High Fidelity Pressure Wires Provide Accurate Validation of Non-Invasive Central Blood Pressure and Pulse Wave Velocity Measurements
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
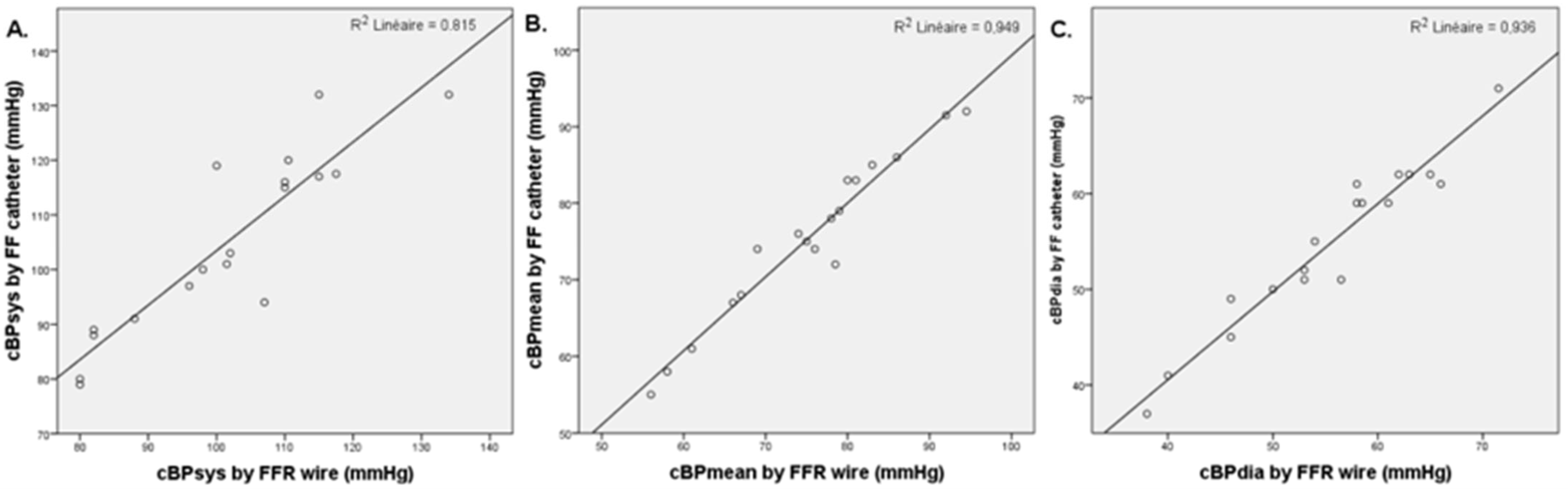
3.2. Invasive and Non-Invasive Central Blood Pressure Comparison
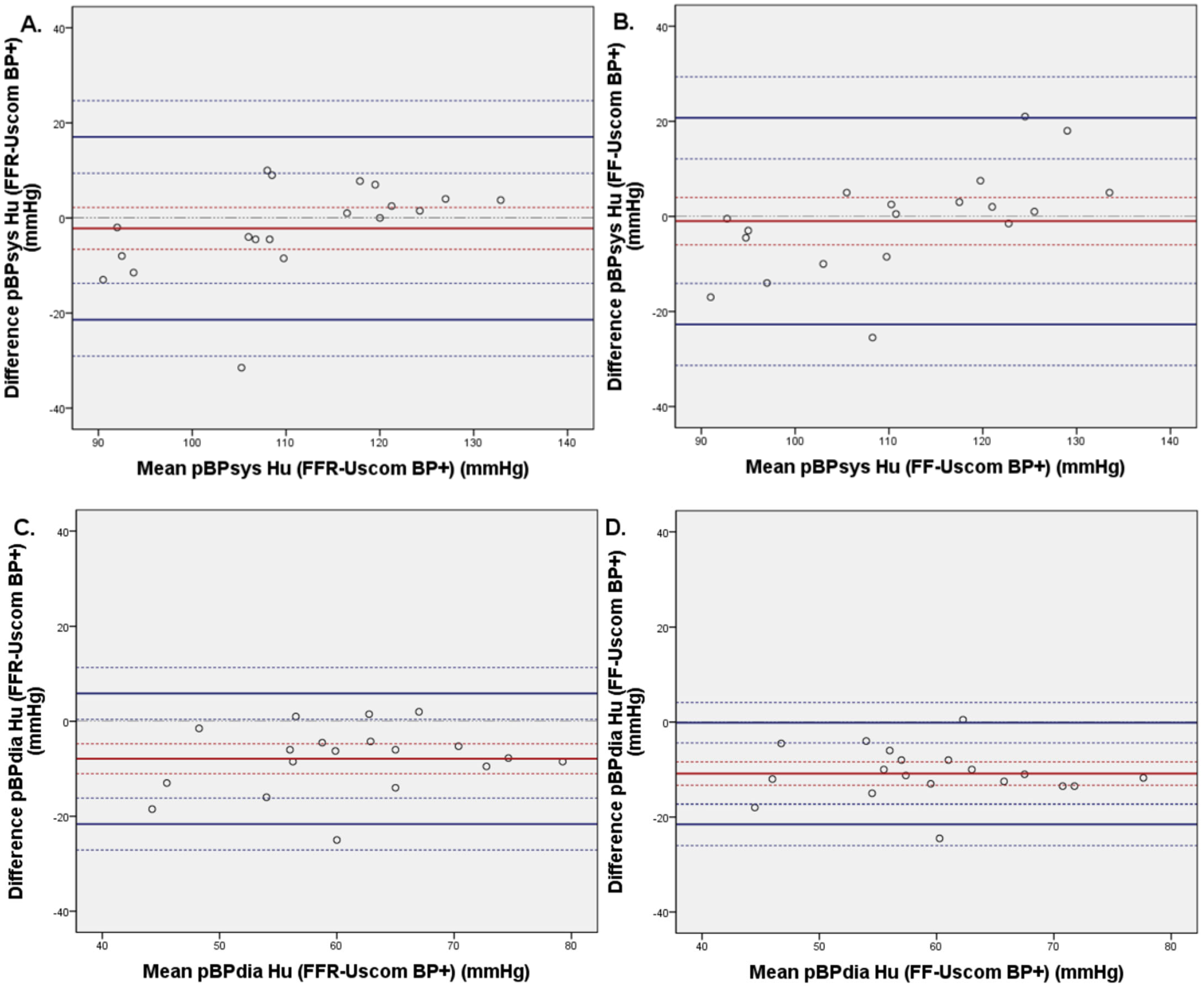
3.3. Invasive and Non-Invasive Brachial Blood Pressure Comparison
3.4. Invasive FFR and FF Central Blood Pressure Comparison
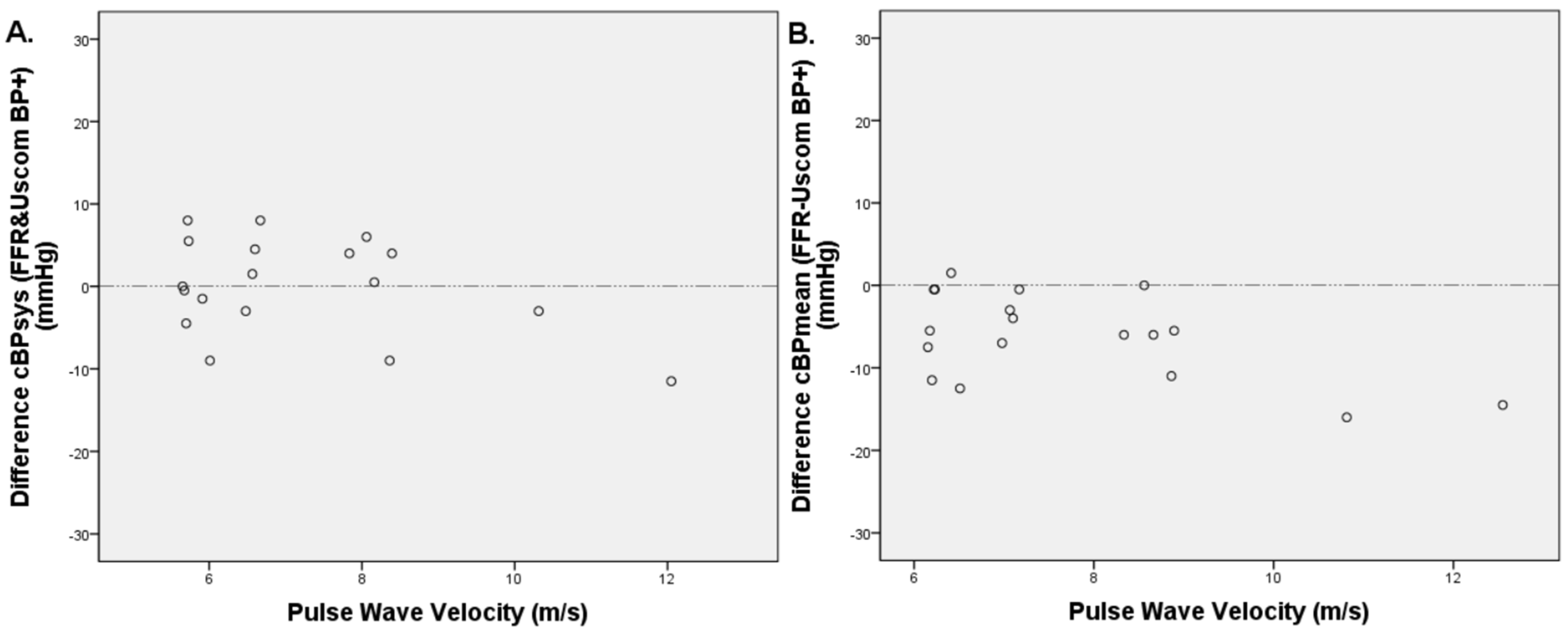
3.5. PWV Measurements
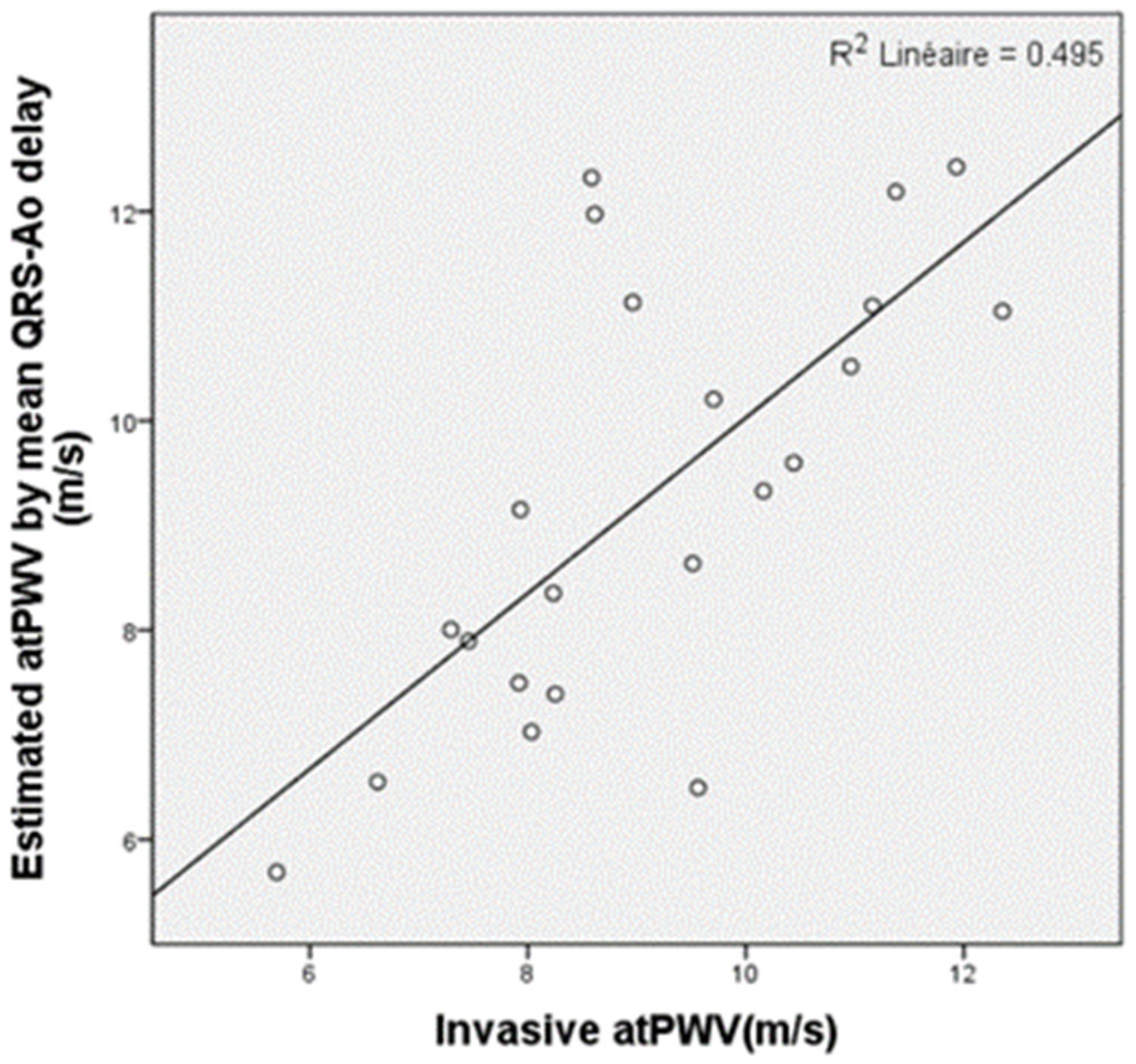
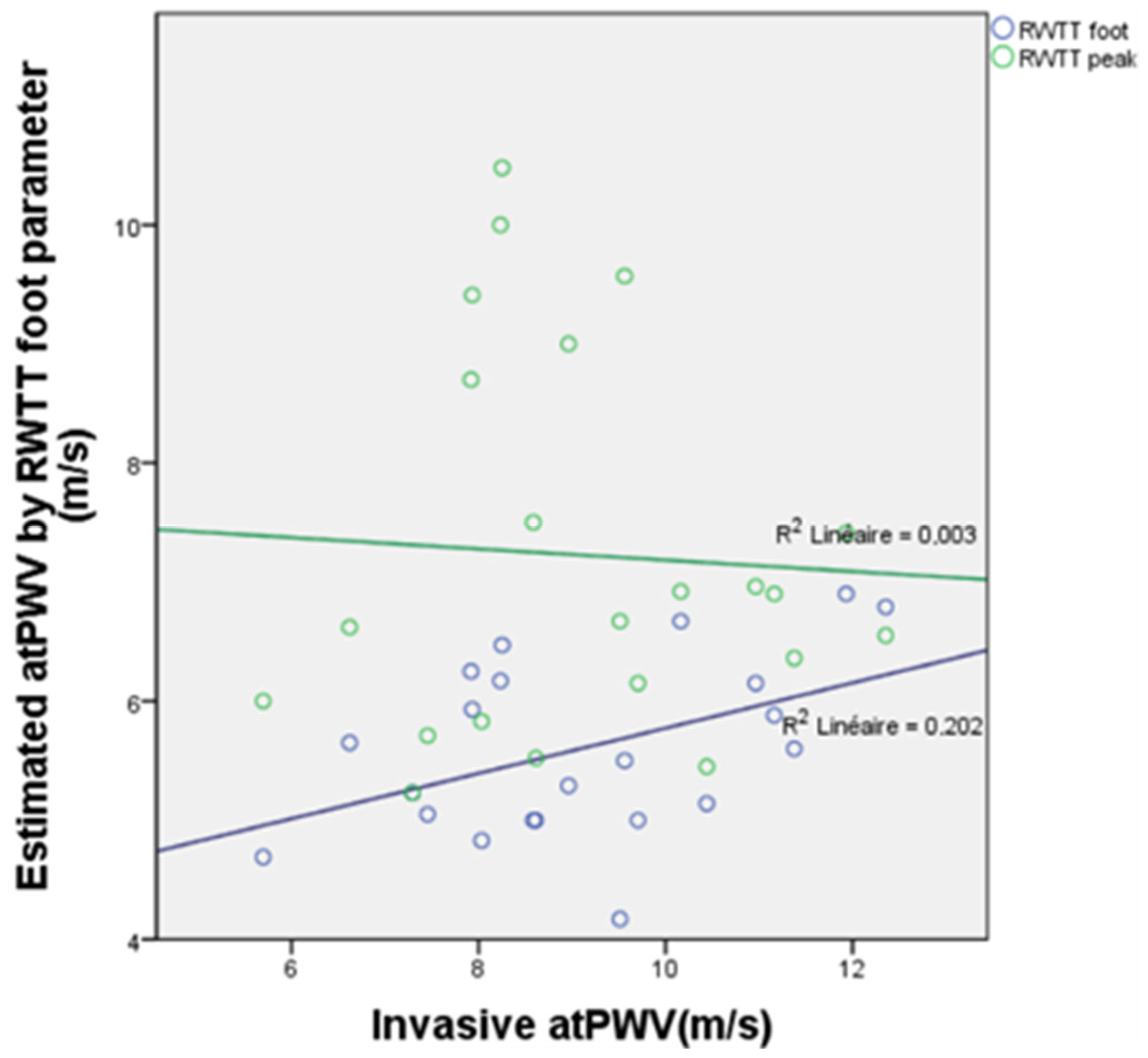
3.6. PWV Measurements Derived from Reflected Wave Transit Time (RWTT)
3.7. Pulse Wave Velocity, Central Blood Pressure Estimation, and Cardiovascular Risk Factors
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison, H.C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Picone, D.S.; Campbell, N.R.C.; Schutte, A.E.; Olsen, M.H.; Ordunez, P.; Whelton, P.K.; Sharman, J.E. Validation Status of Blood Pressure Measuring Devices Sold Globally. JAMA 2022, 327, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Lee, E.T.; Galloway, J.M.; Ali, T.; Umans, J.G.; Howard, B.V. Central Pressure More Strongly Relates to Vascular Disease and Outcome Than Does Brachial Pressure. Hypertension 2007, 50, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, L. Central Blood Pressure as an Index of Antihypertensive Control: Determinants and Potential Value. Can. J. Cardiol. 