The Use of Wound Infiltration for Postoperative Pain Management after Breast Cancer Surgery: A Randomized Clinical Study
Abstract
1. Summary
2. Data Description
3. Methods
4. Personal Technique
5. Primary Outcomes
6. Secondary Outcomes
7. Patient Autonomy/Ethical Consent
8. Results
8.1. Group A
8.2. Group B
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Park, K.U.; Kyrish, K.; Yi, M.; Bedrosian, I.; Caudle, A.S.; Kuerer, H.M.; Hunt, K.K.; Miggins, M.V.; DeSnyder, S.M. Opioid use after breast-conserving surgery: Prospective evaluation of risk factors for high opioid use. Ann. Surg. Oncol. 2023, 27, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.; Spahn, V.; Stein, C. Novel opioid analgesics and side effects. ACS Chem. Neurosci. 2023, 8, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
- Duthie, D.J.; Nimmo, W.S. Adverse effects of opioid analgesic drugs. Br. J. Anaesth. 2021, 59, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Naja, Z.M.; Naccache, N.; Ziade, F.; El-Rajab, M.; Itani, T.; Baraka, A. Multilevel nerve stimulator-guided paravertebral block as a sole anesthetic technique for breast cancer surgery in morbidly obese patients. J. Anesth. 2020, 25, 760. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.; Hutchinson, S.P.; Edbrooke, D.L.; Wrench, I.; Kohlhardt, S.R. Evaluation of a local anaesthesia regimen following mastectomy. Anesthesia 2019, 59, 664–667. [Google Scholar] [CrossRef]
- Nirmala, J.; Harsh, K.; Padmaja, D.; Gopinath, R. Role of wound instillation with bupivacaine through surgical drains for postoperative analgesia in modified radical mastectomy. Indian J. Anesth. 2019, 59, 15–20. [Google Scholar]
- Vigneau, A.; Salengro, A.; Berger, J.; Rouzier, R.; Barranger, E.; Marret, E.; Bonnet, F. A double blind randomized trial of wound infiltration with ropivacaine after breast cancer surgery with axillary nodes dissection. BMC Anesth. 2019, 11, 23. [Google Scholar] [CrossRef]
