Influence of Various Light Regimes on Morphofunctional Condition of Transplantable Melanoma B16
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Objects
2.2. Study Design
2.3. Morphological and Morphometric Methods
2.4. Immunohistochemical Methods
- PER2–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:200;
- BMAL1–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:200;
- Clock–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:200.
2.5. Measurement of Serum Melatonin Concentration
2.6. Methods of Statistical Processing
3. Results
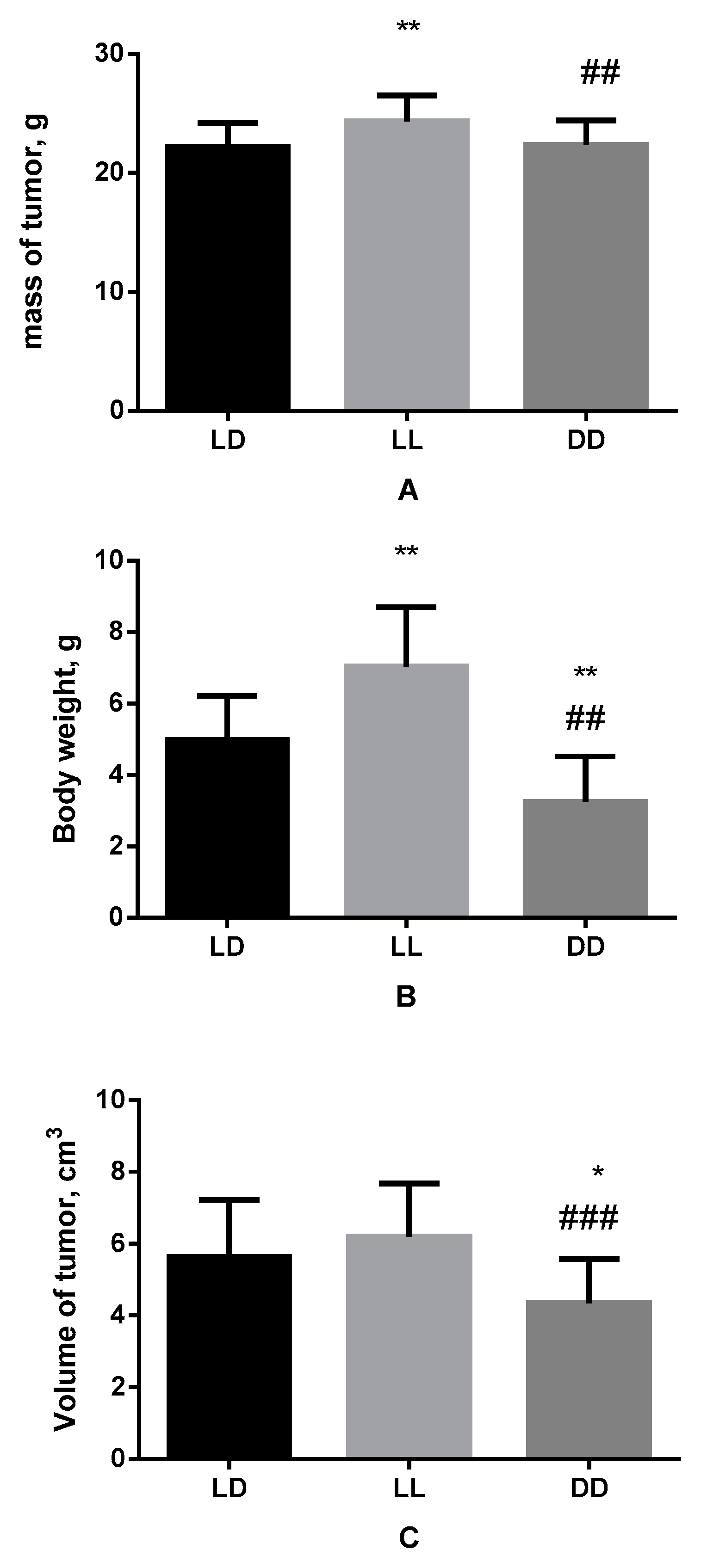
3.1. Influence of Various Light Regimes on Body Weight, Mass and Volume of the Tumor
3.2. Influence of Various Light Regimes on Morphological Characteristics of B16 Melanoma
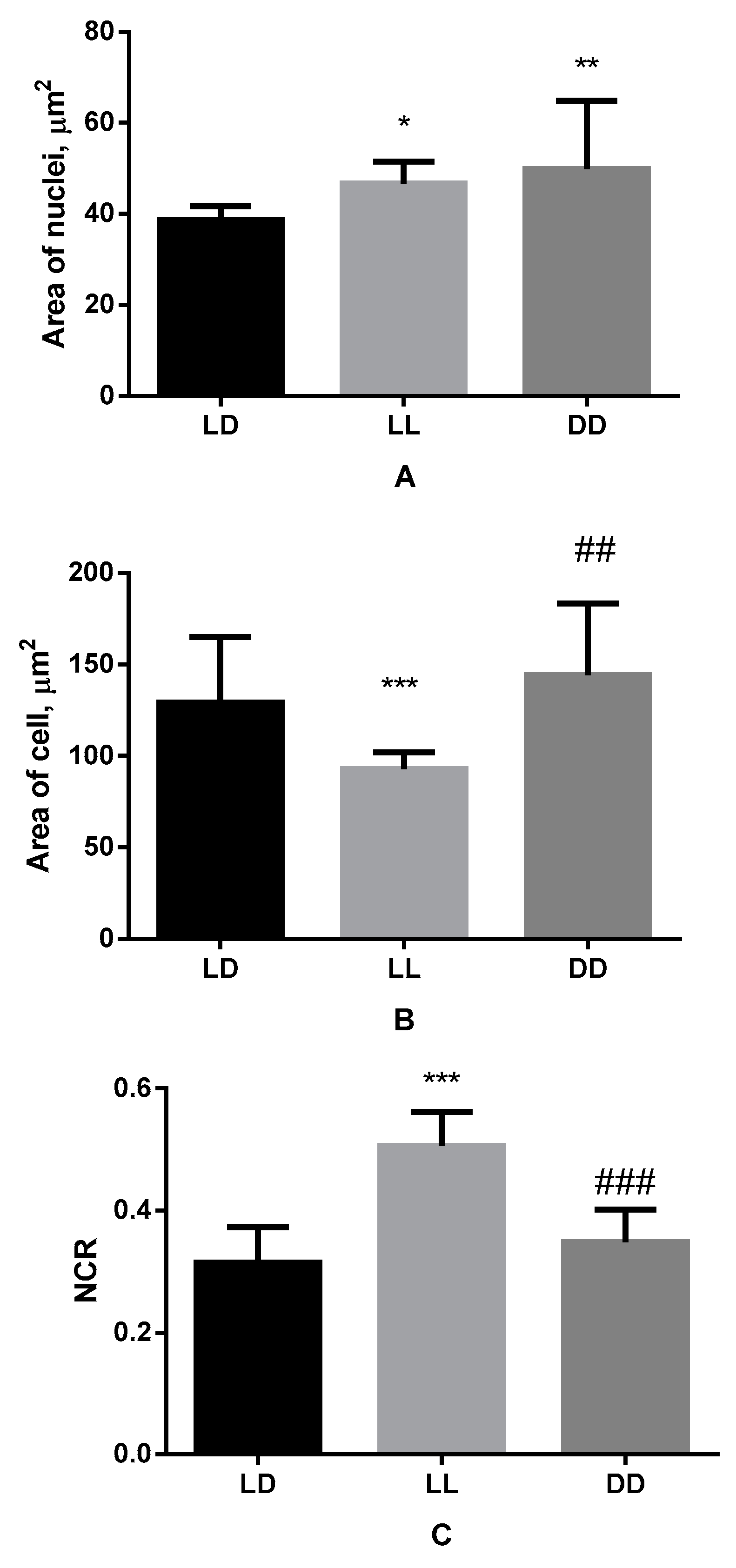
3.3. Influence of Various Lighting Regimes on Micromorphometric Parameters of Cells of B16 Melanoma
3.4. Serum Melatonin Concentration
3.5. Influence of Various Lighting Regimes on Clock Genes Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKenna, H.; Van Der Horst, G.T.J.; Reiss, I.; Martin, D. Clinical chronobiology: A timely consideration in critical care medicine. Crit. Care 2018, 22, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hernández, R.; Escobar, C.; Buijs, R.M. Suprachiasmatic Nucleus–Arcuate Nucleus Axis: Interaction Between Time and Metabolism Essential for Health. Obesity 2020, 28 (Suppl. 1), S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Oster, H.; Lehnert, H.; Schmid, S.; Salamat, N.; Barclay, J.L.; Maronde, E.; Inder, W.; Rawashdeh, O. Coupling the Circadian Clock to Homeostasis: The Role of Period in Timing Physiology. Endocr. Rev. 2019, 40, 66–95. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.; Kinouchi, K.; Sassone-Corsi, P. Circadian clocks, epigenetics, and cancer. Curr. Opin. Oncol. 2015, 27, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Sulli, G.; Lam, M.T.Y.; Panda, S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer 2019, 5, 475–494. [Google Scholar] [CrossRef]
- Shafi, A.A.; Knudsen, K.E. Cancer and the Circadian Clock. Cancer Res. 2019, 79, 3806–3814. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.G. Artificial Lighting in the Industrialized World: Circadian Disruption and Breast Cancer. Cancer Causes Control 2006, 17, 501–507. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; Manucha, W.; Chuffa, L.G.D.A.; Zuccari, D.A.P.D.C. Melatonin and Pathological Cell Interactions: Mitochondrial Glucose Processing in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 12494. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Baturin, D.A.; Popovich, I.G.; Zabezhinski, M.A.; Manton, K.G.; Semenchenko, A.V.; Yashin, A.I. Effect of exposure to light-at-night on life span and spontaneous carcinogenesis in female CBA mice. Int. J. Cancer 2004, 111, 475–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baturin, D.A.; Alimova, I.N.; Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A.; Provinciali, M.; Mancini, R.; Franceschi, C. The effect of light regimen and melatonin on the development of spontaneous mammary tumors in HER-2/neu transgenic mice is related to a downregulation of HER-2/neu gene expression. Neuroendocrinol. Lett. 2001, 22, 441–447. [Google Scholar] [PubMed]
- Yu, H.S.; Reiter, R.J. Reiter Melatonin Biosynthesis, Physiological Effects, and Clinical Applications; CRC Press: Boca Raton, FL, USA, 1993; 560p. [Google Scholar]
- Van Den Heiligenberg, S.; Deprés-Brummer, P.; Barbason, H.; Claustrat, B.; Reynes, M.; Lévi, F. The tumor promoting effect of constant light exposure on diethylnitrosamine-induced hepatocarcinogenesis in rats. Life Sci. 1999, 64, 2523–2534. [Google Scholar] [CrossRef]
- Blask, D.; Sauer, L.; Dauchy, R.; Holowachuk, E.; Ruhoff, M. New Actions of Melatonin on Tumor Metabolism and Growth. Biol. Signals Recept. 1999, 8, 49–55. [Google Scholar] [CrossRef]
- Jasser, S.A.; Blask, D.E.; Brainard, G.C. Light During Darkness and Cancer: Relationships in Circadian Photoreception and Tumor Biology. Cancer Causes Control 2006, 17, 515–523. [Google Scholar] [CrossRef]
- Li, J.; Xu, F. Influences of Light-Dark Shifting on the Immune System, Tumor Growth and Life Span of Rats, Mice and Fruit Flies as well as on the Counteraction of Melatonin. Biol. Signals 1997, 6, 77–89. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E.J.; Won, G.; Lee, J.; Lee, S.; Kim, S.-H. Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic Properties of Melatonin in Hypoxic PC-3 Prostate Cancer Cells. J. Cancer 2015, 6, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Domínguez, N.; Méndez-Blanco, C.; Carbajo-Pescador, S.; Fondevila, F.; García-Palomo, A.; González-Gallego, J.; Mauriz, J.L. Melatonin enhances sorafenib actions in human hepatocarcinoma cells by inhibiting mTORC1/p70S6K/HIF-1α and hypoxia-mediated mitophagy. Oncotarget 2017, 8, 91402–91414. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Antoniello, N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: Review and remarks. Aging Clin. Exp. Res. 2013, 25, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zharinov, G.M.; Bogomolov, O.A.; Chepurnaya, I.V.; Neklasova, N.Y.; Anisimov, V.N. Melatonin increases overall survival of prostate cancer patients with poor prognosis after combined hormone radiation treatment. Oncotarget 2020, 11, 3723–3729. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Yaribeygi, H.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Antitumor and Protective Effects of Melatonin: The Potential Roles of MicroRNAs. Adv Exp Med Biol. 2021, 1328, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, S.; Liu, Z.; Li, C.; Li, M.; Zhao, X. Melatonin Inhibits 17β-Estradiol-Induced Epithelial-Mesenchymal Transition in Endometrial Adenocarcinoma Cells via Upregulating Numb Expression. Gynecol. Obstet. Investig. 2022, 87, 89–99. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.V.M.; Moraes, M.N.; Castrucci, A.M.D.L. The molecular clock in the skin, its functionality, and how it is disrupted in cutaneous melanoma: A new pharmacological target? Cell. Mol. Life Sci. 2019, 76, 3801–3826. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How cancer hijacks the body’s homeostasis through the neuroendocrine system. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- de Assis, L.V.M.; Moraes, M.N.; Mendes, D.; Silva, M.M.; Menck, C.F.M.; Castrucci, A.M.L. Loss of Melanopsin (OPN4) Leads to a Faster Cell Cycle Progression and Growth in Murine Melanocytes. Curr. Issues Mol. Biol. 2021, 43, 101. [Google Scholar] [CrossRef]
- Lubov, J.; Cvammen, W.; Kemp, M. The Impact of the Circadian Clock on Skin Physiology and Cancer Development. Int. J. Mol. Sci. 2021, 22, 6112. [Google Scholar] [CrossRef]
- Fischer, T.W.; Slominski, A.; Zmijewski, M.A.; Reiter, R.J.; Paus, R. Melatonin as a major skin protectant: From free radical scavenging to DNA damage repair. Exp. Dermatol. 2008, 17, 713–730. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Mubashshir, M.; Ahmad, N.; Sköld, H.N.; Ovais, M. An exclusive review of melatonin effects on mammalian melanocytes and melanoma. Exp. Dermatol. 2022, 32, 324–330. [Google Scholar] [CrossRef]
- Jansen, R.; Wang, S.Q.; Burnett, M.; Osterwalder, U.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2013, 69, 853.e1–853.e12. [Google Scholar] [CrossRef] [PubMed]
- Colin-Gonzalez, A.; Aguilera, G.; Serratos, I.; Escribano, B.; Santamaria, A.; Tunez, I. On the Relationship Between the Light/Dark Cycle, Melatonin and Oxidative Stress. Curr. Pharm. Des. 2015, 21, 3477–3488. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Pruski, D. Melatonin Inhibits Proliferation and Melanogenesis in Rodent Melanoma Cells. Exp. Cell Res. 1993, 206, 189–194. [Google Scholar] [CrossRef]
