COVID-19, Blood Lipid Changes, and Thrombosis
Abstract
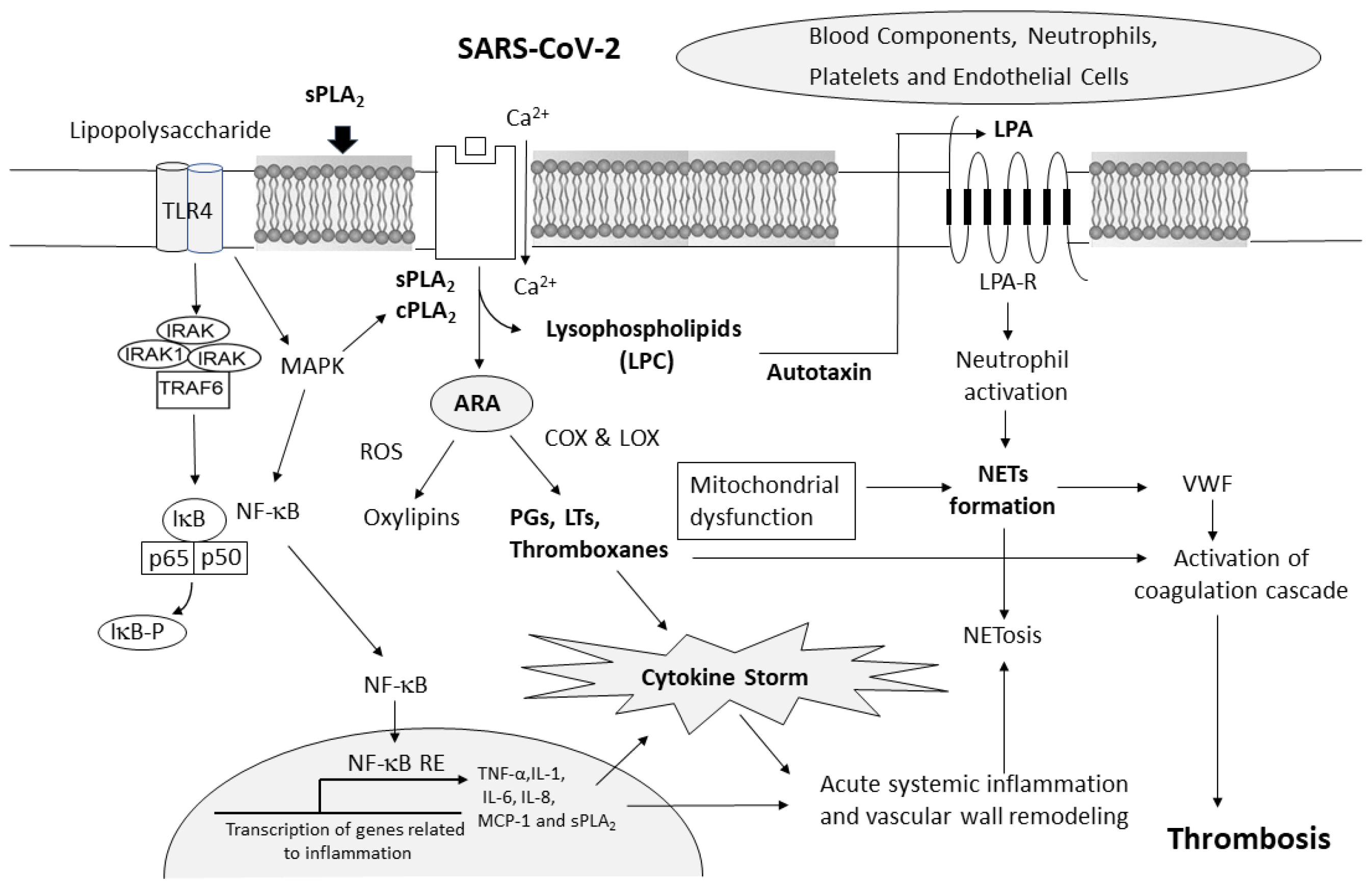
:1. Introduction
2. SARS-CoV-2 and Induction of Cytokine Storm
3. COVID-19-Associated Coagulopathy (CAC)
4. COVID-19 and Changes in Phospholipases A2
5. COVID-19-Associated Changes in Arachidonic Acid (ARA) and Docosahexaenoic Acid (DHA)
6. COVID-19-Associated Changes in Serum Glycerophospholipids and Proinflammatory Lipid Mediators
6.1. Changes in Glycerophospholipids and Sphingolipids/Ceramide
6.2. Thrombxane A2 (TxA2)
6.3. Leukotriene A4 (LTA4) and Lipoxin A4
6.4. Prostaglandin E2 (PGE2)
6.5. Prostacyclin (PGI2)
7. COVID-19-Associated Changes in Lysophospholipids
8. COVID-19-Associated Changes in Autotaxin
9. Lysophosphatidic Acid (LPA) and Platelets in NETs—Contribution to Thrombosis
10. COVID-19 and Inflammation in Blood Vessel Walls
11. Nonenzymatic Effects of sPLA2s on the Coagulation Pathway
12. COVID-19 and Risk of Thrombosis, e.g., Stroke
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO China. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Khan, S.; Ali, A.; Siddique, R.; Nabi, G. Novel Coronavirus Is Putting the Whole World on Alert. J. Hosp. Infect. 2020, 104, 252–253. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Hlh Across Speciality Collaboration, U.K. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Vaninov, N. In the Eye of the COVID-19 Cytokine Storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of Pro-inflammatory Cytokines (Il-1 and Il-6) And Lung Inflammation by Coronavirus-19(COVI-19 or SARS-CoV-2): Anti-Inflammatory Strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Swartzwelter, B.; Koenders, M.I.; Azam, T.; Tengesdal, I.W.; Powers, N.; de Graaf, D.M.; Dinarello, C.A.; Joosten, L.A.B. NLRP3 Inflammasome Inhibitor OLT1177 Suppresses Joint Inflammation in Murine Models of Acute Arthritis. Arthritis Res. Ther. 2018, 20, 169. [Google Scholar] [CrossRef] [Green Version]
- Schonrich, G.; Raftery, M.J.; Samstag, Y. Devilishly Radical NETwork in COVID-19: Oxidative Stress, Neutrophil Extracellular Traps (Nets), and T Cell Suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Chen, I.Y.; Moriyama, M.; Chang, M.F.; Ichinohe, T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M. Clinical Features of Cytokine Storm Syndrome; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Farooqui, A.A. Ischemic and Traumatic Brain and Spinal Cord Injuries; Academic Press Elsevier: Cambridge, MA, USA, 2018. [Google Scholar]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Nurmohamed, N.S.; Collard, D.; Reeskamp, L.F.; Kaiser, Y.; Kroon, J.; Tromp, T.R.; Amsterdam, U.M.C.C.-B.; van den Born, B.H.; Coppens, M.; Vlaar, A.P.J.; et al. Lipoprotein(a), venous thromboembolism and COVID-19: A pilot study. Atherosclerosis 2022, 341, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef]
- Ali, N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med. Virol. 2020, 92, 2409–2411. [Google Scholar] [CrossRef]
- Agrati, C.; Bordoni, V.; Sacchi, A.; Petrosillo, N.; Nicastri, E.; Del Nonno, F.; D’Offizi, G.; Palmieri, F.; Marchioni, L.; Capobianchi, M.R.; et al. Elevated P-Selectin in Severe COVID-19: Considerations for Therapeutic Options. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021016. [Google Scholar] [CrossRef] [PubMed]
- Bouck, E.G.; Denorme, F.; Holle, L.A.; Middelton, E.A.; Blair, A.M.; de Laat, B.; Schiffman, J.D.; Yost, C.C.; Rondina, M.T.; Wolberg, A.S.; et al. COVID-19 and Sepsis Are Associated With Different Abnormalities in Plasma Procoagulant and Fibrinolytic Activity. Arter. Thromb Vasc. Biol. 2021, 41, 401–414. [Google Scholar] [CrossRef]
- Campbell, R.A.; Hisada, Y.; Denorme, F.; Grover, S.P.; Bouck, E.G.; Middleton, E.A.; Wolberg, A.S.; Rondina, M.T.; Mackman, N. Comparison of the coagulopathies associated with COVID-19 and sepsis. Res. Pract. Thromb. Haemost. 2021, 5, e12525. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Grobbelaar, L.M.; Kruger, A.; Venter, C.; Burger, E.M.; Laubscher, G.J.; Maponga, T.G.; Kotze, M.J.; Kwaan, H.C.; Miller, J.B.; Fulkerson, D.; et al. Relative Hypercoagulopathy of the SARS-CoV-2 Beta and Delta Variants when Compared to the Less Severe Omicron Variants Is Related to TEG Parameters, the Extent of Fibrin Amyloid Microclots, and the Severity of Clinical Illness. Semin. Thromb. Hemost. 