Making the Best Use of Available Weapons for the Inevitable Rivalry-Resistance to EGFR-TKIs
Abstract
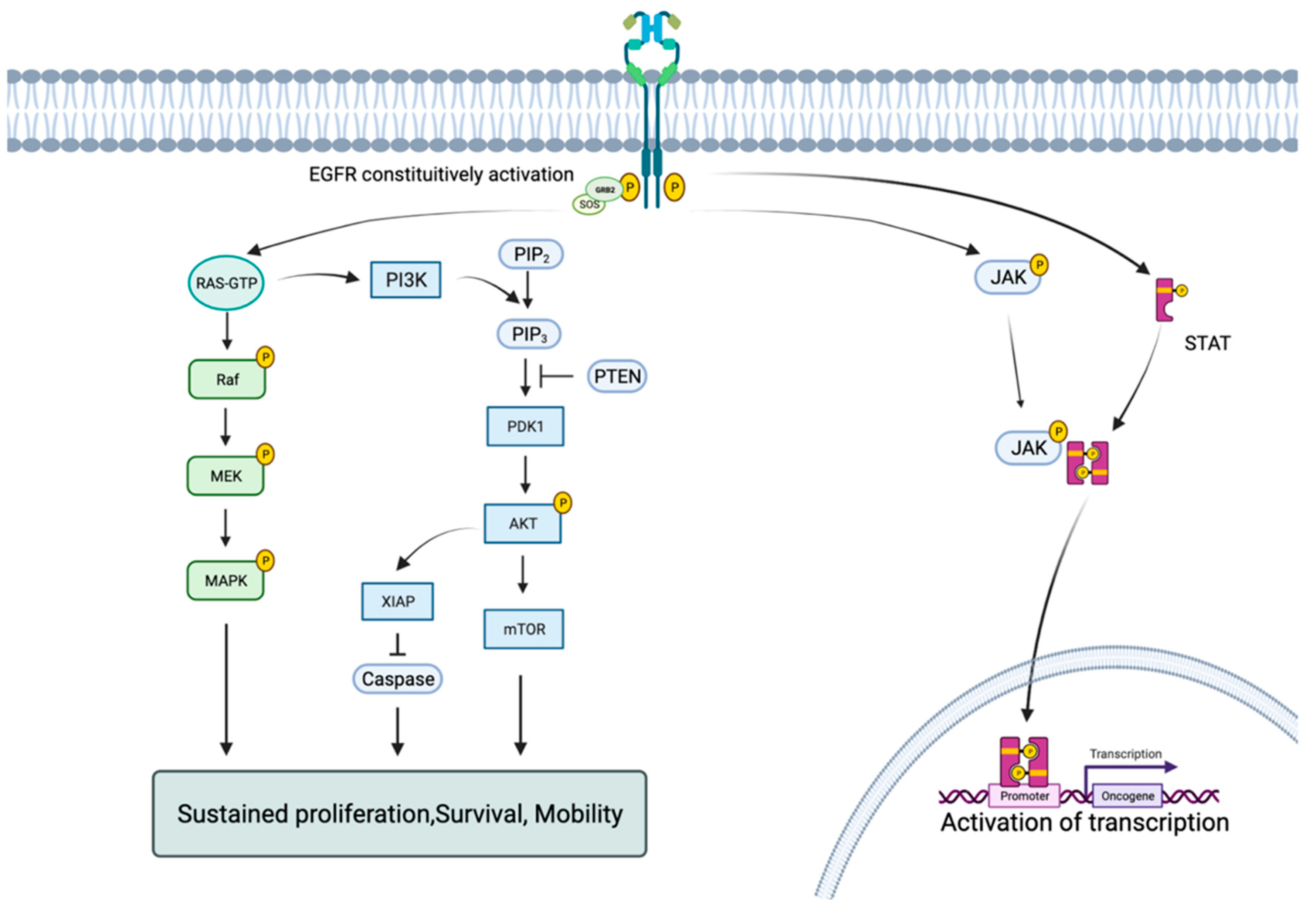
:1. Introduction
2. Conversant with Our Weapons: Clinical Data of Third-Generation EGFR-TKIs
3. Drug Arrangement in Sequential Treatment-Maximizing Benefits for Patients
4. Facing the Inevitable Rivalry: Mechanisms and Strategies against Them
4.1. EGFR Dependent Mutations
4.1.1. C797S Mutations
4.1.2. EGFR Independent Mechanisms of Resistance
4.1.3. Small Cell Lung Cancer (SCLC) Transformation
4.1.4. MET Amplification
4.1.5. HER2 Amplification
4.1.6. Overexpression of AXL
4.1.7. Downstream BRAFV600E Mutations
4.1.8. Agnostic-Based Strategies: New Hopes
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Tsui, S.T.; Liu, C.; Song, Y.; Liu, D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, N.; Zhang, Y.; Li, L.; Han, R.; Zhu, M.; Feng, M.; Chen, H.; Lizaso, A.; Qin, T.; et al. Effective Treatment of Lung Adenocarcinoma Harboring EGFR-Activating Mutation, T790M, and cis-C797S Triple Mutations by Brigatinib and Cetuximab Combination Therapy. J. Thorac. Oncol. 2020, 15, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, D.; To, C.; Shpilsky, J.E.; VanderLaan, P.A.; Kobayashi, S.S.; Mushajiang, M.; Lau, C.J.; Paweletz, C.P.; Oxnard, G.R.; Janne, P.A.; et al. EGFR-Mutated Lung Cancers Resistant to Osimertinib through EGFR C797S Respond to First-Generation Reversible EGFR Inhibitors but Eventually Acquire EGFR T790M/C797S in Preclinical Models and Clinical Samples. J. Thorac. Oncol. 2019, 14, 1995–2002. [Google Scholar] [CrossRef]
- Niederst, M.J.; Hu, H.; Mulvey, H.E.; Lockerman, E.L.; Garcia, A.R.; Piotrowska, Z.; Sequist, L.V.; Engelman, J.A. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin. Cancer Res. 2015, 21, 3924–3933. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zou, M.; Lv, J.; Han, Y.; Wang, G.; Wang, G. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: A case report. Onco Targets Ther. 2018, 11, 5545–5550. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Liu, S.; Jiang, Y.; Hua, L.; Wen, L. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, cis-G796s/cis-C797s by osimertinib, brigatinib, and bevacizumab combination therapy: A case report. Respir. Med. Case Rep. 2022, 36, 101582. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Ma, L.; Yang, L.; Yang, G.; Zhang, S.; Ai, X.; Zhang, S.; Wang, Y. Possibility of brigatinib-based therapy, or chemotherapy plus anti-angiogenic treatment after resistance of osimertinib harboring EGFR T790M-cis-C797S mutations in lung adenocarcinoma patients. Cancer Med. 2021, 10, 8328–8337. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.J.; Huang, J.; Ye, J.Y.; Zhang, X.C.; Tu, H.Y.; Han-Zhang, H.; Wu, Y.L. Lung Adenocarcinoma Harboring EGFR T790M and In Trans C797S Responds to Combination Therapy of First- and Third-Generation EGFR TKIs and Shifts Allelic Configuration at Resistance. J. Thorac. Oncol. 2017, 12, 1723–1727. [Google Scholar] [CrossRef] [Green Version]
