere, E.C.; Araujo, J.C.; Fox, P.S.; Swanston, N.M.; Macapinlac, H.A.; Rohren, E.M. Prognostic Factors in Patients Treated with 223Ra: The Role of Skeletal Tumor Burden on Baseline 18F-Fluoride PET/CT in Predicting Overall Survival. J. Nucl. Med. 2015, 56, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Fosbøl, M.; Petersen, P.M.; Kjaer, A.; Mortensen, J. 223Ra Therapy of Advanced Metastatic Castration-Resistant Prostate Cancer: Quantitative Assessment of Skeletal Tumor Burden for Prognostication of Clinical Outcome and Hematologic Toxicity. J. Nucl. Med. 2018, 59, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Dittman, H.; Campi, C.; Morbelli, S.; Marini, C.; Brignone, M.; Bauckneht, M.; Piva, R.; Massone, A.M.; Piana, M.; et al. Assessment of Skeletal Tumor Load in Metastasized Castration-Resistant Prostate Cancer Patients: A Review of Available Methods and an Overview on Future Perspectives. Bioengineering 2018, 5, 58. [Google Scholar] [CrossRef]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of Baseline and Post-Therapy 18F-FDG PET in the Prognostic Stratification of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients Treated with Radium-223. Cancers 2019, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Rebuzzi, S.E.; Signori, A.; Frantellizzi, V.; Murianni, V.; Lodi Rizzini, E.; Mascia, M.; Lavelli, V.; Donegani, M.I.; Ponzano, M.; et al. The prognostic power of inflammatory indices and clinical factors in metastatic castration-resistant prostate cancer patients treated with radium-223 (BIO-Ra study). Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1063–1074. [Google Scholar] [CrossRef]
- Bauckneht, M.; Lai, R.; D’Amico, F.; Miceli, A.; Donegani, M.I.; Campi, C.; Schenone, D.; Raffa, S.; Chiola, S.; Lanfranchi, F.; et al. Opportunistic skeletal muscle metrics as prognostic tools in metastatic castration-resistant prostate cancer patients candidates to receive Radium-223. Ann. Nucl. Med. 2022, 36, 373–383. [Google Scholar] [CrossRef]
- Bauckneht, M.; Bertagna, F.; Donegani, M.I.; Durmo, R.; Miceli, A.; De Biasi, V.; Laudicella, R.; Fornarini, G.; Berruti, A.; Baldari, S.; et al. The prognostic power of 18F-FDG PET/CT extends to estimating systemic treatment response duration in metastatic castration-resistant prostate cancer (mCRPC) patients. Prostate Cancer Prostatic Dis. 2021, 24, 1198–1207. [Google Scholar] [CrossRef]
- Sabbatini, P.; Larson, S.M.; Kremer, A.; Zhang, Z.F.; Sun, M.; Yeung, H.; Imbriaco, M.; Horak, I.; Conolly, M.; Ding, C.; et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 948–957. [Google Scholar] [CrossRef]
- Mota, J.M.; Armstrong, A.J.; Larson, S.M.; Fox, J.J.; Morris, M.J. Measuring the unmeasurable: Automated bone scan index as a quantitative endpoint in prostate cancer clinical trials. Prostate Cancer Prostatic Dis. 2019, 22, 522–530. [Google Scholar] [CrossRef]
- Anand, A.; Morris, M.J.; Larson, S.M.; Minarik, D.; Josefsson, A.; Helgstrand, J.T.; Oturai, P.S.; Edenbrandt, L.; Røder, M.A.; Bjartell, A. Automated Bone Scan Index as a quantitative imaging biomarker in metastatic castration-resistant prostate cancer patients being treated with enzalutamide. EJNMMI Res. 2016, 6, 23. [Google Scholar] [CrossRef]
