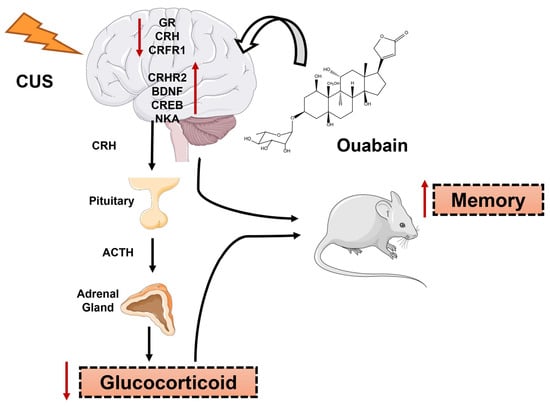
Ouabain Reverts CUS-Induced Disruption of the HPA Axis and Avoids Long-Term Spatial Memory Deficits
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Chronic Unpredictable Stress (CUS)
2.2. Measurement of Corticosterone, CRH, ACTH, and Cytokine Levels
2.3. Measurement of Neuronal (nNOS) and Inducible (iNOS) NOS Activity
2.4. Real-Time PCR
2.5. Novel Object Recognition (NOR)
2.6. Contextual Fear Conditioning and Memory Extinction
2.7. Statistics
3. Results
3.1. Ouabain Interferes with HPA Axis Hyperactivity Induced by CUS
3.2. Effect of Chronic Unpredictable Stress (CUS) and Ouabain on Inflammatory Parameters in the Hippocampus and Hypothalamus
3.3. OUA Did Not Alter the Effects of CUS-Exposure on Antioxidant Enzymes Expression
3.4. The Crh, Crhr1, and Crhr2 Gene Expression was Modified by Both CUS and Chronic Intermittent Ouabain Treatment in the Hippocampus and Hypothalamus
3.5. CUS-Induced Long-Term Memory Impairment Reduced by OUA
3.6. OUA Promotes A Rapid Extinction of Fear Memory without Interfering with the Acquisition of Aversive Memory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasckow, J.W.; Baker, D.; Geracioti, T.D. Corticotropin-Releasing Hormone in Depression and Post-Traumatic Stress Disorder. Peptides 2001, 22, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; Vale, W.W. The Neurobiology of Depression: Inroads to Treatment and New Drug Discovery. J. Clin. Psychiatry 2005, 66 (Suppl. S7), 5–13. [Google Scholar]
- Itoi, K.; Suda, T.; Tozawa, F.; Dobashi, I.; Ohmori, N.; Sakai, Y.; Abe, K.; Demura, H. Microinjection of Norepinephrine into the Paraventricular Nucleus of the Hypothalamus Stimulates Corticotropin-Releasing Factor Gene Expression in Conscious Rats. Endocrinology 1994, 135, 2177–2182. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–Pituitary–Adrenal Axis, Neuroendocrine Factors and Stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.V.; Rivier, C.L. Regulation of the Hypothalamic-Pituitary-Adrenal Axis by Cytokines: Actions and Mechanisms of Action. Physiol. Rev. 1999, 79, 1–71. [Google Scholar] [CrossRef]
- Majzoub, J.A. Corticotropin-Releasing Hormone Physiology. Eur. J. Endocrinol. 2006, 155 (Suppl. S1), S71–S76. [Google Scholar] [CrossRef]
- Stenzel-Poore, M.; Heinrichs, S.; Rivest, S.; Koob, G.; Vale, W. Overproduction of Corticotropin-Releasing Factor in Transgenic Mice: A Genetic Model of Anxiogenic Behavior. J. Neurosci. 1994, 14, 2579–2584. [Google Scholar] [CrossRef]
- van Gaalen, M.M.; Stenzel-Poore, M.; Holsboer, F.; Steckler, T. Reduced Attention in Mice Overproducing Corticotropin-Releasing Hormone. Behav. Brain Res. 2003, 142, 69–79. [Google Scholar] [CrossRef]
- Bale, T.L.; Vale, W.W. CRF and CRF Receptors: Role in Stress Responsivity and Other Behaviors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 525–557. [Google Scholar] [CrossRef]
- Heinrichs, S.C.; Lapsansky, J.; Lovenberg, T.W.; De Souza, E.B.; Chalmers, D.T. Corticotropin-Releasing Factor CRF1, but Not CRF2, Receptors Mediate Anxiogenic-like Behavior. Regul. Pept. 1997, 71, 15–21. [Google Scholar] [CrossRef]