- Albi-Feldzer, A.; Mouret-Fourme, E.E.; Hamouda, S.; Motamed, C.; Dubois, P.Y.; Jouanneau, L.; Jayr, M.D.C. A double-blind randomized trial of wound and intercostal space infiltration with ropivacaine during breast cancer surgery: Effects on chronic postoperative pain. Anesthesiology 2018, 118, 318–326. [Google Scholar] [CrossRef]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Tara, L.; Emily, D.; Nantthasorn, Z.; Tari, A.; Laura, D.; Rob, R.; Golshan, M.; Schreiber, K.L. Chronic pain after breast surgery: A prospective, observational study. Ann. Surg. Oncol. 2018, 25, 2917–2924. [Google Scholar]
- Butterwick, K.J.; Goldman, M.P.; Sriprachya-Anunt, S. Lidocaine levels during the first two hours of infiltration of dilute anesthetic solution for tumescent liposuction: 681 rapid versus slow delivery. Derm. Surg. 1999, 25, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.A.; Ferris, G.; Feng, C.; Cooper, J.Z.; Brown, M.D. Decreasing the pain of local anesthesia: A prospective, double-blind comparison of buffered, premixed 1% lidocaine with epinephrine versus 1% lidocaine freshly mixed with epinephrine. J. Am. Acad. Derm. 2006, 54, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.E.; Vandervaart, S.; Perampaladas, K.; MacHado, M.; Einarson, T.R.; Taddio, A. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann. Emerg. Med. 2011, 58, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, A.; Bajaj, S.; Hasan, M. Novel techniques of local anaesthetic infiltration. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 167–171. [Google Scholar] [CrossRef]
