Abstract
The importance of uric acid, the final metabolite of purines excreted by the kidneys and intestines, was not previously recognized, except for its role in forming crystals in the joints and causing gout. However, recent evidence implies that uric acid is not a biologically inactive substance and may exert a wide range of effects, including antioxidant, neurostimulatory, proinflammatory, and innate immune activities. Notably, uric acid has two contradictory properties: antioxidant and oxidative ones. In this review, we present the concept of “dysuricemia”, a condition in which deviation from the appropriate range of uric acid in the living body results in disease. This concept encompasses both hyperuricemia and hypouricemia. This review draws comparisons between the biologically biphasic positive and negative effects of uric acid and discusses the impact of such effects on various diseases.
1. Introduction
Gout is one of the most well-known and ancient diseases, which continues to affect humans today. Recent data from the 2007–2016 National Health and Nutrition Examination Survey (NHANES) revealed that the prevalence of gout in the United States is 3.9%, which is equivalent to approximately 9.2 million people [1].
Uric acid was first identified in urine by Scheele in 1776 and later extracted from the tophi of gout by Wollastone in 1787 [2]. In 1848, Garrod discovered that patients with gout had elevated blood glucose levels [3]. Gout is a type of inflammatory arthritis that occurs when sodium uric acid crystallizes in the joints and is preceded by hyperuricemia. Historically, hyperuricemia was considered synonymous with gout. Uric acid was thought to be a form of waste excreted from the kidneys and intestinal tract. When serum uric acid rises above the dissolution limit, it crystallizes and precipitates in the joints, causing inflammation. If it precipitates in the urinary tract, it forms stones. Therefore, serum uric acid levels were measured only when gouty arthritis and uric acid urolithiasis were suspected. Traditionally, asymptomatic hyperuricemia was generally considered a benign disease that did not require treatment [4,5]. However, in recent years, it has become increasingly clear that uric acid is not a biologically inert substance but has various biological functions. Therefore, both high and low uric acid levels can affect an organism, even if the condition is asymptomatic.
Hyperuricemia is strongly associated with lifestyle-related diseases such as hypertension [6,7], type 2 diabetes mellitus [8], and metabolic syndrome [9]. It may contribute to myocardial infarction, an atherosclerotic disease [10,11]. In addition, gout caused by hyperuricemia is extremely painful and reduces the quality of life [12] and physical function [13]. Conversely, a study implied that hypouricemia causes exercise-induced renal failure and urinary stones [14]. Therefore, it is now understood that both elevated and reduced uric acid levels can have adverse effects on the living body. In this context, we propose the concept of “uricemia,” which encompasses both hyperuricemia and hypouricemia. This review outlines the conflicting biological roles of uric acid and discusses its impact on various disease states.
2. Definition and Epidemiology
Hyperuricemia is determined by the solubility of uric acid and is defined as a serum uric acid level greater than 7.0 mg/dL in both men and women [14]. In the NHANES, the prevalence rates of gout and hyperuricemia remained substantial in the U.S. between 2007 and 2016, with gout having a prevalence of 3.9% (9.2 million individuals) among U.S. adults from 2015 to 2016. The mean serum uric acid level was 6.0 mg/dL in men and 4.8 mg/dL in women, and the prevalence of hyperuricemia was 20.2% in men and 20.0% in women [1]. The data show that the prevalence of hyperuricemia has been stable over the last 10 years, but it still persists at a considerable rate.
Although there is no established definition of hypouricemia, a value of 2.0 mg/dL or less is generally used as a reference value for this condition [14]. Hypouricemia is less common than hyperuricemia, and in Japan, its prevalence was reported to be 0.2% in men and 0.4% in women [15]. Hypouricemia was also found to be a rare outcome in Germany, affecting 0.09% of the population (0% in men; 0.27% in women) [16]. Regardless of race or region, hypouricemia has been found to be more prevalent in women than in men.
2.1. Serum Uric Acid Regulation
Blood uric acid levels depend on the balance between the exogenous production of uric acid via dietary factors (including purine intake) and its endogenous production via purine metabolism, reabsorption and excretion by the kidney, and excretion by the intestine [17]. Thus, uric acid is homeostasis through the complicated process of production, secretion, and reabsorption in the kidney and excretion in the intestines. Uric acid is produced by the metabolism of endogenous purines (approximately 300–400 mg synthesized daily) and exogenous purines (approximately 300 mg from the diet), totaling 1200 mg in healthy men (600 mg in healthy women) on purine-free diets. The purine nucleobases adenine and guanine are components of both ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Therefore, the breakdown of RNA and DNA increases blood uric acid levels. Many hematological diseases, such as acute myelogenous leukemia, involve the production of excessive uric acid as tumor cells proliferate and collapse, causing hyperuricemia. Hyperuricemia is also caused by the rapid disintegration of tumor cells by antitumor agents. This is called tumor lysis syndrome and is complicated by hyperkalemia and hyperphosphatemia. Hyperuricemia associated with hematological diseases reflects tumor volume. It may also indicate a response to treatment with antitumor agents. Rapidly elevated uric acid levels can lead to renal failure, which is treated with uric acid inhibitors or uric acid-degrading enzymes, depending on the risk of developing the disease [18]. In addition, uric acid is produced by the breakdown of adenosine triphosphate (ATP) through the metabolism of fructose and alcohol. ATP is an important source of energy for intracellular reactions. In terms of dietary effects, animal protein contributes significantly to the intake of this purine. In addition, serum uric acid levels are increased by the intake of glutamic acid, which is metabolized to uric acid in the liver, and by the intake of foods high in purines and uric acid itself. Foods that affect serum uric acid levels include alcohol, meat and seafood, soft drinks, dairy products, coffee, and vitamin C. A purine-free diet reduces uric acid excretion via urine by approximately 40% [19], indicating that dietary purine contributes greatly to serum uric acid levels.