- Goto, A.; Yamada, K.; Nagoshi, H.; Terano, Y.; Omata, M. Stress-Induced Elevation of Ouabainlike Compound in Rat Plasma and Adrenal. Hypertension 1995, 26, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in Structure, Diversity in Function. Am. J. Physiol. Ren. Physiol. 1998, 275, F633–F650. [Google Scholar] [CrossRef] [PubMed]
- Gloor, S.M. Relevance of Na,K-ATPase to Local Extracellular Potassium Homeostasis and Modulation of Synaptic Transmission. FEBS Lett. 1997, 412, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Lax, E.; Gispan-Herman, I.; Ovadia, H.; Rosen, H.; Yadid, G.; Lichtstein, D. Neutralization of Endogenous Digitalis-like Compounds Alters Catecholamines Metabolism in the Brain and Elicits Anti-Depressive Behavior. Eur. Neuropsychopharmacol. 2012, 22, 72–79. [Google Scholar] [CrossRef]
- Goldstein, I.; Lerer, E.; Laiba, E.; Mallet, J.; Mujaheed, M.; Laurent, C.; Rosen, H.; Ebstein, R.P.; Lichtstein, D. Association between Sodium- and Potassium-Activated Adenosine Triphosphatase Alpha Isoforms and Bipolar Disorders. Biol. Psychiatry 2009, 65, 985–991. [Google Scholar] [CrossRef]
- Goldstein, I.; Levy, T.; Galili, D.; Ovadia, H.; Yirmiya, R.; Rosen, H.; Lichtstein, D. Involvement of Na+, K+-ATPase and Endogenous Digitalis-like Compounds in Depressive Disorders. Biol. Psychiatry 2006, 60, 491–499. [Google Scholar] [CrossRef]
- Munhoz, C.D.; Lepsch, L.B.; Kawamoto, E.M.; Malta, M.B.; Lima, L.D.S.; Avellar, M.C.W.; Sapolsky, R.M.; Scavone, C. Chronic Unpredictable Stress Exacerbates Lipopolysaccharide-Induced Activation of Nuclear Factor- B in the Frontal Cortex and Hippocampus via Glucocorticoid Secretion. J. Neurosci. 2006, 26, 3813–3820. [Google Scholar] [CrossRef]
- Leite, J.A.; Cavalcante-Silva, L.H.A.; Ribeiro, M.R.; de Morais Lima, G.; Scavone, C.; Rodrigues-Mascarenhas, S. Neuroinflammation and Neutrophils: Modulation by Ouabain. Front. Pharmacol. 2022, 13, 824907. [Google Scholar] [CrossRef]
- Kinoshita, P.F.; Yshii, L.M.; Vasconcelos, A.R.; Orellana, A.M.M.; Lima, L.D.S.; Davel, A.P.C.; Rossoni, L.V.; Kawamoto, E.M.; Scavone, C. Signaling Function of Na,K-ATPase Induced by Ouabain against LPS as an Inflammation Model in Hippocampus. J. Neuroinflam. 2014, 11, 218. [Google Scholar] [CrossRef]
- McKee, M.; Scavone, C.; Nathanson, J.A. Nitric Oxide, CGMP, and Hormone Regulation of Active Sodium Transport. Proc. Natl. Acad. Sci. USA 1994, 91, 12056–12060. [Google Scholar] [CrossRef]
- Roozendaal, B.; Okuda, S.; Van der Zee, E.A.; McGaugh, J.L. Glucocorticoid Enhancement of Memory Requires Arousal-Induced Noradrenergic Activation in the Basolateral Amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- Novaes, L.S.; Bueno-de-Camargo, L.M.; Munhoz, C.D. Environmental Enrichment Prevents the Late Effect of Acute Stress-Induced Fear Extinction Deficit: The Role of Hippocampal AMPA-GluA1 Phosphorylation. Transl. Psychiatry 2021, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural Regulation of Endocrine and Autonomic Stress Responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Karagkouni, A.; Alevizos, M.; Theoharides, T.C. Effect of Stress on Brain Inflammation and Multiple Sclerosis. Autoimmun. Rev. 2013, 12, 947–953. [Google Scholar] [CrossRef]