- White, P.F.; Rawal, S.; Latham, P.; Markowitz, S.; Issioui, T.; Chi, L.; Dellaria, S.; Shi, C.; Morse, L.; Ing, C. Use of a continuous local anesthetic infusion for pain management after median sternotomy. Anesthesiology 2003, 99, 918–923. [Google Scholar] [CrossRef]
- Yadav, U.; Srivastava, S.; Srivastav, D. Postoperative analgesic effect of bupivacaine alone and with dexmedetomidine in wound instillation for lumbar laminectomy: A randomized control trial. Anesth. Essays Res. 2020, 14, 149. [Google Scholar]
- Bharti, N.; Dontukurthy, S.; Bala, I.; Singh, G. Postoperative analgesic effect of intravenous (i.v.) clonidine compared with clonidine administration in wound infiltration for open cholecystectomy. Br. J. Anaesth. 2013, 111, 656–661. [Google Scholar] [CrossRef]
- Kaki, A.M.; Al Marakbi, W. Post-herniorrhaphy infiltration of tramadol versus bupivacaine for postoperative pain relief: A randomized study. Ann. Saudi Med. 2008, 28, 165–168. [Google Scholar] [CrossRef]
- Rømsing, J.; Mysager, S.; Vilmann, P.; Sonne, J.; Larsen, N.E.; Øtergaard, D. Postoperative analgesia is not different after local vs. systemic administration of meloxicam in patients undergoing inguinal hernia repair. Can. J. Anesth. 2001, 48, 978–984. [Google Scholar] [CrossRef]
- Karamanlioglu, B.; Turan, A.; Memis, D.; Kaya, G.; Ozata, S.; Ture, M. Infiltration with ropivacaine plus lornoxicam reduces postoperative pain and opioid consumption. Can. J. Anesth. 2005, 52, 1047–1053. [Google Scholar] [CrossRef]
- Loh, J.W.; Taib, N.A.; Cheong, Y.T.; Tin, T.S. A Double-Blind, Randomized Controlled Trial of Pre-incision Wound Infiltration Using Diclofenac Versus Bupivacaine for Post-operative Pain Relief in Open Thyroid and Parathyroid Surgery. World J. Surg. 2020, 44, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, K.B.; Dogan, L.; Nalbant, H.; Akinci, M.; Karaman, N.; Ozaslan, C.; Kulacoglu, H. Comparing scalpel, electrocautery