- Uemura, K.; Miyoshi, Y.; Kawahara, T.; Yoneyama, S.; Hattori, Y.; Teranishi, J.; Kondo, K.; Moriyama, M.; Takebayashi, S.; Yokomizo, Y.; et al. Prognostic value of a computer-aided diagnosis system involving bone scans among men treated with docetaxel for metastatic castration-resistant prostate cancer. BMC Cancer 2016, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Ukai, R.; Hashimoto, K. Bone scan index can be a useful biomarker of survival outcomes in patients with metastatic castration-resistant prostate cancer treated with radium-223. Cancer Rep. 2019, 2, e1203. [Google Scholar] [CrossRef]
- Ulmert, D.; Kaboteh, R.; Fox, J.J.; Savage, C.; Evans, M.J.; Lilja, H.; Abrahamsson, P.A.; Björk, T.; Gerdtsson, A.; Bjartell, A.; et al. A novel automated platform for quantifying the extent of skeletal tumour involvement in prostate cancer patients using the Bone Scan Index. Eur. Urol. 2012, 62, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M. EXINI Quantitative Bone Scan Index: Expanded Utility for the Planar Radionuclide Bone Scan. J. Nucl. Med. 2016, 57, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Trägårdh, E.; Edenbrandt, L.; Beckman, L.; Svensson, J.H.; Thellenberg, C.; Widmark, A.; Kindblom, J.; Ullén, A.; Bjartell, A. Assessing Radiographic Response to 223Ra with an Automated Bone Scan Index in Metastatic Castration-Resistant Prostate Cancer Patients. J. Nucl. Med. 2020, 61, 671–675. [Google Scholar] [CrossRef]
- Kitajima, K.; Igeta, M.; Kuyama, J.; Kawahara, T.; Suga, T.; Otani, T.; Sugawara, S.; Kono, Y.; Tamaki, Y.; Seko-Nitta, A.; et al. Novel nomogram developed for determining suitability of metastatic castration-resistant prostate cancer patients to receive maximum benefit from radium-223 dichloride treatment-Japanese Ra-223 Therapy in Prostate Cancer using Bone Scan Index (J-RAP-BSI) Trial. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1487–1498. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Costa, R.; Mascia, M.; Spanu, A.; Farcomeni, A.; Licari, M.; Cindolo, L.; Nuvoli, S.; Pontico, M.; De Vincentis, G. Primary Radical Prostatectomy or Ablative Radiotherapy as Protective Factors for Patients With mCRPC Treated With Radium-223 Dichloride: An Italian Multicenter Study. Clin. Genitourin Cancer 2020, 18, 185–191. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Monari, F.; Mascia, M.; Costa, R.; Rubini, G.; Spanu, A.; Farcomeni, A.; Lodi Rizzini, E.; Cindolo, L.; Licari, M.; et al. Overall survival in mCPRC patients treated with Radium-223 in association with bone health agents: A national multicenter study. Int. J. Radiat. Biol. 2020, 96, 1608–1613. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Monari, F.; Mascia, M.; Costa, R.P.; Rubini, G.; Spanu, A.; Farcomeni, A.; Lodi Rizzini, E.; Cindolo, L.; Tripoli, V.; et al. Radium-223 in mCPRC patients: A large real-life Italian multicenter study. Minerva Urol. Nephrol. 2022, 74, 21–28. [Google Scholar] [CrossRef]
- Even-Sapir, E.; Metser, U.; Mishani, E.; Lievshitz, G.; Lerman, H.; Leibovitch, I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J. Nucl. Med. 2006, 47, 287–297. [Google Scholar]
- Yang, H.L.; Liu, T.; Wang, X.M.; Xu, Y.; Deng, S.M. Diagnosis of bone metastases: A meta-analysis comparing ¹⁸FDG PET, CT, MRI and bone scintigraphy. Eur. Radiol. 2011, 21, 2604–2617. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean (SD) | Patients | Percentage |
|---|---|---|---|
| Age (years) | 73.6 ± 8.1 | ||
| Gleason score | 7.9 ± 1.0 | ||
| 5 | 2 | 0.5% | |
| 6 | 18 | 4.9% | |
| 7 | 105 | 28.4% | |
| 8 | 83 | 22.4% | |
| 9 | 98 | 26.5% | |
| 10 | 7 | 1.9% | |
| unknown | 57 | 15.4% | |
| ECOG PS | 0.7 ± 0.8 | ||