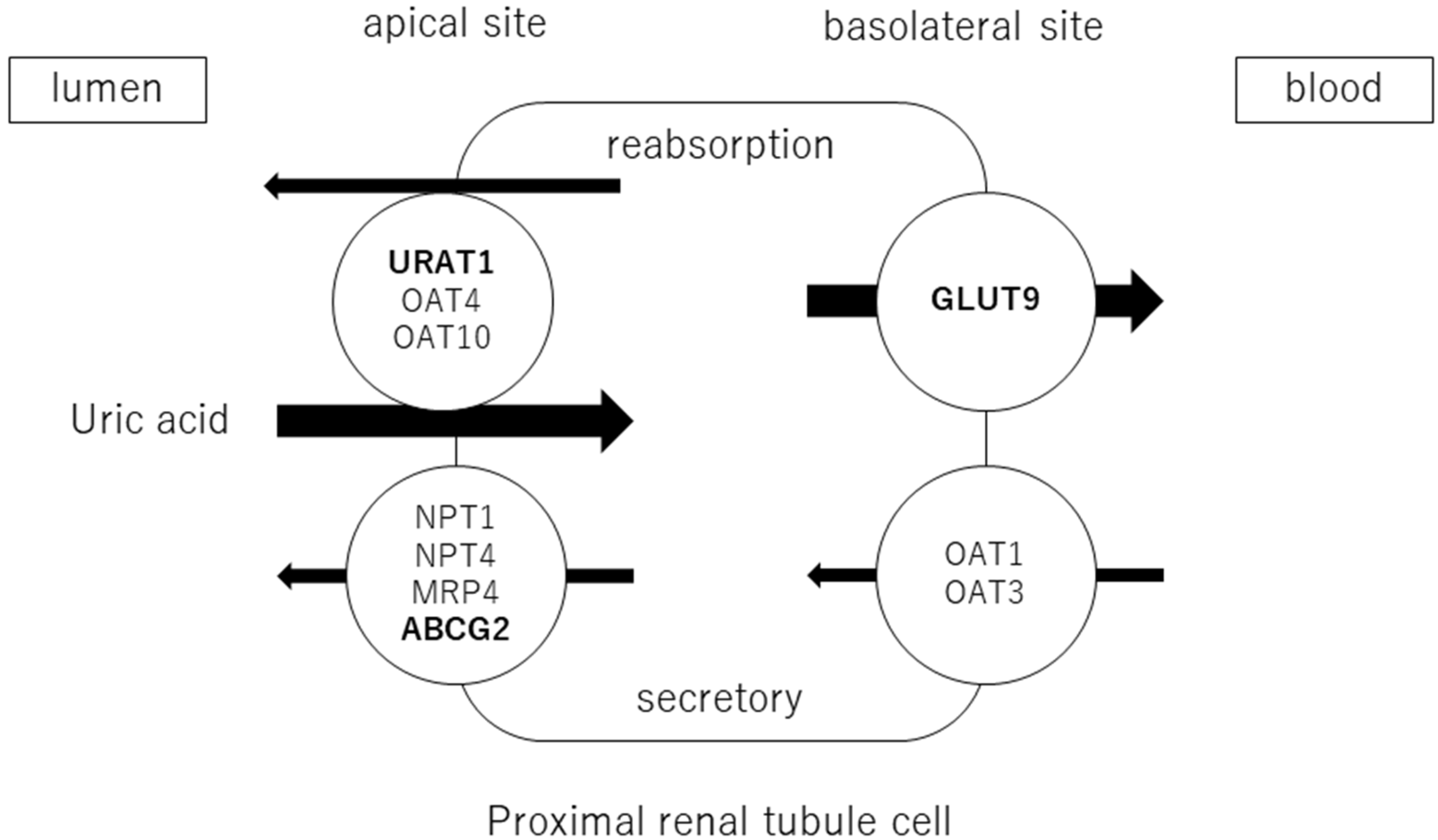
Adenosine products are converted to hypoxanthine, and hypoxanthine is oxidized to xanthine and then uric acid by xanthine oxidoreductase (XOR). Approximately two-thirds of uric acid is excreted by the kidneys and one-third by the small intestine. Uric acid is filtered through the glomeruli and reabsorbed in the proximal tubules. The excretion rate of uric acid in the proximal tubules of the kidney is approximately 10% [20] and is regulated by transporters expressed at the apical and basolateral sides. Uric acid reabsorption is mediated by URAT1/SLC22A12, OAT4/SLC22A11, and OAT10/SLC22A3 transporters at apical sides [21], and by GLUT9/SLC2A9 transporter at basolateral sides. Uric acid secretion is mediated by OAT1/SLC22A11 and OAT3/SLC22A8 transporters at the basolateral sides and by NPT1/SLC17A1, NPT4/SLC17A3, MRP4/ABCC4, and BCRP/ABCG2 at the apical sides [22,23] (Figure 1). URAT1 and GLUT9 are transporters for uric acid reabsorption, and their dysfunction causes hypouricemia. Patients with URAT1 and GLUT9 dysfunctional variants are called hereditary renal hypouricemia types 1 and 2, respectively. In the intestine, BCRP/ABCG2 secretes uric acid into the intestinal lumen [24]. Genome-wide association studies have reported over 30 genetic variants in uric acid transporters that affect serum uric acid. Three uric acid transporters, URAT1, GLUT9, and ABCG2, play key roles in the regulation of serum uric acid, and their dysfunctions lead to dysuricemia (hypouricemia and hyperuricemia). The role of ABCG2 variants has been shown to be more important for the risk of hyperuricemia than environmental factors such as obesity and intense alcohol consumption [25]. Nonsynonymous allelic variants of ABCG2 were shown to significantly hasten the onset of hyperuricemia and increase the likelihood of gout and the presence of a family history of gout. ABCG2 dysfunction was known to be a risk factor for pediatric-onset hyperuricemia and gout [26]. Furthermore, Nakayama et al. reported a significant increase in the selection of the ABCG2 and ALDH2 loci in gout patients in Japan [27].