2022, 48, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Nougier, C.; Benoit, R.; Simon, M.; Desmurs-Clavel, H.; Marcotte, G.; Argaud, L.; David, J.S.; Bonnet, A.; Negrier, C.; Dargaud, Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV-2 associated thrombosis. J. Thromb. Haemost. 2020, 18, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- Whyte, C.S.; Simpson, M.; Morrow, G.B.; Wallace, C.A.; Mentzer, A.J.; Knight, J.C.; Shapiro, S.; Curry, N.; Bagot, C.N.; Watson, H.; et al. The suboptimal fibrinolytic response in COVID-19 is dictated by high PAI-1. J. Thromb. Haemost. 2022, 20, 2394–2406. [Google Scholar] [CrossRef]
- Miltiades, A.; Houck, P.J.; Monteleone, M.; Harrison, N.L.; Cabrera-Garcia, D.; Roh, D.; Wagener, G. Insights into Fibrinogen-Mediated COVID-19 Hypercoagubility in Critically Ill Patients. J. Neurosurg. Anesthesiol. 2022, 34, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: Evidence, mechanisms, and therapeutic implications. Biochem. J. 2022, 479, 1653–1708. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, L.M.; Venter, C.; Vlok, M.; Ngoepe, M.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. Biosci. Rep. 2021, 41, BSR20210611. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.R.; van der Wal, A.C.; Pabittei, D.R.; Mackaaij, C.; van Leeuwen, M.B.; Li, X.; de Boer, O.J. Neutrophil Extracellular Traps Participate in All Different Types of Thrombotic and Haemorrhagic Complications of Coronary Atherosclerosis. Thromb. Haemost. 2018, 118, 1078–1087. [Google Scholar] [CrossRef]
- Stiel, L.; Mayeur-Rousse, C.; Helms, J.; Meziani, F.; Mauvieux, L. First visualization of circulating neutrophil extracellular traps using cell fluorescence during human septic shock-induced disseminated intravascular coagulation. Thromb. Res. 2019, 183, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun. 2018, 10, 414–421. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Thierry, A.R.; Roch, B. Neutrophil Extracellular Traps and By-Products Play a Key Role in COVID-19: Pathogenesis, Risk Factors, and Therapy. J. Clin. Med. 2020, 9, 2942. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demopoulos, C.A.; Pinckard, R.N.; Hanahan, D.J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar] [CrossRef] [PubMed]
- Demopoulos, C.; Antonopoulou, S.; Theoharides, T.C. COVID-19, microthromboses, inflammation, and platelet activating factor. BioFactors 2020, 46, 927–933. [Google Scholar] [CrossRef]
- de Carvalho, J.C.S.; da Silva-Neto, P.V.; Toro, D.M.; Fuzo, C.A.; Nardini, V.; Pimentel, V.E.; Pérez, M.M.; Fraga-Silva, T.F.C.; Oliveira, C.N.S.; Degiovani, A.M.; et al. The Interplay among Glucocorticoid Therapy, Platelet-Activating Factor and Endocannabinoid Release Influences the Inflammatory Response to COVID-19. Viruses 2023, 15, 573. [Google Scholar] [CrossRef]
- Li, T.; Peng, R.; Wang, F.; Hua, L.; Liu, S.; Han, Z.; Pei, J.; Pei, S.; Zhao, Z.; Jiang, X.; et al. Lysophosphatidic acid promotes thrombus stability by inducing rapid formation of neutrophil extracellular traps: A new mechanism of thrombosis. J. Thromb. Haemost. 2020, 18, 1952–1964. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Nagashima, S.; Mendes, M.C.; Camargo Martins, A.P.; Borges, N.H.; Godoy, T.M.; Miggiolaro, A.; da Silva Dezidério, F.; Machado-Souza, C.; de Noronha, L. Endothelial Dysfunction and Thrombosis in Patients With COVID-19-Brief Report. Arter. Thromb. Vasc. Biol. 2020, 40, 2404–2407. [Google Scholar] [CrossRef]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues Prestes, T.R.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L.; Simoes, E.S.A.C. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr. Drug Targets 2017, 18, 1301–1313. [Google Scholar] [CrossRef]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef]
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A central role for amyloid fibrin microclots in long COVID/PASC: Origins and therapeutic implications. Biochem. J. 2022, 479, 537–559. [Google Scholar] [CrossRef]
- Ripon, M.A.R.; Bhowmik, D.R.; Amin, M.T.; Hossain, M.S. Role of arachidonic cascade in COVID-19 infection: A review. Prostaglandins Other Lipid Mediat. 2021, 154, 106539. [Google Scholar] [CrossRef]