2014, 30, S23–S28. [Google Scholar] [CrossRef]
- Ohno, Y.; Kanno, Y.; Takenaka, T. Central blood pressure and chronic kidney disease. World J. Nephrol. 2016, 5, 90–100. [Google Scholar] [CrossRef]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central blood pressure: Current evidence and clinical importance. Eur. Heart J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef]
- Segers, P.; Mahieu, D.; Kips, J.; Rietzschel, E.; De Buyzere, M.; De Bacquer, D.; Bekaert, S.; De Backer, G.; Gillebert, T.; Verdonck, P.; et al. Amplification of the Pressure Pulse in the Upper Limb in Healthy, Middle-Aged Men and Women. Hypertension 2009, 54, 414–420. [Google Scholar] [CrossRef]
- McEniery, C.M.; Yasmin, N.; McDonnell, B.; Munnery, M.; Wallace, S.M.; Rowe, C.V.; Cockcroft, J.R.; Wilkinson, I.B. Central Pressure: Variability and Impact of Cardiovascular Risk Factors. Hypertension 2008, 51, 1476–1482. [Google Scholar] [CrossRef]
- Avolio, A.P.; Van Bortel, L.M.; Boutouyrie, P.; Cockcroft, J.R.; McEniery, C.M.; Protogerou, A.D.; Roman, M.J.; Safar, M.E.; Segers, P.; Smulyan, H. Role of Pulse Pressure Amplification in Arterial Hypertension. Hypertension 2009, 54, 375–383. [Google Scholar] [CrossRef]