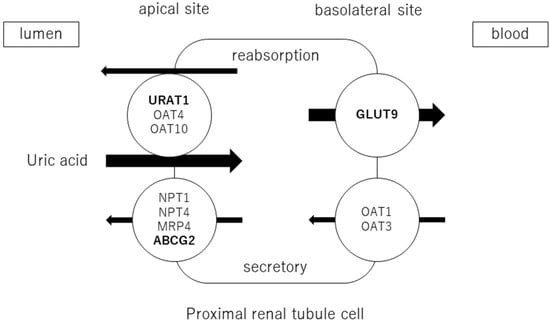
Figure 1.
Uric acid transport molecules in the proximal renal tubules. Uric acid is regulated by several transporters expressed at the apical and basolateral sides. Uric acid reabsorption is mediated by URAT1/SLC22A12, OAT4/SLC22A11, and OAT10/SLC22A3 transporters at the apical side, and GLUT9/SLC2A9 transporter at the basolateral side. Uric acid secretion is mediated by OAT1/SLC22A11 and OAT3/SLC22A8 transporters at the basolateral side, and NPT1/SLC17A1, NPT4/SLC17A3, MRP4/ABCC4, and BCRP/ABCG2 at the apical side. URAT1, GLUT9, and ABCG2 were bolded, as they play crucial roles in the regulation of serum uric acid, and their dysfunctions cause uric acid transport disorders (hypouricemia and hyperuricemia).
Serum uric acid levels are affected by several drugs through transporters. Loop diuretics and thiazide diuretics decrease uric acid excretion by both decreasing extracellular fluid volume and glomerular filtration rate and inhibiting uric acid secretion via NPT4 [28,29]. Losartan, an angiotensin II receptor blocker (ARB) [23,30], and calcium channel blockers (CCBs) [31] also inhibit URAT1 and increase uric acid excretion. Cyclosporin A promotes uric acid uptake via OAT10 [32]. Sodium/glucose co-transporter-2 (SGLT2) inhibitors are agents that increase urinary glucose. Since glucose and uric acid are co-transported with glucose, serum uric acid levels decreased [14,33]. Sacubitril/valsartan also reduces serum uric acid [34]. However, the mechanism by which they lower uric acid remains unclear.
Uric acid is a weak acid with a pKa of 5.8. Uric acid occurs primarily as an anionic urate at physiological pH of 7.4. The reference range for serum uric acid in humans is 1.5–6.0 mg/dL for women and 2.5–7.0 mg/dL for men. There is a difference between the sexes in that hyperuricemia is more common in men. Uric acid has low solubility in water, so the average concentration of uric acid in human serum is at the dissolution limit (6.8 mg/dL). When this level is exceeded, it is crystallized as monosodium urate (MSU) [17]. In most species, uric acid is broken down into allantoin by urate oxidase (uricase). However, humans have lost uricase, so they exhibit higher levels of uric acid than other mammals [35].