- Arulananda, S.; Do, H.; Musafer, A.; Mitchell, P.; Dobrovic, A.; John, T. Combination Osimertinib and Gefitinib in C797S and T790M EGFR-Mutated Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 1728–1732. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, Y.L. Re-emerging C797S In Trans and Rechallenge of Osimertinib With Erlotinib. J. Thorac. Oncol. 2019, 14, e81–e82. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Kashima, K.; Kawauchi, H.; Tanimura, H.; Tachibana, Y.; Chiba, T.; Torizawa, T.; Sakamoto, H. CH7233163 Overcomes Osimertinib-Resistant EGFR-Del19/T790M/C797S Mutation. Mol. Cancer Ther. 2020, 19, 2288–2297. [Google Scholar] [CrossRef]
- Dong, R.F.; Zhu, M.L.; Liu, M.M.; Xu, Y.T.; Yuan, L.L.; Bian, J.; Xia, Y.Z.; Kong, L.Y. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharm. Res. 2021, 167, 105583. [Google Scholar] [CrossRef]
- Mambetsariev, I.; Arvanitis, L.; Fricke, J.; Pharaon, R.; Baroz, A.R.; Afkhami, M.; Koczywas, M.; Massarelli, E.; Salgia, R. Small Cell Lung Cancer Transformation following Treatment in EGFR-Mutated Non-Small Cell Lung Cancer. J. Clin. Med. 2022, 11, 1429. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Wang, H.; Jia, T.; Wang, E.; Luo, Y.; Wei, Y.; Qin, Z.; Ma, X. Small cell lung cancer transformation: From pathogenesis to treatment. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Marcoux, N.; Gettinger, S.N.; O’Kane, G.; Arbour, K.C.; Neal, J.W.; Husain, H.; Evans, T.L.; Brahmer, J.R.; Muzikansky, A.; Bonomi, P.D.; et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J. Clin. Oncol. 2019, 37, 278–285. [Google Scholar] [CrossRef]
- Bai, W.; Zhen, C.; Zhang, R.; Yu, W.; Zhou, Z. Clinicopathological features of patients with transformation from EGFR mutant lung adenocarcinoma to small cell lung cancer. Transl. Cancer Res. 2021, 10, 3694–3704. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Prim. 2021, 7, 3. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Xu, W.; Li, J.; Liu, Y.; Zeng, X.; Zhong, M.; Zhu, Y. The Multi-Omics Analysis of Key Genes Regulating EGFR-TKI Resistance, Immune Infiltration, SCLC Transformation in EGFR-Mutant NSCLC. J. Inflamm. Res. 2022, 15, 649–667. [Google Scholar] [CrossRef]
- Pros, E.; Saigi, M.; Alameda, D.; Gomez-Mariano, G.; Martinez-Delgado, B.; Alburquerque-Bejar, J.J.; Carretero, J.; Tonda, R.; Esteve-Codina, A.; Catala, I.; et al. Genome-wide profiling of non-smoking-related lung cancer cells reveals common RB1 rearrangements associated with histopathologic transformation in EGFR-mutant tumors. Ann. Oncol. 2020, 31, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xu, L.; Wang, B.; Kong, W.; Chen, Y.; Yu, Z. Outcomes in Patients With Lung Adenocarcinoma With Transformation to Small Cell Lung Cancer After EGFR Tyrosine Kinase Inhibitors Resistance: A Systematic Review and Pooled Analysis. Front. Oncol. 