- Bilici, M.; Efe, H.; Köroğlu, M.A.; Uydu, H.A.; Bekaroğlu, M.; Değer, O. Antioxidative Enzyme Activities and Lipid Peroxidation in Major Depression: Alterations by Antidepressant Treatments. J. Affect. Disord. 2001, 64, 43–51. [Google Scholar] [CrossRef]
- Lucca, G.; Comim, C.M.; Valvassori, S.S.; Réus, G.Z.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Gavioli, E.C.; Quevedo, J. Effects of Chronic Mild Stress on the Oxidative Parameters in the Rat Brain. Neurochem. Int. 2009, 54, 358–362. [Google Scholar] [CrossRef]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, Memory and the Amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- de Quervain, D.; Wolf, O.T.; Roozendaal, B. Glucocorticoid-Induced Enhancement of Extinction—From Animal Models to Clinical Trials. Psychopharmacology 2019, 236, 183–199. [Google Scholar] [CrossRef]
- Munhoz, C.D.; Sorrells, S.F.; Caso, J.R.; Scavone, C.; Sapolsky, R.M. Glucocorticoids Exacerbate Lipopolysaccharide-Induced Signaling in the Frontal Cortex and Hippocampus in a Dose-Dependent Manner. J. Neurosci. 2010, 30, 13690–13698. [Google Scholar] [CrossRef]
- Okuda, S.; Roozendaal, B.; McGaugh, J.L. Glucocorticoid Effects on Object Recognition Memory Require Training-Associated Emotional Arousal. Proc. Natl. Acad. Sci. USA 2004, 101, 853–858. [Google Scholar] [CrossRef]
- Featherstone, R.E.; Gifford, R.L.; Crown, L.M.; Amirfathi, F.; Alaniz, J.P.; Yi, J.; Tran, A.; Adomian, D.; Schwenk, A.; Melnychenko, O.; et al. Early Life Social Instability Stress Causes Lasting Cognitive Decrement and Elevated Hippocampal Stress-Related Gene Expression. Exp. Neurol. 2022, 354, 114099. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr. Rev. 2020, 41, 470–490. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Iranmanesh, A.; Naftolowitz, D.; Tatham, N.; Cassidy, F.; Carroll, B.J. Corticotropin Secretory Dynamics in Humans under Low Glucocorticoid Feedback. J. Clin. Endocrinol. Metab. 2001, 86, 5554–5563. [Google Scholar] [CrossRef]
- Gibbison, B.; Spiga, F.; Walker, J.J.; Russell, G.M.; Stevenson, K.; Kershaw, Y.; Zhao, Z.; Henley, D.; Angelini, G.D.; Lightman, S.L. Dynamic Pituitary-Adrenal Interactions in Response to Cardiac Surgery. Crit. Care Med. 2015, 43, 791–800. [Google Scholar] [CrossRef] [PubMed]
- McIlmoil, S.; Strickland, J.; Judd, A.M. Interleukin 6 Increases the in Vitro Expression of Key Proteins Associated with Steroidogenesis in the Bovine Adrenal Zona Fasciculata. Domest. Anim. Endocrinol. 2016, 55, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Spiga, F.; Zavala, E.; Walker, J.J.; Zhao, Z.; Terry, J.R.; Lightman, S.L. Dynamic Responses of the Adrenal Steroidogenic Regulatory Network. Proc. Natl. Acad. Sci. USA 2017, 114, E6466–E6474. [Google Scholar] [CrossRef]
- López-López, A.L.; Jaime, H.B.; Escobar Villanueva, M.D.C.; Padilla, M.B.; Palacios, G.V.; Aguilar, F.J.A. Chronic Unpredictable Mild Stress Generates Oxidative Stress and Systemic Inflammation in Rats. Physiol. Behav. 2016, 161, 15–23. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef]