2.1.1. The Pros and Cons of Uric Acid: The Pros
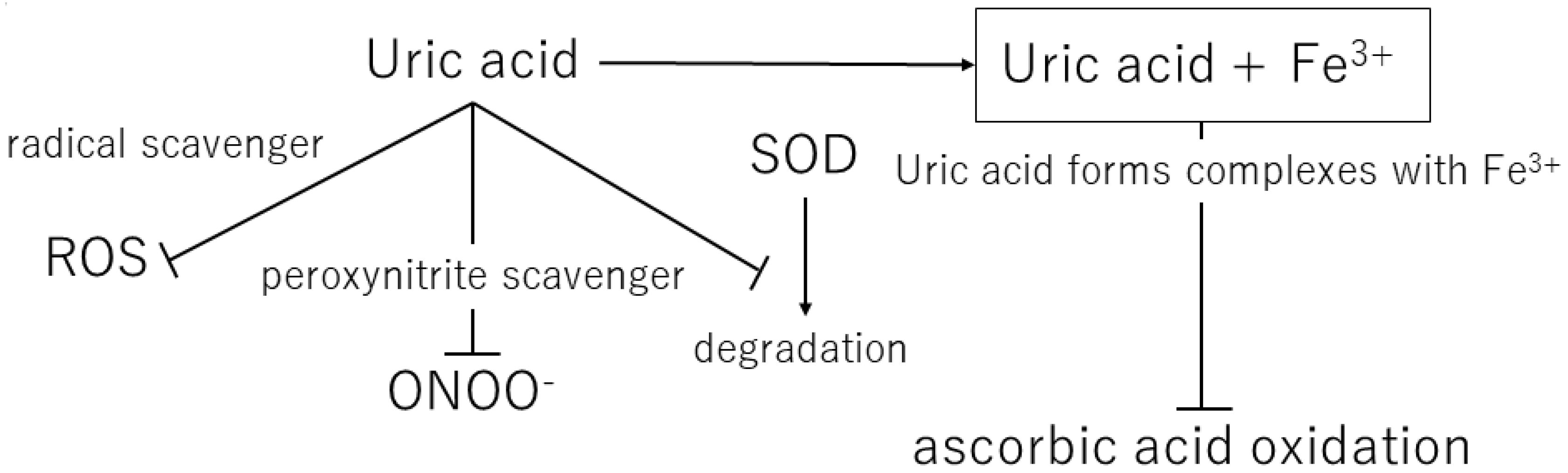
Over the course of human evolution, mutations in genes involved in ascorbic acid synthesis caused loss of function. Simultaneously, mutations in the uricase gene led to uric acid becoming the final metabolite in this pathway. Ames et al. showed that uric acid might act as an antioxidant in various redox reactions [36]. Uric and ascorbic acids are considered the most important water-soluble antioxidants [37], and the plasma level of uric acid is approximately six times that of ascorbic acid [38]. Ascorbic acid has a tendency to undergo oxidization and mutation, suggesting that the resulting mutated metabolite may contain peroxide radicals. Therefore, uric acid is considered a better antioxidant than ascorbic acid [39]. In addition, Yeum et al. measured the antioxidant capacity of the human body and suggested that uric acid accounts for 60% of the total antioxidant capacity in plasma [40]. This suggests that uric acid may provide the major endogenous defense against oxidative damage in the body [41]. Fabbrini showed that uric acid is a major antioxidant that may protect against oxidative damage caused by free radicals [42]. It is widely accepted that high levels of blood uric acid are an evolutionary advantage in humans because uric acid protects the heart, blood vessels, and nerve cells from oxidative damage. Uric acid also acts as a defense against aging and cancer by preventing oxidative damage caused by ROS [43] (Table 1) (Figure 2).

Table 1.
Mechanisms of uric acid: antioxidant effects and pro-oxidant effects.
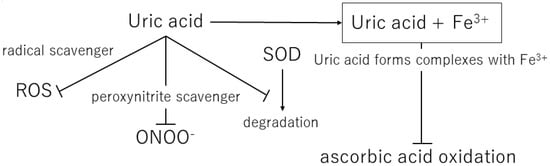
Figure 2.
Mechanisms of the antioxidant effects of extracellular uric acid. ROS, reactive oxygen species; ONOO−, peroxynitrite; SOD, superoxide dismutase.
Uric acid reacts with a variety of oxidants, among which it has been reported that it preferentially reacts with peroxynitrite to form triuret [44]. Peroxynitrite is thought to contribute to the development of cardiovascular disease and has been shown to cause oxidative damage via tissue nitration [45]. Uric acid has been suggested to act as a scavenger of peroxynitrite [46]. In addition, uric acid can help keep the levels of superoxide dismutase, an enzyme playing a key role in protecting the extracellular environment from oxidative stress. In the extracellular environment, uric acid can scavenge hydroxyl radicals and peroxynitrite, but it has been shown to lose antioxidant capacity in a hydrophobic environment [47,48]. A prospective case-control study by Nieto et al. showed an association between elevated total serum antioxidant capacity and elevated serum uric acid levels in patients with atherosclerosis and concluded that this elevated uric acid level is a compensatory mechanism to counteract the oxidative damage associated with atherosclerosis in humans [41].
It is well known that uric acid levels increase under starvation conditions [49]. First, fasting causes rapid weight loss, followed by a period of lipid utilization and a subsequent period of protein degradation. Serum uric acid levels can be markedly elevated during the proteolytic phase with weight loss, and these elevated uric acid levels are associated with increased exercise, decreased water excretion, and elevated cortisol levels [49]. Acute increases in uric acid levels induce locomotor activity, exploratory activity, and impulsivity in rats [50]. When food is scarce and sodium intake is low, uric acid may promote sodium retention and blood pressure maintenance, thus favoring survival [51]. Studies have shown that the increase in locomotor activity associated with feeding is mediated by an increase in corticosterone levels, which is associated with an increase in uric acid levels [52]. Because uric acid is chemically similar to caffeine, it has potential neurostimulatory effects, increasing human cognition, alertness, and motivation. Increased uric acid levels may be advantageous for survival [53]. In fact, we found a weak association among uric acid levels, IQ test results, and school performance [54,55].