- Fischer, T.W.; Zmijewski, M.A.; Zbytek, B.; Sweatman, T.W.; Slominski, R.M.; Wortsman, J.; Slominski, A. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 2006, 29, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Artime, A.; Cernuda-Cernuda, R.; Naveda, F.A.; Cepas, V.; Gonzalez-Menendez, P.; Fernadez-Vega, S.; Quiros-Gonzalez, I.; Sainz, R.M.; Mayo, J.C. Melatonin-Induced Cytoskeleton Reorganization Leads to Inhibition of Melanoma Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; López-Valverde, A.; Arias-Santiago, S.; De Vicente, J.; De Diego, R.G. Role of melatonin in cancer treatment. Anticancer. Res. 2012, 32, 2747–2753. [Google Scholar] [PubMed]
- Pathipaka, R.; Thyagarajan, A.; Sahu, R.P. Melatonin as a Repurposed Drug for Melanoma Treatment. Med. Sci. 2023, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 2003, 270, 3335–3344. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Treschalina, E.M.; Zhukova, O.S.; Gerasimova, G.K.; Andronova, N.G.; Garin, A.M. Methodical recommendations for the preclinical study of the antitumor activity of drugs. In Guidelines for Conducting Preclinical Studies of Drugs. Part One; Grif i K: Moscow, Russia, 2012; pp. 642–657. [Google Scholar]
- Burghoff, S.; Gong, X.; Viethen, C.; Jacoby, C.; Flögel, U.; Bongardt, S.; Schorr, A.; Hippe, A.; Homey, B.; Schrader, J. Growth and metastasis of B16-F10 melanoma cells is not critically dependent on host CD73 expression in mice. BMC Cancer 2014, 14, 898. [Google Scholar] [CrossRef] [Green Version]
- Underwood, W.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D. AVMA Guidelines for the Euthanasia of Animals; American Veterinary Medical Association: Schaumburg, IL, USA, 2013. [Google Scholar]
- Overwijk, W.W.; Restifo, N.P. B16 as a Mouse Model for Human Melanoma. Curr. Protoc. Immunol. 2001, 39, 20.1.1–20.1.29. [Google Scholar] [CrossRef]
- Ishikawa, M.; Koga, Y.; Hosokawa, M.; Kobayashi, H. Augmentation of B16 melanoma lung colony formation in C57BL/6 mice having marked granulocytosis. Int. J. Cancer 1986, 37, 919–924. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef]
- Broeke, J.; Pérez, J.M.M.; Pascau, J. Image Processing with ImageJ; Packt Publishing: Birmingham, UK, 2015; p. 346. [Google Scholar]
- Smitha, T.; Sharada, P.; Girish, H. Morphometry of the basal cell layer of oral leukoplakia and oral squamous cell carcinoma using computer-aided image analysis. J. Oral Maxillofac. Pathol. 2011, 15, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cree, I.A.; Tan, P.H.; Travis, W.D.; Wesseling, P.; Yagi, Y.; White, V.A.; Lokuhetty, D.; Scolyer, R.A. Counting mitoses: SI(ze) matters! Mod. Pathol. 2021, 34, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Roh, J.; Park, C.-S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Rubin Grandis, J.; Melhem, M.F.; Barnes, E.L.; Tweardy, D.J. Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 1996, 78, 1284–1292. [Google Scholar] [CrossRef]