- Cecelja, M.; Chowienczyk, P. Arterial stiffening: Causes and consequences. Artery Res. 2013, 7, 22–27. [Google Scholar] [CrossRef]
- Quinn, U.; Tomlinson, L.A.; Cockcroft, J.R. Arterial stiffness. JRSM Cardiovasc. Dis. 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Schmitt, M.; Avolio, A.; Qasem, A.; McEniery, C.M.; Butlin, M.; Wilkinson, I.B.; Cockcroft, J.R. Basal NO Locally Modulates Human Iliac Artery Function In Vivo. Hypertension 2005, 46, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, D.; Zhang, Y.; Borghi, C.; Blacher, J.; Safar, M.E. Effects of Antihypertensive Drugs on Central Blood Pressure in Humans: A Preliminary Observation. Am. J. Hypertens. 2013, 26, 1045–1052. [Google Scholar] [CrossRef]
- Pauca, A.L.; O’Rourke, M.F.; Kon, N.D. Prospective Evaluation of a Method for Estimating Ascending Aortic Pressure From the Radial Artery Pressure Waveform. Hypertension 2001, 38, 932–937. [Google Scholar] [CrossRef]
- Sztrymf, B.; Jacobs, F.; Chemla, D.; Richard, C.; Millasseau, S.C. Validation of the new Complior sensor to record pressure signals non-invasively. J. Clin. Monit. Comput. 