- Ong, W.Y.; Go, M.L.; Wang, D.Y.; Cheah, I.K.; Halliwell, B. Effects of Antimalarial Drugs on Neuroinflammation-Potential Use for Treatment of COVID-19-Related Neurologic Complications. Mol. Neurobiol. 2021, 58, 106–117. [Google Scholar] [CrossRef]
- Kramer, R.M.; Stephenson, D.T.; Roberts, E.F.; Clemens, J.A. Cytosolic phospholipase A2 (cPLA2) and lipid mediator release in the brain. J. Lipid Mediat. Cell Signal. 1996, 14, 3–7. [Google Scholar] [CrossRef]
- Sun, G.Y.; Sun, A.Y.; Horrocks, L.A.; Simonyi, A. Roles of cytosolic and secretory phospholipases A2 in oxidative and inflammatory signaling pathways in the CNS. Handb. Neurochem. Mol. Neurobiol. 2008, 8, C4.1. [Google Scholar]
- Sun, G.Y.; Geng, X.; Teng, T.; Yang, B.; Appenteng, M.K.; Greenlief, C.M.; Lee, J.C. Dynamic Role of Phospholipases A2 in Health and Diseases in the Central Nervous System. Cells 2021, 10, 2963. [Google Scholar] [CrossRef]
- Lin, T.N.; Wang, Q.; Simonyi, A.; Chen, J.J.; Cheung, W.M.; He, Y.Y.; Xu, J.; Sun, A.Y.; Hsu, C.Y.; Sun, G.Y. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J. Neurochem. 2004, 90, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.M.; You, J.K.; Wang, X.; Snider, A.J.; Hallmark, B.; Zec, M.M.; Seeds, M.C.; Sergeant, S.; Johnstone, L.; Wang, Q.; et al. Group IIA secreted phospholipase A2 is associated with the pathobiology leading to COVID-19 mortality. J. Clin. Investig. 2021, 131, e149236. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; Ismail, N.E.M.; Mohamed, A.H.; Borik, R.M.; Ali, A.A.; Mosaad, Y.O. Plasma Phospholipids: A Promising Simple Biochemical Parameter to Evaluate COVID-19 Infection Severity. Bioinform. Biol. Insights 2021, 15, 11779322211055891. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, F.A.; Rostad, C.A.; Anderson, E.J.; Chahroudi, A.; Jaggi, P.; Wrammert, J.; Mantus, G.; Basu, R.; Harris, F.; Hanberry, B.; et al. Secretory phospholipase A2 in SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-C). Exp. Biol. Med. 2021, 246, 2543–2552. [Google Scholar] [CrossRef] [PubMed]
- Diorio, C.; Shraim, R.; Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Canna, S.W.; Henrickson, S.E.; McNerney, K.O.; Balamuth, F.; et al. Proteomic profiling of MIS-C patients indicates heterogeneity relating to interferon gamma dysregulation and vascular endothelial dysfunction. Nat. Commun. 2021, 12, 7222. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Dempsy, R.; Hatcher, J.F. Integration of cytokine biology and lipid metabolism in stroke. Front. Biosci. A J. Virtual Libr. 2008, 13, 1250–1270. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.Q.; Zhong, C.Y.; Sun, W.W.; Xiao, H.; Zhu, P.; Lin, Y.Z.; Zhang, C.L.; Gao, H.; Song, Z.Y. Elevated Type II Secretory Phospholipase A2 Increases the Risk of Early Atherosclerosis in Patients with Newly Diagnosed Metabolic Syndrome. Sci. Rep. 2016, 6, 34929. [Google Scholar] [CrossRef] [Green Version]
- Beck, G.; Yard, B.A.; Schulte, J.; Haak, M.; van Ackern, K.; van der Woude, F.J.; Kaszkin, M. Secreted phospholipases A2 induce the expression of chemokines in microvascular endothelium. Biochem. Biophys. Res. Commun. 2003, 300, 731–737. [Google Scholar] [CrossRef]
- Sonoki, K.; Iwase, M.; Sasaki, N.; Ohdo, S.; Higuchi, S.; Takata, Y.; Iida, M. Secretory PLA2 inhibitor indoxam suppresses LDL modification and associated inflammatory responses in TNFalpha-stimulated human endothelial cells. Br. J. Pharm. 2008, 153, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, L.H.; Duchez, A.C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Pare, A.; Rousseau, M.; Naika, G.S.; Levesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183. [Google Scholar] [CrossRef] [Green Version]
- Neidlinger, N.A.; Larkin, S.K.; Bhagat, A.; Victorino, G.P.; Kuypers, F.A. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J. Biol. Chem. 2006, 281, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Vijay, R.; Hua, X.; Meyerholz, D.K.; Miki, Y.; Yamamoto, K.; Gelb, M.; Murakami, M.; Perlman, S. Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection. J. Exp. Med. 2015, 212, 1851–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Meyerholz, D.; Wong, L.R.; Gelb, M.; Murakami, M.; Perlman, S. Coronavirus-specific antibody production in middle-aged mice requires phospholipase A2G2D. J. Clin. Invest. 2021, 131, e147201. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.R.; Zheng, J.; Wilhelmsen, K.; Li, K.; Ortiz, M.E.; Schnicker, N.J.; Thurman, A.; Pezzulo, A.A.; Szachowicz, P.J.; Li, P.; et al. Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19. Nature 2022, 605, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Y.; Zhang, Y.; Li, N.; Yin, Q.; Liu, L.; Lv, X.; Liu, Y.; Li, A.; Fang, B.; et al. Abnormal upregulation of cardiovascular disease biomarker PLA2G7 induced by proinflammatory macrophages in COVID-19 patients. Sci. Rep. 2021, 11, 6811. [Google Scholar] [CrossRef]