Shi et al. reported that uric acid is an endogenous danger signal released from damaged cells. Damaged cells release uric acid, which stimulates dendritic cell maturation and promotes CD8+ T-cell responses [56]. In addition, Shi et al. identified uric acid as one of the endogenous adjuvants and revealed that the removal of uric acid reduced the production of cytotoxic T lymphocytes against antigens in the transplanted cells [57]. Thus, uric acid is involved in T-cell activation in various contexts, such as tumors, transplants, and autoimmunity.
2.1.2. The Pros and Cons of Uric Acid: The Cons
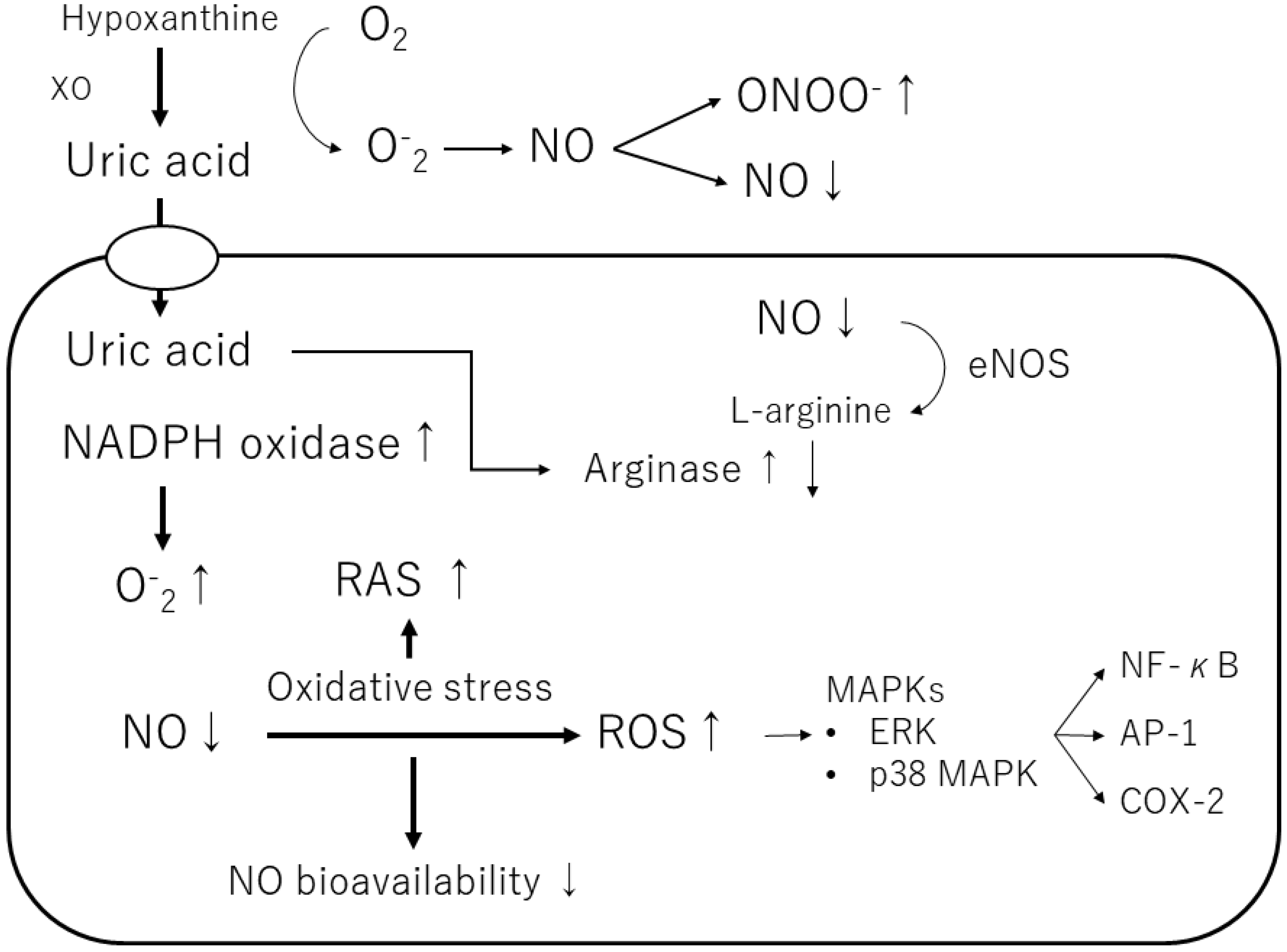
Uric acid produces allantoin and peroxynitrite via superoxide radicals to form triuret. The chemical reaction of uric acid with peroxynitrite produces aminocarbonyl and triurethanol radicals, which react with myeloperoxidase to produce the pro-oxidant uric acid hydroperoxide [58]. In contrast, uric acid in cells stimulates reduced NADPH oxidase and functions as a pro-oxidant [46] (Table 1) (Figure 3). The effects of exogenous uric acid on cells can be prevented by inhibiting the uptake of uric acid into the cells. Similarly, the biological effects of endogenous uric acid can be prevented by inhibiting its synthesis using XOR inhibitors [59]. Uric acid exhibits autocrine, paracrine, and endocrine activities. Intracellular uric acid stimulates proinflammatory transcription factors, growth factors, vasoconstrictive substances (angiotensin II, thromboxane, and endothelin), and chemokines, leading to mitochondrial dysfunction [58,59]. Uric acid also reduces endothelial NO bioavailability through various mechanisms, causing endothelial cell damage [60,61]. Thus, depending on the chemical environment, uric acid may not be an antioxidant but a pro-oxidant.