2013, 27, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Carlier, S.; Pasquet, A.; Rabben, S.I.; Hellevik, L.R.; Remme, E.; De Backer, T.; De Sutter, J.; Thomas, J.D.; Verdonck, P. Individualizing the aorto-radial pressure transfer function: Feasibility of a model-based approach. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, H542–H549. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Karageorgopoulou, T.D.; Sergentanis, T.N.; Protogerou, A.D.; Psaltopoulou, T.; Sharman, J.E.; Weber, T.; Blacher, J.; Daskalopoulou, S.S.; Wassertheurer, S.; et al. Accuracy of commercial devices and methods for noninvasive estimation of aortic systolic blood pressure a systematic review and meta-analysis of invasive validation studies. J. Hypertens. 2016, 34, 1237. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Hwang, S.-J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial Stiffness and Cardiovascular Events. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-J.; Jung, I.H.; Kim, J.; Kim, J.H.; Suh, J.; Kim, H.S.; Kim, H.K.; Jung, Y.J.; Kang, J.W.; Lee, S. Association between brachial-ankle pulse wave velocity and occult coronary artery disease detected by multi-detector computed tomography. Int. J. Cardiol. 2012, 157, 227–232. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- Gotzmann, M.; Hogeweg, M.; Seibert, F.S.; Rohn, B.J.; Bergbauer, M.; Babel, N.; Bauer, F.; Mügge, A.; Westhoff, T.H. Accuracy of fully automated oscillometric central aortic blood pressure measurement techniques. J. Hypertens. 2020, 38, 235. [Google Scholar] [CrossRef]