- Theodoropoulou, M.A.; Koutoulogenis, G.S.; Zhang, L.; Akrani, I.; Mikros, E.; Hilgenfeld, R.; Kokotos, G. Identification of a Dual Inhibitor of Secreted Phospholipase A(2) (GIIA sPLA(2)) and SARS-CoV-2 Main Protease. Pharmaceuticals 2022, 15, 961. [Google Scholar] [CrossRef]
- Caterino, M.; Gelzo, M.; Sol, S.; Fedele, R.; Annunziata, A.; Calabrese, C.; Fiorentino, G.; D’Abbraccio, M.; Dell’Isola, C.; Fusco, F.M.; et al. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci. Rep. 2021, 11, 2941. [Google Scholar] [CrossRef]
- Wu, D.; Shu, T.; Yang, X.; Song, J.X.; Zhang, M.; Yao, C.; Liu, W.; Huang, M.; Yu, Y.; Yang, Q.; et al. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl. Sci. Rev. 2020, 7, 1157–1168. [Google Scholar] [CrossRef]
- Barberis, E.; Timo, S.; Amede, E.; Vanella, V.V.; Puricelli, C.; Cappellano, G.; Raineri, D.; Cittone, M.G.; Rizzi, E.; Pedrinelli, A.R.; et al. Large-Scale Plasma Analysis Revealed New Mechanisms and Molecules Associated with the Host Response to SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 8623. [Google Scholar] [CrossRef] [PubMed]
- Castane, H.; Iftimie, S.; Baiges-Gaya, G.; Rodriguez-Tomas, E.; Jimenez-Franco, A.; Lopez-Azcona, A.F.; Garrido, P.; Castro, A.; Camps, J.; Joven, J. Machine learning and semi-targeted lipidomics identify distinct serum lipid signatures in hospitalized COVID-19-positive and COVID-19-negative patients. Metab. Clin. Exp. 2022, 131, 155197. [Google Scholar] [CrossRef]
- Masoodi, M.; Peschka, M.; Schmiedel, S.; Haddad, M.; Frye, M.; Maas, C.; Lohse, A.; Huber, S.; Kirchhof, P.; Nofer, J.R.; et al. Disturbed lipid and amino acid metabolisms in COVID-19 patients. J. Mol. Med. 2022, 100, 555–568. [Google Scholar] [CrossRef]
- Perez, M.M.; Pimentel, V.E.; Fuzo, C.A.; da Silva-Neto, P.V.; Toro, D.M.; Fraga-Silva, T.F.C.; Gardinassi, L.G.; Oliveira, C.N.S.; Souza, C.O.S.; Torre-Neto, N.T.; et al. Acetylcholine, Fatty Acids, and Lipid Mediators Are Linked to COVID-19 Severity. J. Immunol. 2022, 209, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Bourredjem, A.; Piroth, L.; Bouhemad, B.; Jalil, A.; Pallot, G.; Le Guern, N.; Thomas, C.; Pilot, T.; Bergas, V.; et al. High plasma concentration of non-esterified polyunsaturated fatty acids is a specific feature of severe COVID-19 pneumonia. Sci. Rep. 2021, 11, 10824. [Google Scholar] [CrossRef]
- Verduci, E.; Rise, P.; Di Profio, E.; Fiori, L.; Vizzuso, S.; Dilillo, D.; Mannarino, S.; Zoia, E.; Calcaterra, V.; Pinna, C.; et al. Blood Fatty Acids Profile in MIS-C Children. Metabolites 2021, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Karu, N.; Kindt, A.; Lamont, L.; van Gammeren, A.J.; Ermens, A.A.M.; Harms, A.C.; Portengen, L.; Vermeulen, R.C.H.; Dik, W.A.; Langerak, A.W.; et al. Plasma Oxylipins and Their Precursors Are Strongly Associated with COVID-19 Severity and with Immune Response Markers. Metabolites 2022, 12, 619. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef]
- Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Gu, Z.; Lee, J.C.; Folk, W.R.; Greenlief, C.M.; Sun, G.Y. Yin-Yang Mechanisms Regulating Lipid Peroxidation of Docosahexaenoic Acid and Arachidonic Acid in the Central Nervous System. Front. Neurol. 2019, 10, 642. [Google Scholar] [CrossRef] [Green Version]
- Baral, P.K.; Amin, M.T.; Rashid, M.M.O.; Hossain, M.S. Assessment of Polyunsaturated Fatty Acids on COVID-19-Associated Risk Reduction. Rev. Bras. Farmacogn. Orgao Of. Soc. Bras. Farmacogn. 2022, 32, 50–64. [Google Scholar] [CrossRef]
- Sun, Y.; Chatterjee, R.; Ronanki, A.; Ye, K. Circulating Polyunsaturated Fatty Acids and COVID-19: A Prospective Cohort Study and Mendelian Randomization Analysis. Front. Med. 2022, 9, 923746. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.A.; De La Rosa, X.; Santos, F.G.; Rizo, J.M.; Gracia Banzo, R.; Silva, R.S. Acute severe SARS COVID-19 patients produce pro-resolving lipids mediators and eicosanoids. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6782–6796. [Google Scholar] [CrossRef] [PubMed]