2021, 11, 766148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C. Durable response to durvalumab-based immunochemotherapy in small-cell lung carcinoma transformation from EGFR-mutant non-small cell lung cancer: A case report. Thorac. Cancer 2022, 13, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.; Brennan, C.; Shih, J.Y.; Riely, G.; Viale, A.; Wang, L.; Chitale, D.; Motoi, N.; Szoke, J.; Broderick, S.; et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA 2007, 104, 20932–20937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Inui, N.; Karayama, M.; Inoue, Y.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; Takeuchi, K.; Sugimura, H.; Suda, T. Successful crizotinib monotherapy in EGFR-mutant lung adenocarcinoma with acquired MET amplification after erlotinib therapy. Respir. Med. Case Rep. 2017, 20, 160–163. [Google Scholar] [CrossRef]
- Ou, S.I.; Govindan, R.; Eaton, K.D.; Otterson, G.A.; Gutierrez, M.E.; Mita, A.C.; Argiris, A.; Brega, N.M.; Usari, T.; Tan, W.; et al. Phase I Results from a Study of Crizotinib in Combination with Erlotinib in Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 145–151. [Google Scholar] [CrossRef] [Green Version]
- De Mello, R.A.; Neves, N.M.; Amaral, G.A.; Lippo, E.G.; Castelo-Branco, P.; Pozza, D.H.; Tajima, C.C.; Antoniou, G. The Role of MET Inhibitor Therapies in the Treatment of Advanced Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 1918. [Google Scholar] [CrossRef]
- Wu, Y.L.; Cheng, Y.; Zhou, J.; Lu, S.; Zhang, Y.; Zhao, J.; Kim, D.W.; Soo, R.A.; Kim, S.W.; Pan, H.; et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): An open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir. Med. 2020, 8, 1132–1143. [Google Scholar] [CrossRef]
- Sequist, L.V.; Han, J.Y.; Ahn, M.J.; Cho, B.C.; Yu, H.; Kim, S.W.; Yang, J.C.; Lee, J.S.; Su, W.C.; Kowalski, D.; et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020, 21, 373–386. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.Y.; Kim, S.W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Nagasaka, M.; Balmanoukian, A.S.; Madison, R.; Zhang, S.S.; Klempner, S.J.; Ou, S.I. Amivantamab (JNJ-61186372) induces clinical, biochemical, molecular, and radiographic response in a treatment-refractory NSCLC patient harboring amplified triple EGFR mutations (L858R/T790M/G796S) in cis. Lung Cancer 2022, 164, 52–55. [Google Scholar] [CrossRef]
- Cho, B.C.; Felip, E.; Hayashi, H.; Thomas, M.; Lu, S.; Besse, B.; Sun, T.; Martinez, M.; Sethi, S.N.; Shreeve, S.M.; et al. MARIPOSA: Phase 3 study of first-line amivantamab + lazertinib versus osimertinib in EGFR-mutant non-small-cell lung cancer. Future Oncol. 2022, 18, 639–647. [Google Scholar] [CrossRef]
- Shah, M.P.; Neal, J.W. Targeting Acquired and Intrinsic Resistance Mechanisms in Epidermal Growth Factor Receptor Mutant Non-Small-Cell Lung Cancer. Drugs 2022, 82, 649–662. [Google Scholar] [CrossRef]