- Kowalski, C.; Yang, K.; Charron, T.; Doucet, M.; Hatem, R.; Kouz, R.; Palisaitis, D.; Schampaert, E.; Terriault, P.; Tessier, P.; et al. Inaccuracy of brachial blood pressure and its potential impact on treatment and aortic blood pressure estimation. J. Hypertens. 2021, 39, 2370–2378. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, W.; El-Aklouk, E.; Ruygrok, P.; Al-Jumaily, A.M. Non-invasive model-based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J. Biomech. 2009, 42, 2111–2115. [Google Scholar] [CrossRef]
- Gardner, R.M. Direct Blood Pressure Measurement—Dynamic Response Requirements. Anesthesiology 1981, 54, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M. Principles of pressure transducers, resonance, damping and frequency response. Anaesth. Intensive Care Med. 2012, 13, 1–6. [Google Scholar] [CrossRef]
- Boutaleb, A.M.; Ghafari, C.; Ungureanu, C.; Carlier, S. Fractional flow reserve and non-hyperemic indices: Essential tools for percutaneous coronary interventions. World J. Clin. Cases 2023, 11, 2123–2139. [Google Scholar] [CrossRef]
- Sharman, J.E.; Avolio, A.P.; Baulmann, J.; Benetos, A.; Blacher, J.; Blizzard, C.L.; Boutouyrie, P.; Chen, C.-H.; Chowienczyk, P.; Cockcroft, J.R.; et al. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur. Heart J. 2017, 38, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Fiori, G.; Fuiano, F.; Scorza, A.; Conforto, S.; Sciuto, S.A. Non-Invasive Methods for PWV Measurement in Blood Vessel Stiffness Assessment. IEEE Rev. Biomed. Eng. 2022, 15, 169–183. [Google Scholar] [CrossRef]
- Trachet, B.; Reymond, P.; Kips, J.; Swillens, A.; De Buyzere, M.; Suys, B.; Stergiopulos, N.; Segers, P. Numerical Validation of a New Method to Assess Aortic Pulse Wave Velocity from a Single Recording of a Brachial Artery Waveform with an Occluding Cuff. Ann. Biomed. Eng. 2010, 38, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. J. R. Stat. Soc. Ser. Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Joffe, R.; Duff, J.; Garcia Guerra, G.; Pugh, J.; Joffe, A.R. The accuracy of blood pressure measured by arterial line and non-invasive cuff in critically ill children. Crit. Care 2016, 20, 177. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Nakagomi, A.; Okada, S.; Ohno, Y.; Kobayashi, Y. Invasive validation of a novel brachial cuff-based oscillometric device (SphygmoCor XCEL) for measuring central blood pressure. J. Hypertens. 2017, 35, 69. [Google Scholar] [CrossRef]