- Leite, J.A.; Alves, A.K.D.A.; Galvão, J.G.M.; Teixeira, M.P.; Cavalcante-Silva, L.H.A.; Scavone, C.; Morrot, A.; Rumjanek, V.M.; Rodrigues-Mascarenhas, S. Ouabain Modulates Zymosan-Induced Peritonitis in Mice. Mediat. Inflamm. 2015, 2015, 265798. [Google Scholar] [CrossRef]
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain Interleukin-1 Mediates Chronic Stress-Induced Depression in Mice via Adrenocortical Activation and Hippocampal Neurogenesis Suppression. Mol. Psychiatry 2007, 13, 717–728. [Google Scholar] [CrossRef]
- Grippo, A.J.; Francis, J.; Beltz, T.G.; Felder, R.B.; Johnson, A.K. Neuroendocrine and Cytokine Profile of Chronic Mild Stress-Induced Anhedonia. Physiol. Behav. 2005, 84, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Sze, J.; Marin, T.; Arevalo, J.M.G.; Doll, R.; Ma, R.; Cole, S.W. A Functional Genomic Fingerprint of Chronic Stress in Humans: Blunted Glucocorticoid and Increased NF-ΚB Signaling. Biol. Psychiatry 2008, 64, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Dinkel, K.; MacPherson, A.; Sapolsky, R.M. Novel Glucocorticoid Effects on Acute Inflammation in the CNS. J. Neurochem. 2003, 84, 705–716. [Google Scholar] [CrossRef]
- Gonzalez, P.; Machado, I.; Vilcaes, A.; Caruso, C.; Roth, G.A.; Schiöth, H.; Lasaga, M.; Scimonelli, T. Molecular Mechanisms Involved in Interleukin 1-Beta (IL-1β)-Induced Memory Impairment. Modulation by Alpha-Melanocyte-Stimulating Hormone (α-MSH). Brain Behav. Immun. 2013, 34, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Karalis, K.; Sano, H.; Redwine, J.; Listwak, S.; Wilder, R.L.; Chrousos, G.P. Autocrine or Paracrine Inflammatory Actions of Corticotropin-Releasing Hormone in Vivo. Science 1991, 254, 421–423. [Google Scholar] [CrossRef]
- Wang, N.; Ma, H.; Li, Z.; Gao, Y.; Cao, X.; Jiang, Y.; Zhou, Y.; Liu, S. Chronic Unpredictable Stress Exacerbates Surgery-Induced Sickness Behavior and Neuroinflammatory Responses via Glucocorticoids Secretion in Adult Rats. PLoS ONE 2017, 12, e0183077. [Google Scholar] [CrossRef]
- Elizabeth, A.; Adegbuyi, A.; Olusegun, A.; Benneth, B.-A.; Anthony, E.; Abayomi, A.; Solomon, U. Morin Hydrate Attenuates Chronic Stress-Induced Memory Impairment and Degeneration of Hippocampal Subfields in Mice: The Role of Oxidative, Nitrergic and Neuroinflammatory Pathways. Metab. Brain Dis. 2020, 35, 1145–1156. [Google Scholar] [CrossRef]
- Sevgi, S.; Ozek, M.; Eroglu, L. L-NAME Prevents Anxiety-like and Depression-like Behaviour in Rats Exposed to Restraint Stress. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 95. [Google Scholar] [CrossRef]
- Zhou, Q.-G.; Zhu, L.-J.; Chen, C.; Wu, H.-Y.; Luo, C.-X.; Chang, L.; Zhu, D.-Y. Hippocampal Neuronal Nitric Oxide Synthase Mediates the Stress-Related Depressive Behaviors of Glucocorticoids by Downregulating Glucocorticoid Receptor. J. Neurosci. 2011, 31, 7579–7590. [Google Scholar] [CrossRef]
- Wang, S.-S.; Kamphuis, W.; Huitinga, I.; Zhou, J.-N.; Swaab, D.F. Gene Expression Analysis in the Human Hypothalamus in Depression by Laser Microdissection and Real-Time PCR: The Presence of Multiple Receptor Imbalances. Mol. Psychiatry 2008, 13, 786–799. [Google Scholar] [CrossRef]