- Planchard, D.; Loriot, Y.; Andre, F.; Gobert, A.; Auger, N.; Lacroix, L.; Soria, J.C. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann. Oncol. 2015, 26, 2073–2078. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Chen, Z.H.; Zhang, X.C.; Xu, C.R.; Yan, H.H.; Xie, Z.; Chuai, S.K.; Ye, J.Y.; Han-Zhang, H.; Zhang, Z.; et al. Analysis of resistance mechanisms to abivertinib, a third-generation EGFR tyrosine kinase inhibitor, in patients with EGFR T790M-positive non-small cell lung cancer from a phase I trial. EBioMedicine 2019, 43, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, S.; Hai, J.; Wang, X.; Chen, T.; Quinn, M.M.; Gao, P.; Zhang, Y.; Ji, H.; Cross, D.A.E.; et al. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin. Cancer Res. 2018, 24, 2594–2604. [Google Scholar] [CrossRef] [Green Version]
- Gan, J.; Huang, Y.; Liao, J.; Pang, L.; Fang, W. HER2 Amplification in Advanced NSCLC Patients After Progression on EGFR-TKI and Clinical Response to EGFR-TKI Plus Pyrotinib Combination Therapy. Onco Targets Ther. 2021, 14, 5297–5307. [Google Scholar] [CrossRef]
- Colavito, S.A. AXL as a Target in Breast Cancer Therapy. J. Oncol. 2020, 2020, 5291952. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef]
- Jeong, I.; Song, J.; Bae, S.Y.; Lee, S.K. Overcoming the Intrinsic Gefitinib-resistance via Downregulation of AXL in Non-small Cell Lung Cancer. J. Cancer Prev. 2019, 24, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Bach, D.H.; Fan, Y.H.; Luu, T.T.; Hong, J.Y.; Park, H.J.; Lee, S.K. AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells. Cell Death Dis. 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okura, N.; Nishioka, N.; Yamada, T.; Taniguchi, H.; Tanimura, K.; Katayama, Y.; Yoshimura, A.; Watanabe, S.; Kikuchi, T.; Shiotsu, S.; et al. ONO-7475, a Novel AXL Inhibitor, Suppresses the Adaptive Resistance to Initial EGFR-TKI Treatment in EGFR-Mutated Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Liao, W.Y.; Lin, C.A.; Shih, J.Y.; Yu, C.J.; Yang, J.C. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Osimertinib. J. Thorac. Oncol. 2017, 12, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, T.; Tachihara, M.; Nishimura, Y. Mechanism of Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors and a Potential Treatment Strategy. Cells 2018, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Maniar, R.; Gallitano, S.M.; Husain, S.; Moazami, G.; Weiss, M.J.; Shu, C.A. Unusual Adverse Events in a Patient With BRAF-Mutated Non-Small Cell Lung Cancer Treated With BRAF/MEK Inhibition. J. Natl. Compr. Cancer Netw. 2023, 21, 232–234. [Google Scholar] [CrossRef]
- La Monica, S.; Cretella, D.; Bonelli, M.; Fumarola, C.; Cavazzoni, A.; Digiacomo, G.; Flammini, L.; Barocelli, E.; Minari, R.; Naldi, N.; et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J. Exp. Clin. Cancer Res. 2017, 36, 174. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Yu, D.; Tian, W.; Wu, F. Resistance mechanisms to osimertinib and emerging therapeutic strategies in nonsmall cell lung cancer. Curr. Opin. Oncol. 2022, 34, 54–65. [Google Scholar] [CrossRef]