- Palmas, F.; Clarke, J.; Colas, R.A.; Gomez, E.A.; Keogh, A.; Boylan, M.; McEvoy, N.; McElvaney, O.J.; McElvaney, O.; Alalqam, R.; et al. Dysregulated plasma lipid mediator profiles in critically ill COVID-19 patients. PLoS ONE 2021, 16, e0256226. [Google Scholar] [CrossRef] [PubMed]
- Sedighiyan, M.; Abdollahi, H.; Karimi, E.; Badeli, M.; Erfanian, R.; Raeesi, S.; Hashemi, R.; Vahabi, Z.; Asanjarani, B.; Mansouri, F.; et al. Omega-3 polyunsaturated fatty acids supplementation improve clinical symptoms in patients with COVID-19: A randomised clinical trial. Int. J. Clin. Pract. 2021, 75, e14854. [Google Scholar] [CrossRef]
- Kaur, G.; Ji, X.; Rahman, I. SARS-CoV2 Infection Alters Tryptophan Catabolism and Phospholipid Metabolism. Metabolites 2021, 11, 659. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodriguez, Y.; Gallo, J.E.; Salazar-Uribe, J.C.; Santander, M.J.; Cala, M.P.; Zapata, W.; Zapata, M.I.; et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 2021, 118, 102598. [Google Scholar] [CrossRef]
- Yan, B.; Yuan, S.; Cao, J.; Fung, K.; Lai, P.M.; Yin, F.; Sze, K.H.; Qin, Z.; Xie, Y.; Ye, Z.W.; et al. Phosphatidic acid phosphatase 1 impairs SARS-CoV-2 replication by affecting the glycerophospholipid metabolism pathway. Int. J. Biol. Sci. 2022, 18, 4744–4755. [Google Scholar] [CrossRef]
- Shimura, T.; Kurano, M.; Okamoto, K.; Jubishi, D.; Kano, K.; Igarashi, K.; Shimamoto, S.; Aoki, J.; Moriya, K.; Yatomi, Y. Increase in serum levels of phosphatidylserine-specific phospholipase A(1) in COVID-19 patients. Cell. Mol. Immunol. 2021, 18, 2275–2277. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Mwangi, V.I.; Sartim, M.A.; Delafiori, J.; Sales, G.M.; de Oliveira, A.N.; Busanello, E.N.B.; Val, F.; Xavier, M.S.; Costa, F.T.; et al. Metabolomic Profiling of Plasma Reveals Differential Disease Severity Markers in COVID-19 Patients. Front. Microbiol. 2022, 13, 844283. [Google Scholar] [CrossRef]
- Bruzzone, C.; Bizkarguenaga, M.; Gil-Redondo, R.; Diercks, T.; Arana, E.; Garcia de Vicuna, A.; Seco, M.; Bosch, A.; Palazon, A.; San Juan, I.; et al. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience 2020, 23, 101645. [Google Scholar] [CrossRef]
- Kurano, M.; Okamoto, K.; Jubishi, D.; Hashimoto, H.; Sakai, E.; Saigusa, D.; Kano, K.; Aoki, J.; Harada, S.; Okugawa, S.; et al. Dynamic modulations of sphingolipids and glycerophospholipids in COVID-19. Clin. Transl. Med. 2022, 12, e1069. [Google Scholar] [CrossRef] [PubMed]
- Hornemann, T.; Richard, S.; Rütti, M.F.; Wei, Y.; von Eckardstein, A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J. Biol. Chem. 2006, 281, 37275–37281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Gupta, S.D.; Gable, K.; Niranjanakumari, S.; Moitra, P.; Eichler, F.; Brown, R.H., Jr.; Harmon, J.M.; Dunn, T.M. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. USA 2009, 106, 8186–8191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maula, T.; Artetxe, I.; Grandell, P.M.; Slotte, J.P. Importance of the sphingoid base length for the membrane properties of ceramides. Biophys. J. 2012, 103, 1870–1879. [Google Scholar] [CrossRef] [Green Version]
- Rucker, D.; Dhamoon, A.S. Physiology, Thromboxane A2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G.; Toniato, E. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: Inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J. Biol. Regul. Homeost. Agents 2020, 34, 1623–1627. [Google Scholar] [CrossRef]
- Chiang, K.C.; Rizk, J.G.; Nelson, D.J.; Krishnamurti, L.; Subbian, S.; Imig, J.D.; Khan, I.; Reddy, S.T.; Gupta, A. Ramatroban for chemoprophylaxis and treatment of COVID-19: David takes on Goliath. Expert Opin. Ther. Targets 2022, 26, 13–28. [Google Scholar] [CrossRef]
- Gupta, A.; Kalantar-Zadeh, K.; Reddy, S.T. Ramatroban as a Novel Immunotherapy for COVID-19. J. Mol. Genet. Med. Int. J. Biomed. Res. 2020, 14. [Google Scholar] [CrossRef]
- Ogletree, M.L.; Chander Chiang, K.; Kulshrestha, R.; Agarwal, A.; Agarwal, A.; Gupta, A. Treatment of COVID-19 Pneumonia and Acute Respiratory Distress With Ramatroban, a Thromboxane A(2) and Prostaglandin D(2) Receptor Antagonist: A Four-Patient Case Series Report. Front Pharm. 2022, 13, 904020. [Google Scholar] [CrossRef]