and ultrasonic dissector effects: The impact on wound complications and pro-inflammatory cytokine levels in wound fluid from mastectomy patients. J. Breast Cancer 2011, 14, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Galatius, H.; Okholm, M.; Hoffmann, J. Mastectomy using ultrasonic dissection: Effect on seroma formation. Breast 2003, 12, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ayantunde, A.A.; Cheung, K.L. Concepts of seroma formation and prevention in breast cancer surgery. ANZ J. Surg. 2006, 76, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Sakkary, M.A. The value of mastectomy flap fixation in reducing fluid drainage and seroma formation in breast cancer patients. World J. Surg. Oncol. 2012, 10, 8. [Google Scholar] [CrossRef]
- Jain, P.K.; Sowdi, R.; Anderson, A.D.; MacFie, J. Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. Br. J. Surg. 2004, 91, 54–60. [Google Scholar] [CrossRef]
- Chung, T.L.; Holton, L.H., III; Goldberg, N.H.; Silverman, R.P. Seroma prevention using Mytilus edulis protein in a rat mastectomy model. Breast J. 2006, 12, 442–445. [Google Scholar] [CrossRef]
- Bacilious, N.; Kulber, D.A.; Peters, E.D.; Gayle, L.B.; Chen, M.J.; Harper, A.D.; Hoffman, L. Harvesting of the latissimus dorsi muscle: A small animal model for seroma formation. Microsurgery 1995, 16, 646–649. [Google Scholar] [CrossRef]
- Baudry, G.; Steghens, A.; Laplaza, D.; Koeberle, P.; Bachour, K.; Bettinger, G.; Combier, F.; Samain, E. Ropivacaine infiltration during breast cancer surgery: Postoperative acute and chronic pain effect. Ann. Fr. Anesth. Reanim. 2008, 27, 979–986. [Google Scholar] [CrossRef]
- Johansson, A.; Kornfält, J.; Nordin, L.; Svensson, L.; Ingvar, C.; Lundberg, J. Wound infiltration with ropivacaine and fentanyl: Effects on postoperative pain and PONV after breast surgery. J. Clin. Anesth. 2003, 15, 113–118. [Google Scholar] [CrossRef]