- Mattace-Raso, F.U.; Hofman, A.; Verwoert, G.C.; Wittemana, J.C.; Wilkinson, I.; Cockcroft, J.; McEniery, C.; Yasmin, N.; Laurent, S.; Boutouyrie, P.; et al. The Reference Values for Arterial Stifness? Collaboration. Determinants of Pulse wave velocity in healthy people amd im the presence of cardiovascular rsik factors: “Establishing normal and reference values.” Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar]
- Tanaka, H.; Munakata, M.; Kawano, Y.; Ohishi, M.; Shoji, T.; Sugawara, J.; Tomiyama, H.; Yamashina, A.; Yasuda, H.; Sawayama, T.; et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J. Hypertens. 2009, 27, 2022. [Google Scholar] [CrossRef]
- Picone, D.S.; Schultz, M.G.; Otahal, P.; Aakhus, S.; Al-Jumaily, A.M.; Black, J.A.; Bos, W.J.; Chambers, J.B.; Chen, C.-H.; Cheng, H.-M.; et al. Accuracy of Cuff-Measured Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 572–586. [Google Scholar] [CrossRef]
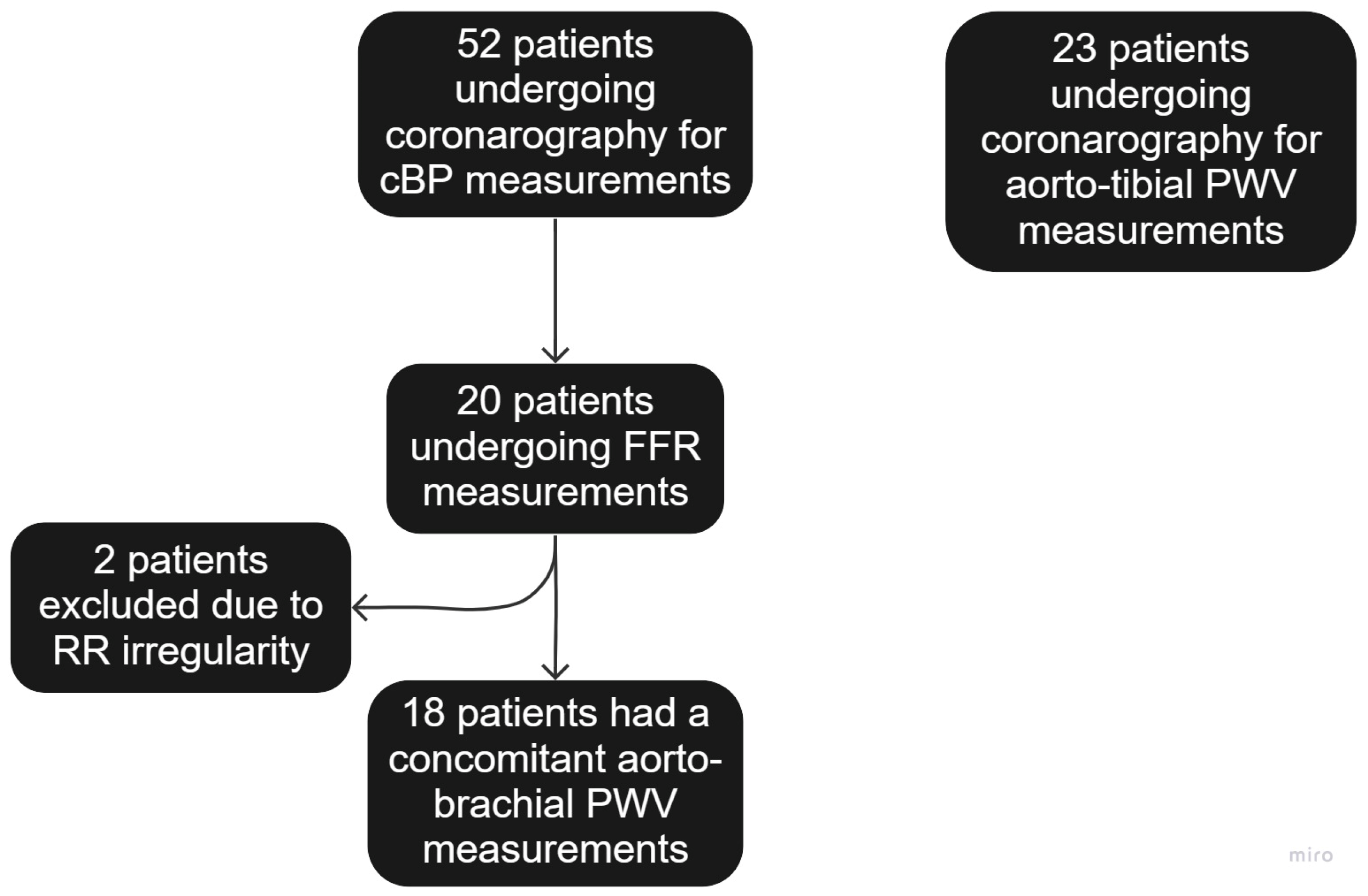
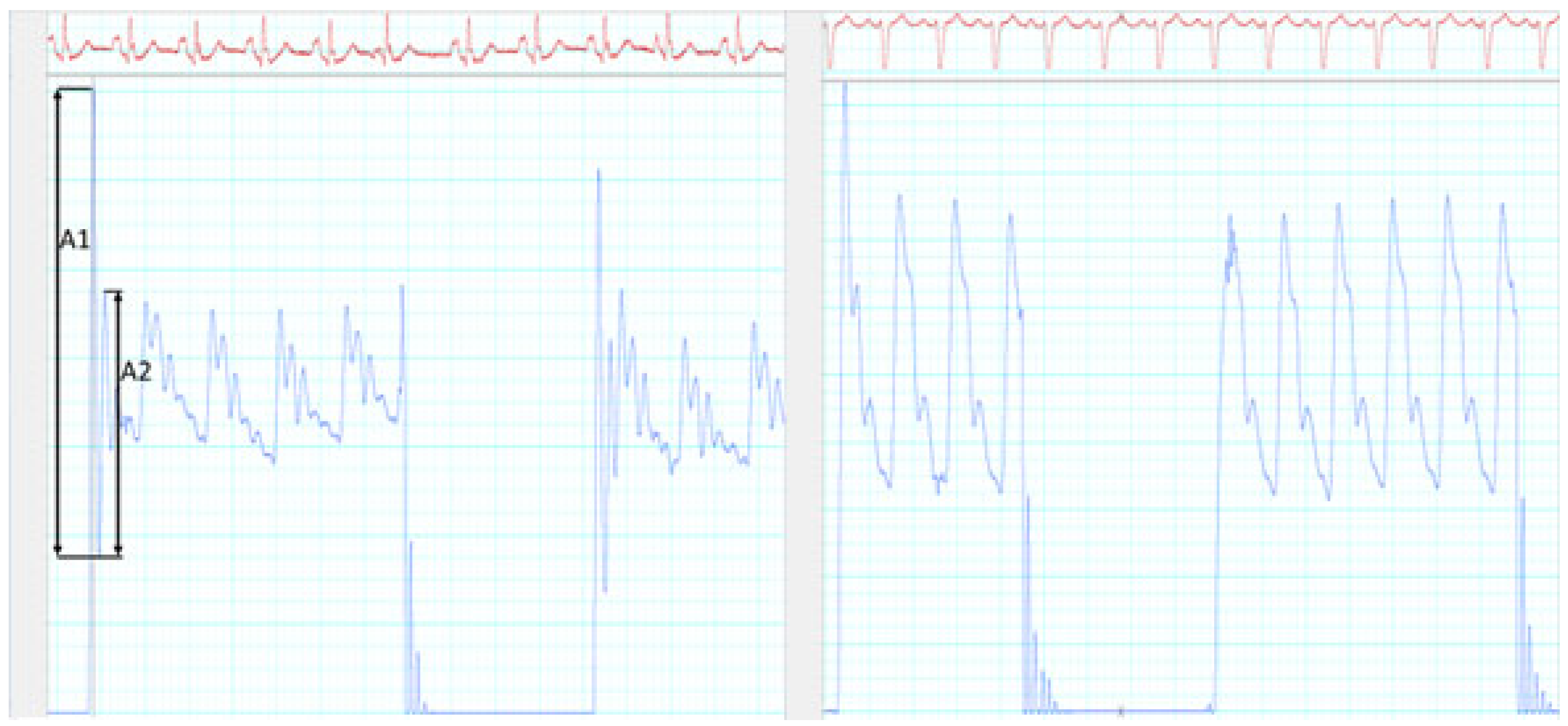

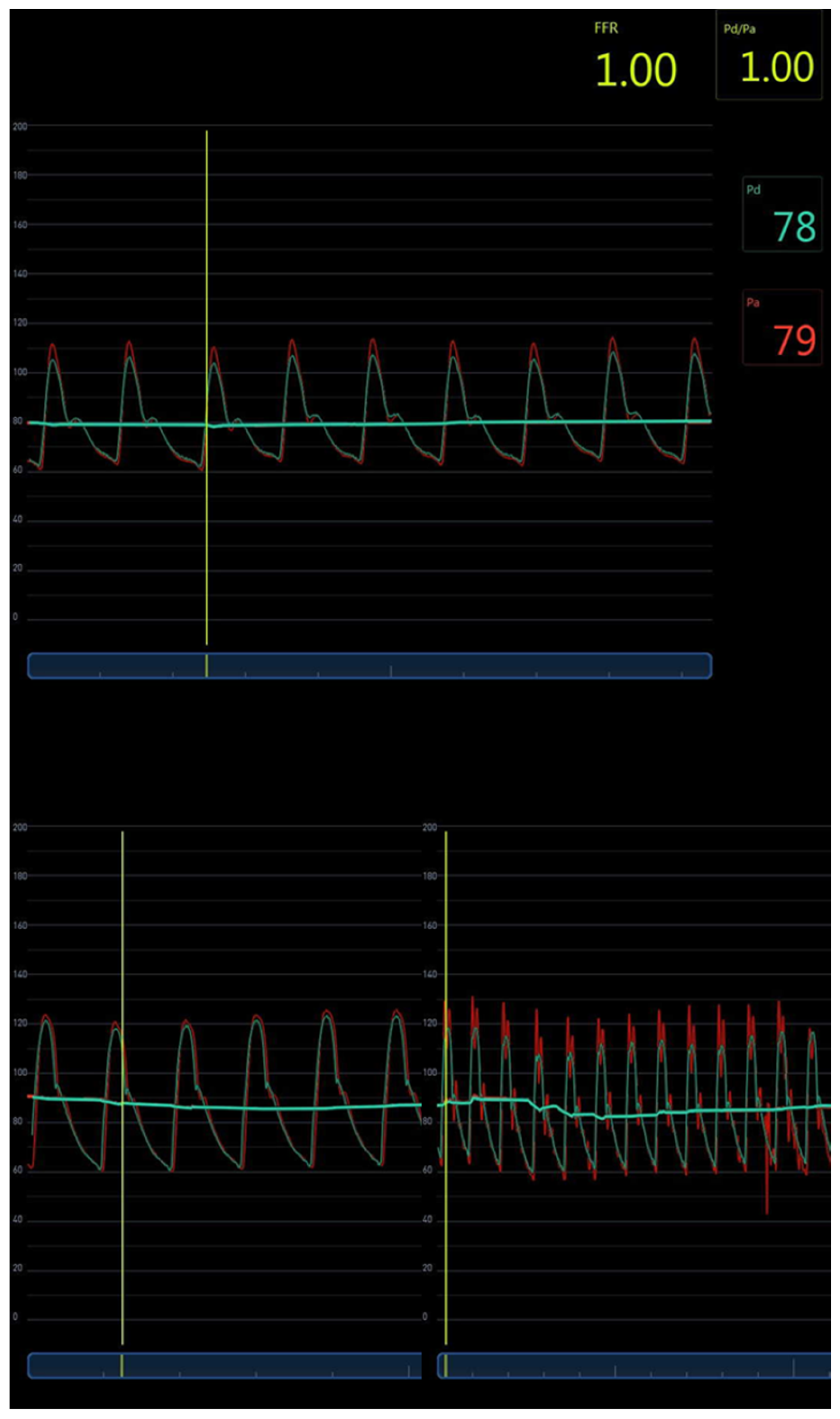
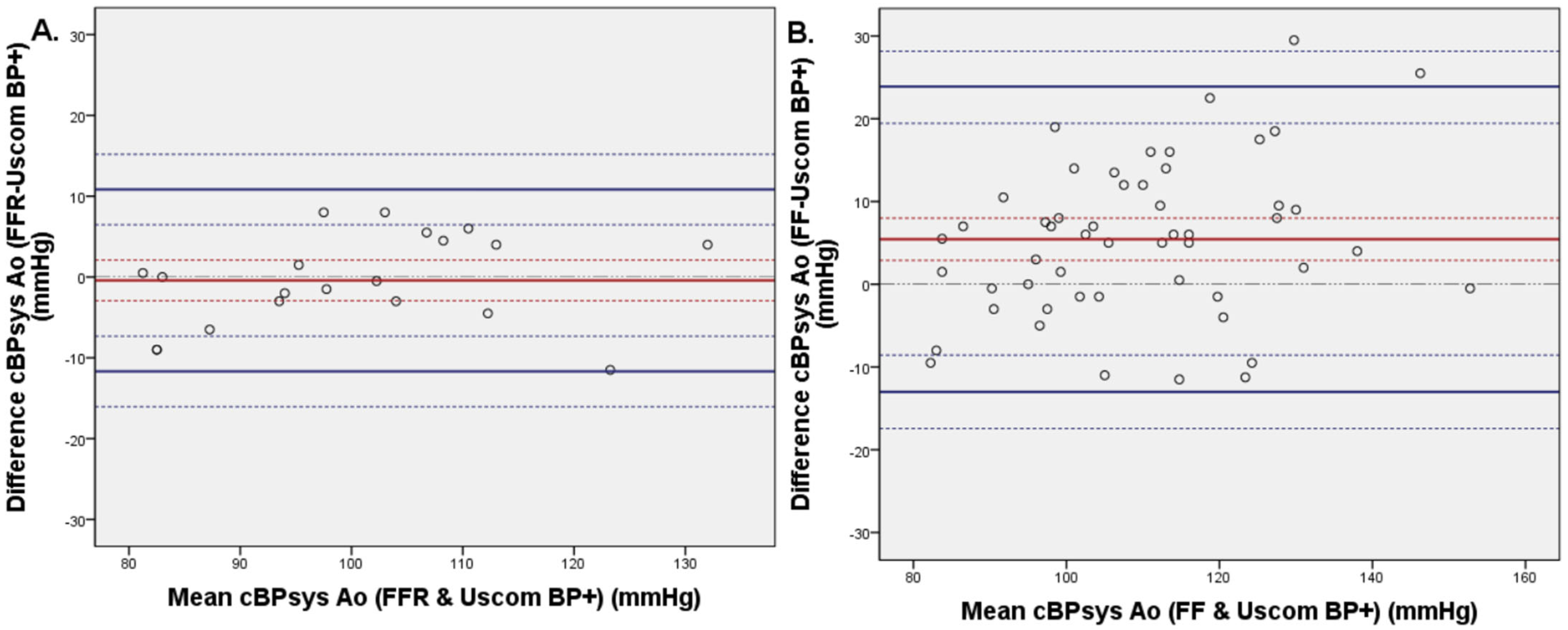




| Characteristic | cBP Comparison and n = 52 (%) | atPWV n = 23 (%) |
|---|---|---|
Smoking history
| 26 (50) 12 (23) | 17 (74) 6 (26) |
| Hypertension | 34 (65) | 12 (52) |
| Diabetes mellitus | 24 (46) | 9 (39) |
| Dyslipidemiae | 50 (96) | 16 (70) |