| 0 | 176 | 47.6% | |
| 1 | 131 | 35.4% | |
| 2 | 58 | 15.7% | |
| 3 | 5 | 1.3% | |
| Previous primary treatment | |||
| Radical prostatectomy/Radiotherapy | 159 | 43% | |
| No | 202 | 54.6% | |
| missing | 9 | 2.4% | |
| Lymphadenopathies | |||
| Yes | 129 | 34.9% | |
| No | 203 | 54.8% | |
| unknown | 38 | 10.3% | |
| Prior chemotherapy | |||
| Yes | 230 | 62.2% | |
| No | 140 | 37.8% | |
| Use of bisphosphonates/denosumab | |||
| Yes | 193 | 52.2% | |
| No | 177 | 47.8% | |
| Number of previous treatments | 1.9 ± 1.4 | ||
| 0 | 51 | 13.8% | |
| 1 | 102 | 27.5% | |
| 2 | 89 | 24.1% | |
| ≥3 | 119 | 32.2% | |
| missing | 9 | 2.4% | |
| Number of cycles of 223RaCl2 received | 5.3 ± 1.3 | ||
| 1 | 8 | 2.2% | |
| 2 | 13 | 3.5% | |
| 3 | 26 | 7% | |
| 4 | 28 | 7.6% | |
| 5 | 33 | 8.9% | |
| 6 | 262 | 70.8% | |
| Baseline tALP (U/L) | 251.1 ± 310.9 | ||
| Baseline PSA (ng/ml) | 230.7 ± 580.1 | ||
| Baseline Hb (g/dL) | 11.9 ± 1.6 | ||
| Baseline BSI (%) | 2.98 ± 2.42 |
| Baseline Clinical Variables | HR | CI. Low | CI. Up | p-Value |
|---|---|---|---|---|
| Age | 1.011 | 1.001 | 1.021 | 0.026 |
| Radical prostatectomy/Radiotherapy | 0.787 | 0.724 | 0.856 | <0.001 |
| Gleason score | 1.115 | 1.016 | 1.225 | 0.022 |
| Lymphadenopathies | 1.437 | 1.014 | 2.037 | 0.042 |
| ECOG | 1.580 | 1.186 | 2.104 | 0.002 |
| Hb | 0.706 | 0.676 | 0.738 | <0.001 |
| tALP | 1.001 | 1.001 | 1.001 | <0.001 |
| PSA | 1.001 | 1.000 | 1.001 | <0.001 |
| BSI | 1.137 | 1.052 | 1.230 | 0.001 |
| Baseline Clinical Variables | HR | CI. Low | CI. Up | p-Value |
|---|---|---|---|---|
| Gleason score | 1.096 | 1.051 | 1.144 | <0.001 |
| Hb | 0.744 | 0.723 | 0.766 | <0.001 |
| tALP | 1.001 | 1.000 | 1.001 | <0.001 |
| PSA | 1.000 | 1.000 | 1.001 | <0.001 |
| BSI | 1.054 | 1.040 | 1.068 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Feo, M.S.; Frantellizzi, V.; Bauckneht, M.; Farcomeni, A.; Filippi, L.; Rizzini, E.L.; Lavelli, V.; Stazza, M.L.; Di Raimondo, T.; Fornarini, G.; et al. The DASciS Software for BSI Calculation as a Valuable Prognostic Tool in mCRPC Treated with 223RaCl2: A Multicenter Italian Study. Biomedicines 2023, 11, 1103. https://doi.org/10.3390/biomedicines11041103
De Feo MS, Frantellizzi V, Bauckneht M, Farcomeni A, Filippi L, Rizzini EL, Lavelli V, Stazza ML, Di Raimondo T, Fornarini G, et al. The DASciS Software for BSI Calculation as a Valuable Prognostic Tool in mCRPC Treated with 223RaCl2: A Multicenter Italian Study. Biomedicines. 2023; 11(4):1103. https://doi.org/10.3390/biomedicines11041103
Chicago/Turabian StyleDe Feo, Maria Silvia, Viviana Frantellizzi, Matteo Bauckneht, Alessio Farcomeni, Luca Filippi, Elisa Lodi Rizzini, Valentina Lavelli, Maria Lina Stazza, Tania Di Raimondo, Giuseppe Fornarini, and et al. 2023. "The DASciS Software for BSI Calculation as a Valuable Prognostic Tool in mCRPC Treated with 223RaCl2: A Multicenter Italian Study" Biomedicines 11, no. 4: 1103. https://doi.org/10.3390/biomedicines11041103
APA StyleDe Feo, M. S., Frantellizzi, V., Bauckneht, M., Farcomeni, A., Filippi, L., Rizzini, E. L., Lavelli, V., Stazza, M. L., Di Raimondo, T., Fornarini, G., Rebuzzi, S. E., Filippo, M., Mammucci, P., Marongiu, A., Monari, F., Rubini, G., Spanu, A., & De Vincentis, G. (2023). The DASciS Software for BSI Calculation as a Valuable Prognostic Tool in mCRPC Treated with 223RaCl2: A Multicenter Italian Study. Biomedicines, 11(4), 1103. https://doi.org/10.3390/biomedicines11041103