- Johansson, A.; Axelson, J.; Ingvar, C.; Luttropp, H.-H.; Lundberg, J. Preoperative ropivacaine infiltration in breast surgery. Acta Anaesthesiol. Scand. 2000, 44, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Rica, M.A.; Norlia, A.; Rohaizak, M.; Naqiyah, I. Preemptive ropivacaine local anesthetic infiltration versus postoperative ropivacaine wound infiltration in mastectomy: Postoperative pain and drain outputs. Asian J. Surg. 2007, 30, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.G.; Rahman, S.; Jafarabai, M. Local Lidocaine 2% in Post-operative Pain Management in Caserean Delivery. J. Fam. Reprod. Health 2015, 9, 19–21. [Google Scholar]
- Walash, D.A.; Mapp, P.I.; Kelly, S. Calcitonin Gene-Related Peptide in the Joint: Contributions to Pain and Inflammation. British J. Clin. Pharmacol. 2015, 80, 965–978. [Google Scholar] [CrossRef]
- Grigoras, A.; Lee, P.; Sattar, F.; Shorten, G. Perioperative Intravenous Lidocaine Decreases the Incidence of Persistent Pain after Breast Surgery. Clin. J. Pain 2012, 28, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Bryson, G.L.; Lui, A.; Watters, J.M.; Taljaard, M.; Nathan, H.J. Reducing Persistent Postoperative Pain and Disability 1 Year after Breast Cancer Surgery: A Randomized, Controlled Trial Comparing Thoracic Paravertebral Block to Local Anaesthesia. Ann. Surg. Oncol. 2014, 21, 795–801. [Google Scholar] [CrossRef]


| Parameters | No. of Cases | % |
|---|---|---|
| Luminal A | 24 | 31.57 |
| Luminal B Her2 - | 11 | 14.47 |
| Luminal B Her2 + | 9 | 11.84 |
| Her2+ | 15 | 19.73 |
| TNBC | 17 | 22.36 |
| Histologic type | ||
| Infiltrative ductal carcinoma | 63 | 82.89 |
| Lobular | 7 | 9.21 |
| Metaplastic | 4 | 5.26 |
| Medullary | 2 | 2.63 |