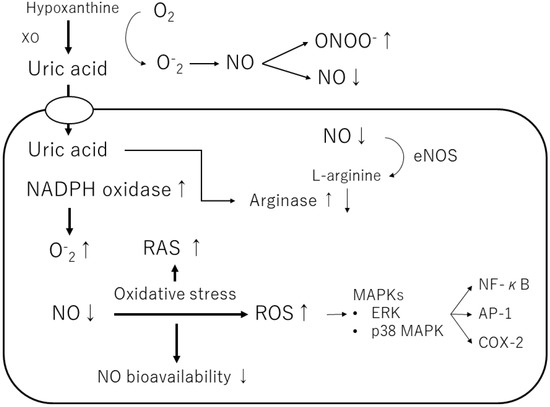
Figure 3.
Mechanisms of the pro-oxidant effect of intracellular uric acid. Intracellular uric acid induces reactive oxygen species (ROS) production and activates several signaling pathways. XO, xanthine oxidase; NO, nitric oxide; ONOO−, peroxynitrite; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; eNOS, endothelial NO synthase; ERK, extracellular signaling-regulated kinase; MAPK, mitogen-activated protein kinase; RAS, renin-angiotensin-aldosterone system; NF-κB, nuclear factor-kappa B; AP-1, activator protein 1; COX-2, cyclooxygenase 2.
Furthermore, uric acid induces the growth of vascular smooth muscle cells (VSMCs) and triggers activation of the extracellular signal-regulated kinase (ERK), mitogen-activated protein (MAP) kinases, and platelet-derived growth factor (PDGF). Uric acid also induces the inflammatory pathway in VSMCs and increases the production of monocyte chemoattractant protein-1 (MCP-1). It has also been shown to cause the activation of cyclooxygenase-2 (COX-2), p38 MAPK, nuclear transcription factor nuclear factor-kappa B (NF-κB), and activator protein-1 (AP-1) [58]. According to a previous study, soluble uric acid, as well as uric acid crystals, can cause inflammation. Corry et al. also showed that uric acid stimulates VSMC proliferation, angiotensin II production, and oxidative stress via the vascular renin-angiotensin system (RAS) in tissues [37]. They also showed that uric acid causes cardiovascular damage by stimulating vascular RAS through the MAP kinase pathway [37]. Sautin et al. reported that uric acid taken up by adipocytes increases intracellular reactive oxygen species (ROS) production in differentiated adipocytes through the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [47].
2.2. Hypertension
Numerous epidemiological studies have reported that elevated uric acid is an independent predictor of hypertension [62]. The relationship between serum uric acid levels and blood pressure was found to be dose-dependent and linear. Hyperuricemia is a strong independent predictor of hypertension with an approximately 2-fold increased risk within 5–10 years [63,64,65]. Hyperuricemia induced by uricase inhibitors in rats leads to hypertension. The mechanism by which hyperuricemia causes hypertension involves the sympathetic nervous system hyperactivity, endothelial dysfunction with reduced NO levels, and renal vasoconstriction mediated by activation of the renin-angiotensin system [66]. Rats with hyperuricemia-induced hypertension were reported to develop hypertension on a high-salt diet even after the uricase inhibitor was discontinued [51]. The mechanism behind this salt sensitivity was considered to involve uric acid entering vascular smooth muscle cells, triggering cell proliferation. In addition, hyperuricemia is thought to activate the renin-angiotensin system and produce inflammatory mediators. As a result, hyperuricemia leads to renal microangiopathy and narrowing of the arterial lumen [51]. Uric acid-lowering drugs can initially ameliorate hypertension; however, as kidney disease progresses, hypertension becomes salt-sensitive and uric acid-independent [67]. In other words, the pathogenesis of hypertension is thought to begin with the development of renal vasoconstriction, ischemia, and oxidative stress by uric acid, followed by persistent inflammation and organic changes (especially tubulointerstitial changes), resulting in increased blood pressure [68].
In a study of new-onset essential hypertension by Feig et al., 90% of subjects with essential hypertension and 30% of those with secondary hypertension had elevated serum uric acid levels (>5.5 mg/dL) compared with 0% of controls, revealing a clear association between uric acid and hypertension [69]. Kuwabara et al. also found that, for every 1 mg/dL decrease in serum uric acid in healthy male subjects, the protective effect against hypertension increased by 18%. In healthy female subjects, each 1 mg/dL decrease in serum uric acid levels was associated with a 31% decrease in the development of hypertension [70]. Elsewhere, the effect of hypouricemic therapy on blood pressure was observed in a randomized, double-blind, placebo-controlled trial. Sixty prehypertensive obese adolescents received allopurinol, probenecid, or a placebo, respectively. After 2 months, systolic blood pressure had increased from baseline by +1.9 mmHg (95% CI: −0.4 to 2.4 mmHg) in the placebo group, while it had decreased by −9.2 mmHg (−6.7 to −11.3 mmHg) in the allopurinol group and by −8.9 mmHg (−7.0 to −10.9 mmHg) in the probenecid group [71]. In addition, in a randomized, double-blind, placebo-controlled crossover trial conducted by Feig et al., allopurinol normalized blood pressure in 19 of 22 subjects who achieved a serum uric acid level of <5.0 mg/dL, whereas only 1 of 30 subjects achieved normal blood pressure with placebo treatment. This indicates that lowering uric acid levels with allopurinol lowers blood pressure in patients with hyperuricemia [72]. In this study, patients with hyperuricemia and hypertension tended to have elevated plasma renin activity, and xanthine oxidase inhibitors reduced plasma renin activity and plasma aldosterone [72,73].