- Ayola-Serrano, N.C.; Roy, N.; Fathah, Z.; Anwar, M.M.; Singh, B.; Ammar, N.; Sah, R.; Elba, A.; Utt, R.S.; Pecho-Silva, S.; et al. The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2021, 70, 877–889. [Google Scholar] [CrossRef]
- Schwarz, B.; Sharma, L.; Roberts, L.; Peng, X.; Bermejo, S.; Leighton, I.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I.; et al. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J. Immunol. 2021, 206, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Camera, M.; Canzano, P.; Brambilla, M.; Rovati, G.E. Montelukast Inhibits Platelet Activation Induced by Plasma From COVID-19 Patients. Front Pharm. 2022, 13, 784214. [Google Scholar] [CrossRef]
- Zhou, Y.; You, C.G. Lipoxin alleviates oxidative stress: A state-of-the-art review. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2022, 71, 1169–1179. [Google Scholar] [CrossRef]
- Ricke-Hoch, M.; Stelling, E.; Lasswitz, L.; Gunesch, A.P.; Kasten, M.; Zapatero-Belinchon, F.J.; Brogden, G.; Gerold, G.; Pietschmann, T.; Montiel, V.; et al. Impaired immune response mediated by prostaglandin E2 promotes severe COVID-19 disease. PLoS ONE 2021, 16, e0255335. [Google Scholar] [CrossRef]
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yucel, C.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and Prostaglandin E2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021, 74, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Filippini, A.; Bna, C.; Bellosta, R.; Bazzani, R.; Luzzani, L.; Pegorer, M.A.; Zandalasini, M.; Baldon, M.; Codazzi, M.; Sabatini, T. COVID-19 acute respiratory distress syndrome: Can iloprost have a role for the treatment? Respir. Med. Case Rep. 2021, 32, 101358. [Google Scholar] [CrossRef]
- Johansson, P.I.; Soe-Jensen, P.; Bestle, M.H.; Clausen, N.E.; Kristiansen, K.T.; Lange, T.; Stensballe, J.; Perner, A. Prostacyclin in Intubated Patients with COVID-19 and Severe Endotheliopathy: A Multicenter, Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 324–329. [Google Scholar] [CrossRef]
- Vigstedt, M.; Soe-Jensen, P.; Bestle, M.H.; Clausen, N.E.; Kristiansen, K.T.; Lange, T.; Stensballe, J.; Perner, A.; Johansson, P.I. The effect of prostacyclin infusion on markers of endothelial activation and damage in mechanically ventilated patients with SARS-CoV-2 infection. J. Crit. Care 2022, 69, 154010. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, T.; Cheng, Z.J.; Guo, B.; Zeng, Y.; Lin, R.; Zheng, P.; Liu, M.; Hu, F.; Li, F.; et al. Effect of a Functional Phospholipid Metabolome-Protein Association Pathway on the Mechanism of COVID-19 Disease Progression. Int. J. Biol. Sci. 2022, 18, 4618–4628. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.R.; Chew, W.S.; Satish, R.L.; Ong, W.Y. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Mol. Neurobiol. 2020, 57, 372–392. [Google Scholar] [CrossRef]
- Herr, D.R.; Ong, J.H.; Ong, W.Y. Potential Therapeutic Applications for Inhibitors of Autotaxin, a Bioactive Lipid-Producing Lysophospholipase D, in Disorders Affecting the Nervous System. ACS Chem. Neurosci. 2018, 9, 398–400. [Google Scholar] [CrossRef] [Green Version]
- Nikitopoulou, I.; Fanidis, D.; Ntatsoulis, K.; Moulos, P.; Mpekoulis, G.; Evangelidou, M.; Vassiliou, A.G.; Dimakopoulou, V.; Jahaj, E.; Tsipilis, S.; et al. Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells. Int. J. Mol. Sci. 2021, 22, 10006. [Google Scholar] [CrossRef] [PubMed]
- Sexton, T.; Chalhoub, G.; Ye, S.; Morris, W.; Annabathula, R.; Dugan, A.; Smyth, S. Autotaxin Activity Predicts 30-Day Mortality in Sepsis Patients and Correlates With Platelet Count and Vascular Dysfunction. Shock 2020, 54, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Karshovska, E.; Mohibullah, R.; Zhu, M.; Zahedi, F.; Thomas, D.; Magkrioti, C.; Geissler, C.; Megens, R.T.A.; Bianchini, M.; Nazari-Jahantigh, M.; et al. Endothelial ENPP2 (Ectonucleotide Pyrophosphatase/Phosphodiesterase 2) Increases Atherosclerosis in Female and Male Mice. Arter. Thromb. Vasc. Biol. 2022, 42, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Strumwasser, A.; Cohan, C.M.; Beattie, G.; Chong, V.; Victorino, G.P. Autotaxin inhibition attenuates endothelial permeability after ischemia-reperfusion injury. Clin. Hemorheol. Microcirc. 2020, 75, 399–407. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- Lim, M.S.; McRae, S. COVID-19 and immunothrombosis: Pathophysiology and therapeutic implications. Crit. Rev. Oncol. Hematol. 2021, 168, 103529. [Google Scholar] [CrossRef]