- Haikala, H.M.; Janne, P.A. Thirty Years of HER3: From Basic Biology to Therapeutic Interventions. Clin. Cancer Res. 2021, 27, 3528–3539. [Google Scholar] [CrossRef]
- Kawano, O.; Sasaki, H.; Endo, K.; Suzuki, E.; Haneda, H.; Yukiue, H.; Kobayashi, Y.; Yano, M.; Fujii, Y. ErbB3 mRNA expression correlated with specific clinicopathologic features of Japanese lung cancers. J. Surg. Res. 2008, 146, 43–48. [Google Scholar] [CrossRef]
- Janne, P.A.; Baik, C.; Su, W.C.; Johnson, M.L.; Hayashi, H.; Nishio, M.; Kim, D.W.; Koczywas, M.; Gold, K.A.; Steuer, C.E.; et al. Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor-Resistant, EGFR-Mutated Non-Small Cell Lung Cancer. Cancer Discov. 2022, 12, 74–89. [Google Scholar] [CrossRef]
- Yang, C.J.; Hung, J.Y.; Tsai, M.J.; Wu, K.L.; Liu, T.C.; Chou, S.H.; Lee, J.Y.; Hsu, J.S.; Huang, M.S.; Chong, I.W. The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy. BMC Pharm. Toxicol 2017, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target Ther. 2022, 7, 93. [Google Scholar] [CrossRef]



| Generic | Trade Name | Chemical Formula | Indications |
|---|---|---|---|
| Erlotinib | Tarceva® |  | First-generation EGFR-TKIs, which could reversibly bind to EGFR. Sensitive mutations include 19del and L858R. They are now the first-line drug choice for EGFR-sensitive mutations. T790M mutations would likely arise and cause resistance. |
| Gefitinib | Iressa® |  | |
| Icotinib | Conmana® |  | |
| Afatinib | Giotrif® |  | Second-generation EGFR-TKIs irreversibly bind to EGFR in a stronger manner. Effectively control major uncommon EGFR mutations. However, adverse events are more severe. |
| Dacomitinib | Vizimpro® |  | |
| Osimertinib | Tagrisso® |  | Third-generation EGFR-TKIs, which irreversibly bind to EGFR and effectively manage T790M mutation. Meanwhile, they can penetrate the Blood–Brain Barrier and thereby control CNS metastasis, providing more choice for the advanced stage. |
| Fumonertinib | Ivesa® |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wang, J.; Liu, C.; Luo, Y.; Xu, H.; Wang, Y.; Sun, N.; He, J. Making the Best Use of Available Weapons for the Inevitable Rivalry-Resistance to EGFR-TKIs. Biomedicines 2023, 11, 1141. https://doi.org/10.3390/biomedicines11041141
Li D, Wang J, Liu C, Luo Y, Xu H, Wang Y, Sun N, He J. Making the Best Use of Available Weapons for the Inevitable Rivalry-Resistance to EGFR-TKIs. Biomedicines. 2023; 11(4):1141. https://doi.org/10.3390/biomedicines11041141
Chicago/Turabian StyleLi, Dongyu, Jingnan Wang, Chengming Liu, Yuejun Luo, Haiyan Xu, Yan Wang, Nan Sun, and Jie He. 2023. "Making the Best Use of Available Weapons for the Inevitable Rivalry-Resistance to EGFR-TKIs" Biomedicines 11, no. 4: 1141. https://doi.org/10.3390/biomedicines11041141
APA StyleLi, D., Wang, J., Liu, C., Luo, Y., Xu, H., Wang, Y., Sun, N., & He, J. (2023). Making the Best Use of Available Weapons for the Inevitable Rivalry-Resistance to EGFR-TKIs. Biomedicines, 11(4), 1141. https://doi.org/10.3390/biomedicines11041141