- Gorman, M.R. Temporal organization of pineal melatonin signaling in mammals. Mol. Cell. Endocrinol. 2020, 503, 110687. [Google Scholar] [CrossRef]
- Rumanova, V.S.; Okuliarova, M.; Zeman, M. Differential Effects of Constant Light and Dim Light at Night on the Circadian Control of Metabolism and Behavior. Int. J. Mol. Sci. 2020, 21, 5478. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Franco, P.I.R.; Neto, J.R.D.C.; Milhomem, A.C.; Machado, J.R.; Miguel, M.P. Antitumor effect of melatonin on breast cancer in experimental models: A systematic review. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188838. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Mirzaei, H.; Asemi, Z. Melatonin: An anti-tumor agent for osteosarcoma. Cancer Cell Int. 2019, 19, 319. [Google Scholar] [CrossRef]
- Shen, D.; Ju, L.; Zhou, F.; Yu, M.; Ma, H.; Zhang, Y.; Liu, T.; Xiao, Y.; Wang, X.; Qian, K. The inhibitory effect of melatonin on human prostate cancer. Cell Commun. Signal. 2021, 19, 34. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. Role and Therapeutic Potential of Melatonin in Various Type of Cancers. Onco Targets Ther. 2021, 14, 2019–2052. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Andrade, L.; Floeter-Winter, L.M.; Rollag, M.D.; Virador, V.; Vieira, W.; Castrucci, A.M.D.L. MT-1 melatonin receptor expression increases the antiproliferative effect of melatonin on S-91 murine melanoma cells. J. Pineal Res. 2004, 36, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.; Dai, J.; Yuan, L.; Dong, C.; Kiefer, T.; Lai, L.; Hill, S. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett. 2002, 179, 141–150. [Google Scholar] [CrossRef] [PubMed]
- García-Navarro, A.; González-Puga, C.; Escames, G.; López, L.C.; López, A.; López-Cantarero, M.; Camacho, E.; Espinosa, A.; Gallo, M.A.; Acuña-Castroviejo, D. Cellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J. Pineal Res. 2007, 43, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, L.; Radogna, F.; Accorsi, A.; Cristina Albertini, M.; Gualandi, G.; Ghibelli, L. Melatonin as a Modulator of Apoptosis in B-Lymphoma Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 345–349. [Google Scholar] [CrossRef]
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhood, B.; Goradel, N.H.; Mortezaee, K.; Khanlarkhani, N.; Najafi, M.; Sahebkar, A. Melatonin and cancer: From the promotion of genomic stability to use in cancer treatment. J. Cell. Physiol. 2019, 234, 5613–5627. [Google Scholar] [CrossRef]