- Genchi, A.; Semerano, A.; Schwarz, G.; Dell’Acqua, B.; Gullotta, G.S.; Sampaolo, M.; Boeri, E.; Quattrini, A.; Sanvito, F.; Diamanti, S.; et al. Neutrophils predominate the immune signature of cerebral thrombi in COVID-19 stroke patients. Acta. Neuropathol. Commun. 2022, 10, 14. [Google Scholar] [CrossRef]
- Iba, T.; Wada, H.; Levy, J.H. Platelet Activation and Thrombosis in COVID-19. Semin. Thromb. Hemost. 2023, 49, 55–61. [Google Scholar] [CrossRef]
- Ebeyer-Masotta, M.; Eichhorn, T.; Weiss, R.; Laukova, L.; Weber, V. Activated Platelets and Platelet-Derived Extracellular Vesicles Mediate COVID-19-Associated Immunothrombosis. Front. Cell Dev. Biol. 2022, 10, 914891. [Google Scholar] [CrossRef]
- Jakobs, K.; Reinshagen, L.; Puccini, M.; Friebel, J.; Wilde, A.B.; Alsheik, A.; Rroku, A.; Landmesser, U.; Haghikia, A.; Krankel, N.; et al. Disease Severity in Moderate-to-Severe COVID-19 Is Associated With Platelet Hyperreactivity and Innate Immune Activation. Front. Immunol. 2022, 13, 844701. [Google Scholar] [CrossRef]
- Bolen, A.L.; Naren, A.P.; Yarlagadda, S.; Beranova-Giorgianni, S.; Chen, L.; Norman, D.; Baker, D.L.; Rowland, M.M.; Best, M.D.; Sano, T.; et al. The phospholipase A1 activity of lysophospholipase A-I links platelet activation to LPA production during blood coagulation. J. Lipid Res. 2011, 52, 958–970. [Google Scholar] [CrossRef] [Green Version]
- le Balle, F.; Simon, M.F.; Meijer, S.; Fourcade, O.; Chap, H. Membrane sidedness of biosynthetic pathways involved in the production of lysophosphatidic acid. Adv. Enzym. Regul. 1999, 39, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ohlinger, T.; Mullner, E.W.; Fritz, M.; Sauer, T.; Werning, M.; Baron, D.M.; Salzer, U. Lysophosphatidic acid-induced pro-thrombotic phosphatidylserine exposure and ionophore-induced microvesiculation is mediated by the scramblase TMEM16F in erythrocytes. Blood Cells Mol. Dis. 2020, 83, 102426. [Google Scholar] [CrossRef] [PubMed]
- Caillon, A.; Trimaille, A.; Favre, J.; Jesel, L.; Morel, O.; Kauffenstein, G. Role of neutrophils, platelets, and extracellular vesicles and their interactions in COVID-19-associated thrombopathy. J. Thromb. Haemost. 2022, 20, 17–31. [Google Scholar] [CrossRef]
- Bhattarai, S.; Sharma, S.; Ara, H.; Subedi, U.; Sun, G.; Li, C.; Bhuiyan, M.S.; Kevil, C.; Armstrong, W.P.; Minvielle, M.T.; et al. Disrupted Blood-Brain Barrier and Mitochondrial Impairment by Autotaxin-Lysophosphatidic Acid Axis in Postischemic Stroke. J. Am. Heart Assoc. 2021, 10, e021511. [Google Scholar] [CrossRef]
- Hanafi, R.; Roger, P.A.; Perin, B.; Kuchcinski, G.; Deleval, N.; Dallery, F.; Michel, D.; Hacein-Bey, L.; Pruvo, J.P.; Outteryck, O.; et al. COVID-19 Neurologic Complication with CNS Vasculitis-Like Pattern. Am. J. Neuroradiol. 2020, 41, 1384–1387. [Google Scholar] [CrossRef]
- Lersy, F.; Anheim, M.; Willaume, T.; Chammas, A.; Brisset, J.C.; Cotton, F.; Kremer, S. Cerebral vasculitis of medium-sized vessels as a possible mechanism of brain damage in COVID-19 patients. J. Neuroradiol. 2021, 48, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Poisson, K.E.; Zygmunt, A.; Leino, D.; Fuller, C.E.; Jones, B.V.; Haslam, D.; Staat, M.A.; Clay, G.; Ting, T.V.; Wesselkamper, K.; et al. Lethal Pediatric Cerebral Vasculitis Triggered by Severe Acute Respiratory Syndrome Coronavirus 2. Pediatr. Neurol. 2022, 127, 1–5. [Google Scholar] [CrossRef]
- Ivanusec, A.; Sribar, J.; Krizaj, I. Secreted Phospholipases A(2)—not just Enzymes: Revisited. Int. J. Biol. Sci. 2022, 18, 873–888. [Google Scholar] [CrossRef]