| NAC therapy | ||
| NAC | 34 | 44.73 |
| Non-NAC | 42 | 55.26 |
| Type of Intervention | % | |
|---|---|---|
| BCS | 44.73% | |
| Total % of BCS + OBCS | 68.41% | |
| OBCS | 23.68% | |
| MRM (Madden procedure) | 31.57% | 31.57% |
| Mammoplasty | No. of Cases | % |
|---|---|---|
| Pitangui–Lejour | 4 | 10.52 |
| Wise pattern | 2 | 5.26 |
| Riberio–Robbins | 3 | 7.89 |
| Total | 9 | 23.68 |
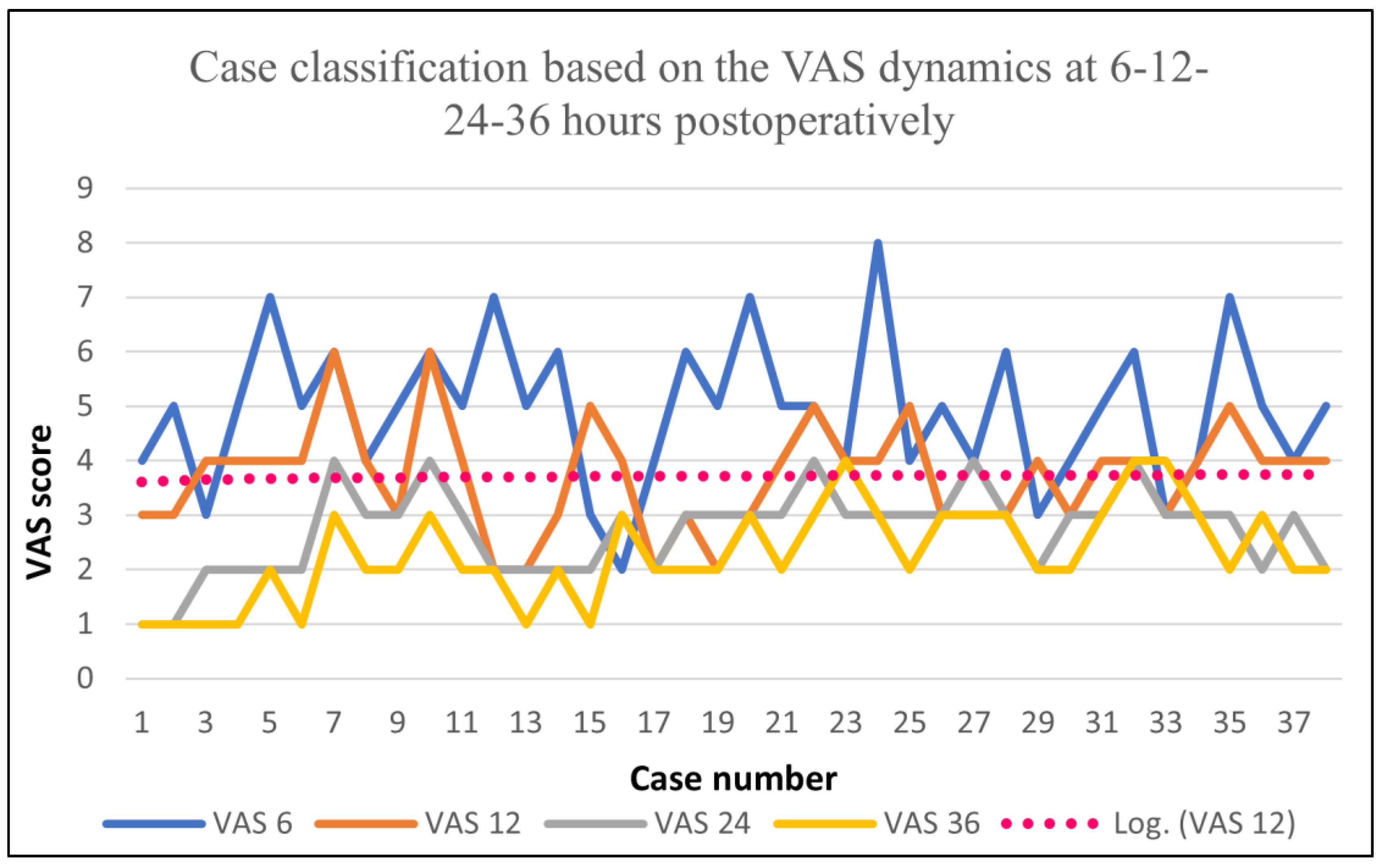
| Function | VAS 6 | VAS 12 | VAS 24 | VAS 36 |
|---|---|---|---|---|
| Average | 0.63 | 0.84 | 0.71 | 1.1 |
| Min | 0 | 0 | 0 | 0 |
| Max | 3 | 3 | 2 | 3 |
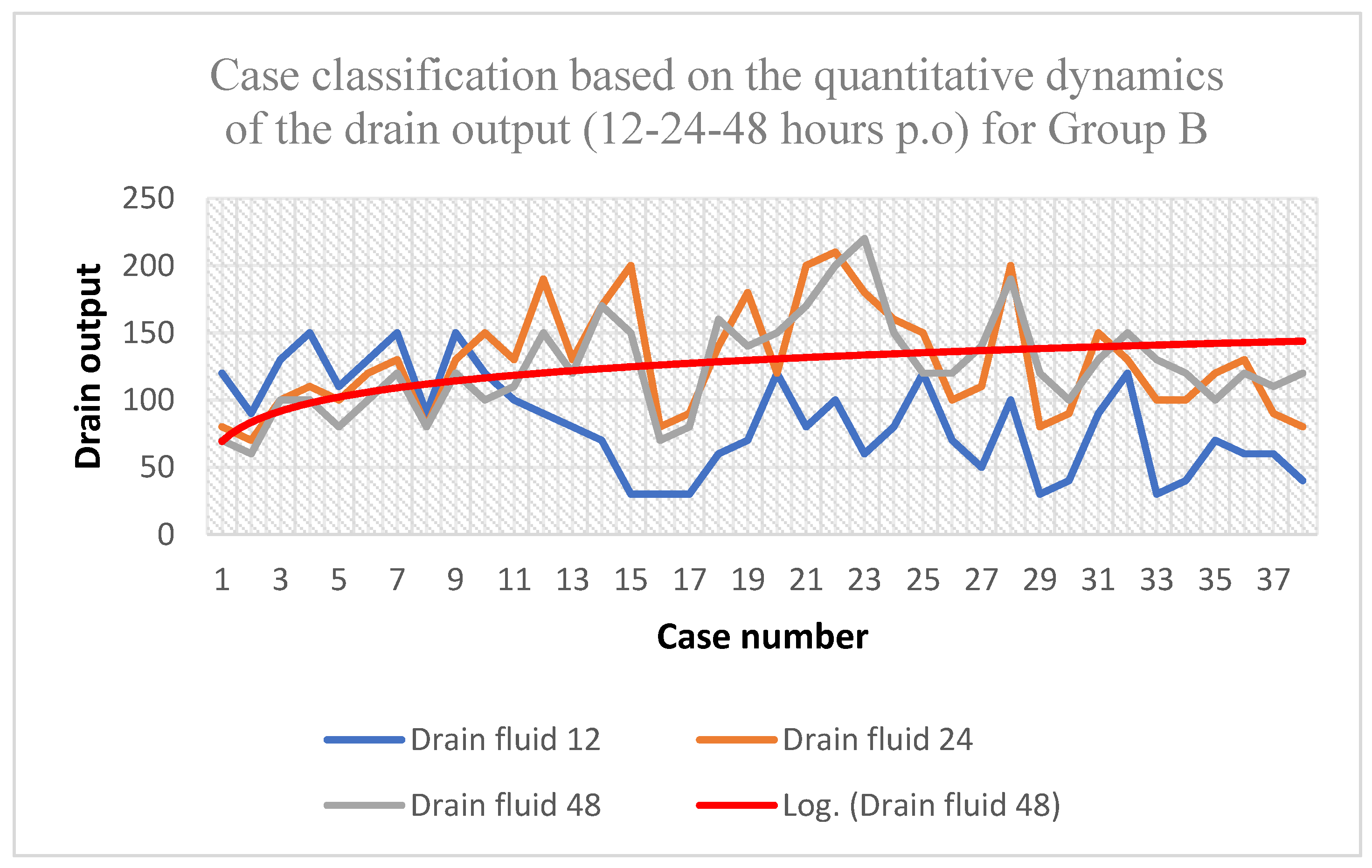
| Drain fluid 12 (mL) | Drain fluid 24 (mL) | Drain fluid 48 (mL) | ||
| Average | 44.73 | 93.68 | 78.68 | |
| Min | 20 | 60 | 40 | |
| Max | 100 | 130 | 110 |
| Type of Intervention | % | Total % |
|---|---|---|
| BCS | 36.84% | |
| 63.15 | ||
| OBCS | 26.31% | |
| MRM (Madden procedure) | 34.21% | 34.21 |
| Mammoplasty Type | Case Number | % |
|---|---|---|
| Pitangui–Lejour | 1 | 2.63 |
| Wise pattern | 2 | 5.26 |
| Riberio–Robbins | 4 | 10.52 |
| Grisotti | 3 | 7.89 |
| Total | 10 | 26.31 |
| Function | VAS 6 | VAS 12 | VAS 24 | VAS 36 |
|---|---|---|---|---|
| Average | 4.92 | 3.71 | 2.71 | 2.28 |
| Min | 2 | 2 | 1 | 1 |
| Max | 8 | 6 | 4 | 4 |
| Output 12 (mL) | Output 24 (mL) | Output 48 (mL) | ||
| Average | 83.16 | 128.42 | 124.74 | |
| Min | 30 | 70 | 60 | |
| Max | 150 | 210 | 220 |
| Type of Surgery | Group A | Group B | p Value |
|---|---|---|---|
| BCS | 44.73% | 36.84% | |
| OBCS | 23.68% | 26.31% | 0.965 |
| RMM | 31.57% | 34.21% | |
| Surgical drain fluid volume (mean value in mL at 12–24–48 p.o) | |||
| 12 h p.o | 44.73 mL | 83.16 | |
| 24 h p.o | 93.68 mL | 128.42 | 0.001 |
| 48 h p.o | 78.68 | 124.74 | |
| Complications | |||
| Haematoma | 1 | 2 | |