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.-H. Severe Life Stress and Oxidative Stress in the Brain: From Animal Models to Human Pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kuhad, A.; Chopra, K. Neuropsychopharmacological Effect of Sesamol in Unpredictable Chronic Mild Stress Model of Depression: Behavioral and Biochemical Evidences. Psychopharmacology 2010, 214, 819–828. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A Review on the Oxidative and Nitrosative Stress (O&NS) Pathways in Major Depression and Their Possible Contribution to the (Neuro)Degenerative Processes in That Illness. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef]
- Garcia, I.J.P.; Kinoshita, P.F.; Silva, L.N.D.; Souza Busch, M.; Atella, G.C.; Scavone, C.; Cortes, V.F.; Barbosa, L.A.; Lima Santos, H. Ouabain Attenuates Oxidative Stress and Modulates Lipid Composition in Hippocampus of Rats in Lipopolysaccharide-Induced Hypocampal Neuroinflammation in Rats. J. Cell. Biochem. 2018, 120, 4081–4091. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Segal, M. Striking Variations in Corticosteroid Modulation of Long-Term Potentiation along the Septotemporal Axis of the Hippocampus. J. Neurosci. 2007, 27, 5757–5765. [Google Scholar] [CrossRef]
- Shavit Stein, E.; Itsekson Hayosh, Z.; Vlachos, A.; Maggio, N. Stress and Corticosteroids Modulate Muscarinic Long Term Potentiation (MLTP) in the Hippocampus. Front. Cell. Neurosci. 2017, 11, 299. [Google Scholar] [CrossRef]
- Orellana, A.M.; Leite, J.A.; Kinoshita, P.F.; Vasconcelos, A.R.; Andreotti, D.Z.; de Sá Lima, L.; Xavier, G.F.; Kawamoto, E.M.; Scavone, C. Ouabain Increases Neuronal Branching in Hippocampus and Improves Spatial Memory. Neuropharmacology 2018, 140, 260–274. [Google Scholar] [CrossRef]
- Kinoshita, P.F.; Leite, J.A.; Orellana, A.M.M.; Vasconcelos, A.R.; Quintas, L.E.M.; Kawamoto, E.M.; Scavone, C. The Influence of Na+, K+-ATPase on Glutamate Signaling in Neurodegenerative Diseases and Senescence. Front. Physiol. 2016, 7, 195. [Google Scholar] [CrossRef]
- de Sá Lima, L.; Kawamoto, E.M.; Munhoz, C.D.; Kinoshita, P.F.; Orellana, A.M.M.; Curi, R.; Rossoni, L.V.; Avellar, M.C.W.; Scavone, C. Ouabain Activates NFκB through an NMDA Signaling Pathway in Cultured Cerebellar Cells. Neuropharmacology 2013, 73, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.M.; Lima, L.S.; Munhoz, C.D.; Yshii, L.M.; Kinoshita, P.F.; Amara, F.G.; Pestana, R.R.F.; Orellana, A.M.M.; Cipolla-Neto, J.; Britto, L.R.G.; et al. Influence of N-Methyl-D-Aspartate Receptors on Ouabain Activation of Nuclear Factor-ΚB in the Rat Hippocampus. J. Neurosci. Res. 2011, 90, 213–228. [Google Scholar] [CrossRef]
- Munhoz, C.D.; Kawamoto, E.M.; de Sá Lima, L.; Lepsch, L.B.; Glezer, I.; Marcourakis, T.; Scavone, C. Glutamate Modulates Sodium-Potassium-ATPase through Cyclic GMP and Cyclic GMP-Dependent Protein Kinase in Rat Striatum. Cell Biochem. Funct. 2004, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.; Blumenthal, L.; Man, H.-Y. Regulation of Neuronal Bioenergy Homeostasis by Glutamate. Neurochem. Int. 2012, 61, 389–396. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chattarji, S. Timing Is Everything: Differential Effects of Chronic Stress on Fear Extinction. Psychopharmacology 2018, 236, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Gale, G.D.; Anagnostaras, S.G.; Godsil, B.P.; Mitchell, S.; Nozawa, T.; Sage, J.R.; Wiltgen, B.; Fanselow, M.S. Role of the Basolateral Amygdala in the Storage of Fear Memories across the Adult Lifetime of Rats. J. Neurosci. 2004, 24, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.-Z. From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits. Front. Neural Circuits 2017, 11, 86. [Google Scholar] [CrossRef]
- Maren, S.; Phan, K.L.; Liberzon, I. The Contextual Brain: Implications for Fear Conditioning, Extinction and Psychopathology. Nat. Rev. Neurosci. 2013, 14, 417–428. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Skórzewska, A.; Wisłowska-Stanek, A.; Lehner, M.; Turzyńska, D.; Sobolewska, A.; Krząścik, P.; Szyndler, J.; Maciejak, P.; Płaźnik, A. The Effect of a Corticotropin-Releasing Factor Receptor 1 Antagonist on the Fear Conditioning Response in Low- and High-Anxiety Rats after Chronic Corticosterone Administration. Stress 2018, 22, 113–122. [Google Scholar] [CrossRef]
- Ferland-Beckham, C.; Chaby, L.E.; Daskalakis, N.P.; Knox, D.; Liberzon, I.; Lim, M.M.; McIntyre, C.; Perrine, S.A.; Risbrough, V.B.; Sabban, E.L.; et al. Systematic Review and Methodological Considerations for the Use of Single Prolonged Stress and Fear Extinction Retention in Rodents. Front. Behav. Neurosci. 2021, 15, 652636. [Google Scholar] [CrossRef]
- Herman, J.P. Neural Control of Chronic Stress Adaptation. Front. Behav. Neurosci. 2013, 7, 61. [Google Scholar] [CrossRef]









| Gene | Primer ID |
|---|---|
| Crh | NM_031019.1 |
| Crhr1 | XM_006247542.2 |
| Crhr2 | NM_022714.2 |
| Sod1 | NM_017050.1 |
| Sod2 | NM_017051.2 |
| Gsr | NM_053906.2 |
| Hprt-1 | NM_012583.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, J.A.; Orellana, A.M.; Andreotti, D.Z.; Matumoto, A.M.; de Souza Ports`, N.M.; de Sá Lima, L.; Kawamoto, E.M.; Munhoz, C.D.; Scavone, C. Ouabain Reverts CUS-Induced Disruption of the HPA Axis and Avoids Long-Term Spatial Memory Deficits. Biomedicines 2023, 11, 1177. https://doi.org/10.3390/biomedicines11041177
Leite JA, Orellana AM, Andreotti DZ, Matumoto AM, de Souza Ports` NM, de Sá Lima L, Kawamoto EM, Munhoz CD, Scavone C. Ouabain Reverts CUS-Induced Disruption of the HPA Axis and Avoids Long-Term Spatial Memory Deficits. Biomedicines. 2023; 11(4):1177. https://doi.org/10.3390/biomedicines11041177
Chicago/Turabian StyleLeite, Jacqueline Alves, Ana Maria Orellana, Diana Zukas Andreotti, Amanda Midori Matumoto, Natacha Medeiros de Souza Ports`, Larissa de Sá Lima, Elisa Mitiko Kawamoto, Carolina Demarchi Munhoz, and Cristoforo Scavone. 2023. "Ouabain Reverts CUS-Induced Disruption of the HPA Axis and Avoids Long-Term Spatial Memory Deficits" Biomedicines 11, no. 4: 1177. https://doi.org/10.3390/biomedicines11041177
APA StyleLeite, J. A., Orellana, A. M., Andreotti, D. Z., Matumoto, A. M., de Souza Ports`, N. M., de Sá Lima, L., Kawamoto, E. M., Munhoz, C. D., & Scavone, C. (2023). Ouabain Reverts CUS-Induced Disruption of the HPA Axis and Avoids Long-Term Spatial Memory Deficits. Biomedicines, 11(4), 1177. https://doi.org/10.3390/biomedicines11041177