- Faure, G.; Gowda, V.T.; Maroun, R.C. Characterization of a human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics. BMC Struct. Biol. 2007, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Yoshihara, K.; Shimbara, S.; Sawada, M.; Inagaki, N.; Nagai, H.; Naito, M.; Tsuruo, T.; Moon, T.C.; Chang, H.W.; et al. Group IID heparin-binding secretory phospholipase A(2) is expressed in human colon carcinoma cells and human mast cells and up-regulated in mouse inflammatory tissues. Eur. J. Biochem. 2002, 269, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Murakami, M.; Valentin, E.; Lambeau, G.; Gelb, M.H.; Kudo, I. Redundant and segregated functions of granule-associated heparin-binding group II subfamily of secretory phospholipases A2 in the regulation of degranulation and prostaglandin D2 synthesis in mast cells. J. Immunol. 2000, 165, 4007–4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.L.; Bayraktutan, U. Risk factors for ischaemic stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2008, 3, 105–116. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Sagris, D.; Papanikolaou, A.; Kvernland, A.; Korompoki, E.; Frontera, J.A.; Troxel, A.B.; Gavriatopoulou, M.; Milionis, H.; Lip, G.Y.H.; Michel, P.; et al. COVID-19 and ischemic stroke. Eur. J. Neurol. 2021, 28, 3826–3836. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef]
- Boehme, A.K.; Luna, J.; Kulick, E.R.; Kamel, H.; Elkind, M.S.V. Influenza-like illness as a trigger for ischemic stroke. Ann. Clin. Transl. Neurol. 2018, 5, 456–463. [Google Scholar] [CrossRef]
- Merkler, A.E.; Parikh, N.S.; Mir, S.; Gupta, A.; Kamel, H.; Lin, E.; Lantos, J.; Schenck, E.J.; Goyal, P.; Bruce, S.S.; et al. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs. Patients With Influenza. JAMA Neurol. 2020, 77, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, T.; Sedghi, A.; Simon, E.; Winzer, S.; Barlinn, J.; de With, K.; Mirow, L.; Wolz, M.; Gruenewald, T.; Schroettner, P.; et al. Increased risk of acute stroke among patients with severe COVID-19: A multicenter study and meta-analysis. Eur. J. Neurol. 2021, 28, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, J.J.; Ye, M.F.; Li, H.M.; Yao, F.R.; Kong, Y.; Xu, Z. The relationship between COVID-19’s severity and ischemic stroke: A systematic review and meta-analysis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 2645–2651. [Google Scholar] [CrossRef]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef]
- Baldini, T.; Asioli, G.M.; Romoli, M.; Carvalho Dias, M.; Schulte, E.C.; Hauer, L.; Aguiar De Sousa, D.; Sellner, J.; Zini, A. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: A systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, X.; Bao, K.; Huang, C. Ischemic stroke associated with COVID-19: A systematic review and meta-analysis. J. Neurol. 2022, 269, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Nannoni, S.; de Groot, R.; Bell, S.; Markus, H.S. Stroke in COVID-19: A systematic review and meta-analysis. Int. J. Stroke Off. J. Int. Stroke Soc. 2021, 16, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Kuno, T.; Mikami, T.; Takagi, H.; Gronseth, G. Clinical Characteristics of Stroke with COVID-19: A Systematic Review and Meta-Analysis. J. Stroke Cereb. Dis. 2020, 29, 105288. [Google Scholar] [CrossRef]
- Tan, Y.K.; Goh, C.; Leow, A.S.T.; Tambyah, P.A.; Ang, A.; Yap, E.S.; Tu, T.M.; Sharma, V.K.; Yeo, L.L.L.; Chan, B.P.L.; et al. COVID-19 and ischemic stroke: A systematic review and meta-summary of the literature. J. Thromb. Thrombolysis 2020, 50, 587–595. [Google Scholar] [CrossRef]
- Knight, J.S.; Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin. Immunopathol. 2022, 44, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Siow, I.; Lee, K.S.; Zhang, J.J.Y.; Saffari, S.E.; Ng, A.; Young, B. Stroke as a Neurological Complication of COVID-19: A Systematic Review and Meta-Analysis of Incidence, Outcomes and Predictors. J. Stroke Cereb. Dis. 2021, 30, 105549. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqui, A.A.; Farooqui, T.; Sun, G.Y.; Lin, T.-N.; Teh, D.B.L.; Ong, W.-Y. COVID-19, Blood Lipid Changes, and Thrombosis. Biomedicines 2023, 11, 1181. https://doi.org/10.3390/biomedicines11041181
Farooqui AA, Farooqui T, Sun GY, Lin T-N, Teh DBL, Ong W-Y. COVID-19, Blood Lipid Changes, and Thrombosis. Biomedicines. 2023; 11(4):1181. https://doi.org/10.3390/biomedicines11041181
Chicago/Turabian StyleFarooqui, Akhlaq A., Tahira Farooqui, Grace Y. Sun, Teng-Nan Lin, Daniel B. L. Teh, and Wei-Yi Ong. 2023. "COVID-19, Blood Lipid Changes, and Thrombosis" Biomedicines 11, no. 4: 1181. https://doi.org/10.3390/biomedicines11041181
APA StyleFarooqui, A. A., Farooqui, T., Sun, G. Y., Lin, T.-N., Teh, D. B. L., & Ong, W.-Y. (2023). COVID-19, Blood Lipid Changes, and Thrombosis. Biomedicines, 11(4), 1181. https://doi.org/10.3390/biomedicines11041181