| Seroma | 1 | 4 | 0.05 |
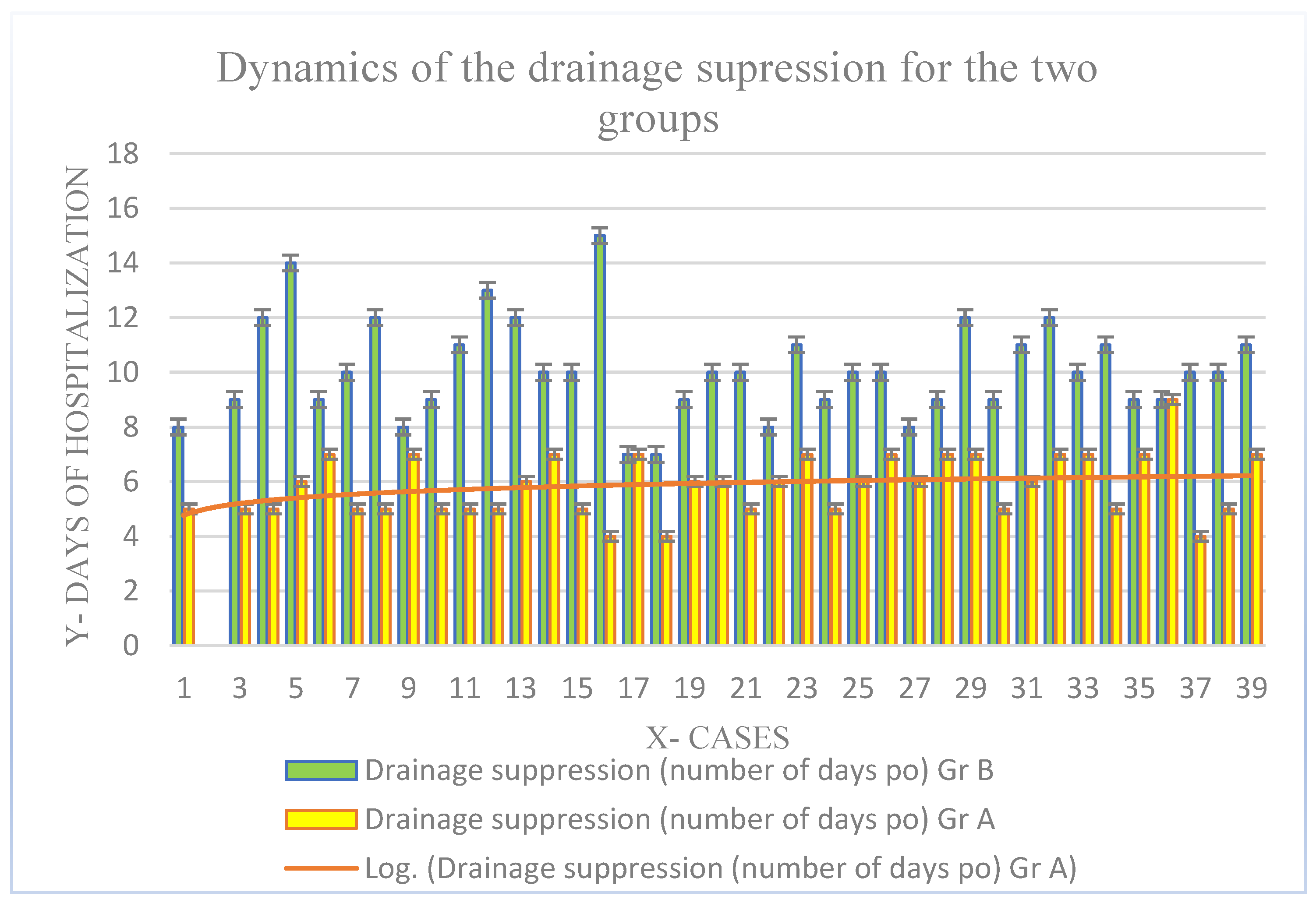
| Days of hospitalization (mean value ± SD) | 2.36 ± 1.2 | 2.86 ± 1.2 | 0.67 |
| The number of analgesics administered p.o (mean value) | |||
| Average | 0.57 | 13 | |
| Min | 0 | 9 | 0.001 |
| Max | 2 | 17 | |
| Smoking | 42.10% (n = 16) | 36.84% (n = 14) | 0.889 |
| No smoking | 57.89% (n = 22) | 63.15 (n = 24) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faur, F.I.; Clim, I.A.; Dobrescu, A.; Isaic, A.; Prodan, C.; Florea, S.; Tarta, C.; Totolici, B.; Duţă, C.; Pasca, P.; et al. The Use of Wound Infiltration for Postoperative Pain Management after Breast Cancer Surgery: A Randomized Clinical Study. Biomedicines 2023, 11, 1195. https://doi.org/10.3390/biomedicines11041195
Faur FI, Clim IA, Dobrescu A, Isaic A, Prodan C, Florea S, Tarta C, Totolici B, Duţă C, Pasca P, et al. The Use of Wound Infiltration for Postoperative Pain Management after Breast Cancer Surgery: A Randomized Clinical Study. Biomedicines. 2023; 11(4):1195. https://doi.org/10.3390/biomedicines11041195
Chicago/Turabian StyleFaur, Flaviu Ionut, Ioana Adelina Clim, Amadeus Dobrescu, Alexandru Isaic, Catalin Prodan, Sabrina Florea, Cristi Tarta, Bogdan Totolici, Ciprian Duţă, Paul Pasca, and et al. 2023. "The Use of Wound Infiltration for Postoperative Pain Management after Breast Cancer Surgery: A Randomized Clinical Study" Biomedicines 11, no. 4: 1195. https://doi.org/10.3390/biomedicines11041195
APA StyleFaur, F. I., Clim, I. A., Dobrescu, A., Isaic, A., Prodan, C., Florea, S., Tarta, C., Totolici, B., Duţă, C., Pasca, P., & Lazar, G. (2023). The Use of Wound Infiltration for Postoperative Pain Management after Breast Cancer Surgery: A Randomized Clinical Study. Biomedicines, 11(4), 1195. https://doi.org/10.3390/biomedicines11041195