Meanwhile, the PIUMA study showed a J-shaped relationship between uric acid levels and the incidence of cardiovascular disease events in both men and women [74]. The association between serum uric acid levels and cardiovascular events was also demonstrated in the Syst-Eur study in elderly subjects with systolic hypertension, in which a J-shaped curve was observed between serum uric acid levels and all-cause mortality [75]. Furthermore, in a cardiovascular study in the elderly (CASTEL), serum uric acid levels independently predicted coronary mortality in a J-shaped manner in the elderly with diabetes [76]. Kawasoe et al. used large-scale health checkup data to examine the association between serum uric acid levels and the prevalence of blood pressure abnormalities, considering the effects of other risk factors. The prevalence of hypertension was lowest in the 2.1–4.0 mg/dL serum uric acid group (second-lowest serum uric acid levels). However, the incidence of hypertension was significantly higher in the 4.1–5.0, 5.1–6.0, and ≥6.1 mg/dL serum uric acid groups, as well as the ≤2.0 mg/dL serum uric acid group (lowest serum uric acid levels). High and low serum uric acid levels were significantly associated with an increased prevalence of hypertension, creating a J-shaped curve [77]. Uric acid has antioxidant effects and contributes significantly to ROS removal. One of the etiologies of hypertension is oxidative stress, which results in the excessive generation of ROS. Elevated ROS levels contribute to vascular dysfunction and remodeling because of oxidative damage [78]. However, there is no clear evidence that oxidative stress alone contributes to the pathogenesis of hypertension; in addition to increased oxidative stress due to hypouricemia, it is probably associated with elevated blood pressure, including other factors of hypertension (salt, the renin-angiotensin system, and symptomatic hyperactivity) [79]. Increased oxidative stress due to decreased uric acid levels may inactivate the nitric oxide released by the endothelium and inhibit vasorelaxation. Therefore, the relationship between blood pressure and serum uric acid levels might have developed a J-shaped curve [77].
2.3. Diabetes Mellitus and Metabolic Syndrome
Hyperuricemia is closely associated with metabolic abnormalities, such as obesity, dyslipidemia, insulin resistance, type 2 diabetes, and metabolic syndrome [80]. Elevated serum uric acid levels are surrogate markers of metabolic syndrome [81]. Japanese researchers surveyed a group of healthy young males for 5 years and revealed that those with high serum uric acid levels gained four times as much weight as those with low levels [6]. Therefore, elevated serum uric acid levels may trigger obesity and metabolic syndrome. Furthermore, increased serum uric acid concentration has been shown to be associated with an increased risk of insulin resistance [82].
Fructose intake induces fatty liver, elevated triglycerides, and elevated serum uric acid [83,84]. Once inside the cell, fructose is rapidly phosphorylated by fructokinase. During fructose metabolism, cellular energy (ATP) is reduced; however, ATP breakdown products (such as AMP) enter the nucleotide degradation pathway to produce uric acid. Serum uric acid levels increase within minutes of fructose ingestion and are often associated with lactate release [85]. Fructose stimulates appetite and induces insulin resistance to raise glucose levels in the body, thereby providing glucose to the brain. Similarly, it stimulates water intake and increases blood pressure to reduce sodium loss [86].
Choi et al. reported a large prospective cohort study of male patients at high cardiovascular risk. The results showed that men with gout had a high risk of developing type 2 diabetes in the future, independent of other risk factors [87]. The Atherosclerosis Risk in Communities Study in the United States, which followed nondiabetic patients with hyperinsulinemia for 11 years, found that hyperuricemia was an independent risk factor for progression to hyperinsulinemia [88].
Another study suggested that the kidney may be involved in the association between hyperuricemia and the risk of type 2 diabetes mellitus [89]. Renal tubular function is affected by hyperinsulinemia, and decreased insulin-mediated glucose metabolism results in decreased uric acid clearance. Thus, reduced uric acid excretion causes hyperuricemia [89,90]. Insulin may promote renal uric acid reabsorption via URAT1 or Na+-dependent anion co-transporter in the proximal renal tubules [91]. In addition, insulin is responsible for inducing hyperuricemia in the renal tubules [92]. Conversely, a decrease in serum uric acid levels has been observed, and there is a bell-shaped relationship between uric acid levels and hemoglobin A1c. This is probably due to the fact that the rate of renal uric acid excretion depends on the level of glycemic control [93]. In addition, Lytvyn et al. reported that glycosuria was induced by the inhibition of sodium/glucose co-transporter 2 (SGLT2) in patients with type 1 diabetes, resulting in a decrease in blood uric acid levels and an increase in the rate of uric acid excretion [94]. Patients whose uric acid levels increase as glycemic control improves are often encountered. This is thought to be due to decreased reabsorption of uric acid in the proximal tubules as insulin levels decrease. Meanwhile, urinary excretion of uric acid has been shown to increase during hyperglycemia, and the mechanism behind this is assumed to involve osmotic diuresis [14]. SGLT2 inhibitors lower blood uric acid levels by increasing urinary glucose and uric acid excretion co-transported with glucose [95,96]. In addition, patients with familial renal diabetes and SGLT2 mutations tend to have lower serum uric acid levels [33]. Chino et al. reported that the SGLT2 inhibitor luseogliflozin administered to healthy subjects caused a decrease in serum uric acid levels and an increase in the excretion rate of urinary uric acid. However, no inhibition of uptake of the major renal tubular uric acid reabsorption transporter was observed [97]. Thus, whether uric acid excretion is due to osmotic pressure or to transporters such as SGLT2 remains unknown.