| CAD history | 19 (37) | |
| CKD | 17 (33) | |
| LVH | 11 (21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalia, A.; Ghafari, C.; Navarre, W.; Delmotte, P.; Phillips, R.; Carlier, S. High Fidelity Pressure Wires Provide Accurate Validation of Non-Invasive Central Blood Pressure and Pulse Wave Velocity Measurements. Biomedicines 2023, 11, 1235. https://doi.org/10.3390/biomedicines11041235
Scalia A, Ghafari C, Navarre W, Delmotte P, Phillips R, Carlier S. High Fidelity Pressure Wires Provide Accurate Validation of Non-Invasive Central Blood Pressure and Pulse Wave Velocity Measurements. Biomedicines. 2023; 11(4):1235. https://doi.org/10.3390/biomedicines11041235
Chicago/Turabian StyleScalia, Alessandro, Chadi Ghafari, Wivine Navarre, Philippe Delmotte, Rob Phillips, and Stéphane Carlier. 2023. "High Fidelity Pressure Wires Provide Accurate Validation of Non-Invasive Central Blood Pressure and Pulse Wave Velocity Measurements" Biomedicines 11, no. 4: 1235. https://doi.org/10.3390/biomedicines11041235
APA StyleScalia, A., Ghafari, C., Navarre, W., Delmotte, P., Phillips, R., & Carlier, S. (2023). High Fidelity Pressure Wires Provide Accurate Validation of Non-Invasive Central Blood Pressure and Pulse Wave Velocity Measurements. Biomedicines, 11(4), 1235. https://doi.org/10.3390/biomedicines11041235