- Akashi, M.; Soma, H.; Yamamoto, T.; Tsugitomi, A.; Yamashita, S.; Yamamoto, T.; Nishida, E.; Yasuda, A.; Liao, J.K.; Node, K. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl. Acad. Sci. USA 2010, 107, 15643–15648. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Ruben, M.D.; Schmidt, R.E.; Francey, L.J.; Smith, D.F.; Anafi, R.C.; Hughey, J.J.; Tasseff, R.; Sherrill, J.D.; Oblong, J.E.; et al. Population-level rhythms in human skin with implications for circadian medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 12313–12318. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, Z.; Lovig, C.; Kommedal, S.; Keszthelyi, R.; Szekeres, G.; Battyáni, Z.; Csernus, V.; Nagy, A.D. Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumor Biol. 2013, 34, 811–819. [Google Scholar] [CrossRef]
- Sandu, C.; Dumas, M.; Malan, A.; Sambakhe, D.; Marteau, C.; Nizard, C.; Schnebert, S.; Perrier, E.; Challet, E.; Pévet, P.; et al. Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cell. Mol. Life Sci. 2012, 69, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Kettner, N.M. The Circadian Clock in Cancer Development and Therapy. Prog. Mol. Biol. Transl. Sci. 2013, 119, 221–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinouchi, K.; Sassone-Corsi, P. Metabolic rivalry: Circadian homeostasis and tumorigenesis. Nat. Rev. Cancer 2020, 20, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, L.; Ferrari, A.; De Mei, C.; Parodi, C.; Wade, M.; Grimaldi, B. Circadian clock: Time for novel anticancer strategies? Pharmacol. Res. 2015, 100, 288–295. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areshidze, D.A.; Kozlova, M.A.; Mnikhovich, M.V.; Bezuglova, T.V.; Chernikov, V.P.; Gioeva, Z.V.; Borisov, A.V. Influence of Various Light Regimes on Morphofunctional Condition of Transplantable Melanoma B16. Biomedicines 2023, 11, 1135. https://doi.org/10.3390/biomedicines11041135
Areshidze DA, Kozlova MA, Mnikhovich MV, Bezuglova TV, Chernikov VP, Gioeva ZV, Borisov AV. Influence of Various Light Regimes on Morphofunctional Condition of Transplantable Melanoma B16. Biomedicines. 2023; 11(4):1135. https://doi.org/10.3390/biomedicines11041135
Chicago/Turabian StyleAreshidze, David A., Maria A. Kozlova, Maxim V. Mnikhovich, Tatyana V. Bezuglova, Valery P. Chernikov, Zarina V. Gioeva, and Aleksey V. Borisov. 2023. "Influence of Various Light Regimes on Morphofunctional Condition of Transplantable Melanoma B16" Biomedicines 11, no. 4: 1135. https://doi.org/10.3390/biomedicines11041135
APA StyleAreshidze, D. A., Kozlova, M. A., Mnikhovich, M. V., Bezuglova, T. V., Chernikov, V. P., Gioeva, Z. V., & Borisov, A. V. (2023). Influence of Various Light Regimes on Morphofunctional Condition of Transplantable Melanoma B16. Biomedicines, 11(4), 1135. https://doi.org/10.3390/biomedicines11041135