2.4. Neurological Disease and Mental Illness
As hyperuricemia is associated with the development of various cardiovascular diseases, it is possible that hyperuricemia and cardiovascular diseases are involved in stroke-related deaths. The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, conducted in 2020, was a case-cohort study performed using a large dataset. The study concluded that hyperuricemia might be a risk factor for stroke [98]. Furthermore, several epidemiological studies have shown a J-shaped association between serum uric acid levels and stroke events [74,99]. A longitudinal national cohort study by Kamei et al. reported that elevated serum uric acid levels of 7.1 mg/dL or higher in men and 5.5 mg/dL or higher in women were independently associated with the incidence of nonfatal stroke. Similarly, in the present study, even the group with low uric acid levels had an increased risk of stroke, showing a J-shaped curve [99].
Reactive oxygen plays a major role in the development of neurodegenerative disease because a large number of reactive oxygen species are produced in the brain. Despite accounting for only 2% of the body’s weight, the brain consumes 20% of the body’s oxygen supply, and most of the oxygen is converted to ROS [100]. The concentration of uric acid in the cerebrospinal fluid is always low, approximately ten times that in the peripheral blood. This concentration in the CSF depends on the blood uric acid level and the integrity of the blood-CSF barrier [37].
Hyperuricemia has been shown to have neuroprotective effects in neurodegenerative diseases, such as Parkinson’s disease and multiple sclerosis, and in dementia, such as Alzheimer’s disease. Elevated blood uric acid levels have been shown to be associated with a reduced risk of developing Parkinson’s disease [101]. Patients with multiple sclerosis and neuromyelitis optica spectrum disorders have lower serum uric acid levels than healthy controls, indicating that serum uric acid levels are a potential diagnostic biomarker [102]. It was also shown that the prevalence of amyotrophic lateral sclerosis in patients with gout was significantly lower than in patients without gout [103]. We also found a reduced risk of dementia in patients with high serum uric acid levels in dementia [104]. There is also an inverse relationship between gout and the risk of developing Alzheimer’s disease, supporting the possible neuroprotective role of uric acid [105].
Patients with Alzheimer’s disease- and Parkinson’s disease-related dementia showed a strong association with high serum uric acid levels; however, no correlation was found in patients with vascular or mixed dementia, suggesting a different relationship with serum uric acid levels, especially in patients with vascular dementia [106]. This suggests that although the antioxidant effect of uric acid prevents nerve degeneration and the development of dementia, both effects need to be considered because hyperuricemia promotes atherosclerosis and adversely affects cognitive function.
Serum uric acid levels are elevated in subjects with anger management issues, while those who exhibit aggressive behavior, including criminal behavior, have higher serum uric acid levels [107]. High urinary uric acid levels have also been associated with aggressive behavior [108]. Moreover, those with Lesch–Nyhan disease, an inherited form of hyperuricemia, show impulsivity, aggression, and a risk of self-harm [109]. It was reported that uric acid-lowering therapy with allopurinol effectively reduces impulsive or aggressive behavior and self-harm in some patients [110]. Conversely, some have suggested that abnormal function of the dopamine pathway in the basal ganglia is a cause of self-injurious behavior, not uric acid [111]. In addition, patients with bipolar mania often have elevated serum uric acid due to dysfunction of the purinergic system, with a decrease in adenosine neurotransmission [112]. Conversely, depression is associated with a decrease in serum uric acid levels, which may represent a marker of the condition [113]. Although there are reports of patients with primary hypouricemia, such as type 1 xanthinuria, an XOR deficiency [114], and renal hypouricemia, a disorder of URAT1 and GLUT9 [115,116], no reports have been published of neurological symptoms in these patients. This suggests that uric acid is not cerebroprotective and may support the value of serum uric acid levels as a diagnostic biomarker in cranial nerve disease.
2.5. Chronic Kidney Disease (CKD)
Elevated serum uric acid levels are widely accepted to predict the development of CKD [117], but whether they are a risk factor or a marker of CKD is controversial. Messerli et al. reported that renal blood flow decreased, and renal vascular resistance and total peripheral resistance increased in patients with hyperuricemia [118]. Elevated serum uric acid is associated with renal lesions such as glomerulosclerosis and interstitial fibrosis because of the deposition of uric acid crystals, suggesting that hyperuricemia in hypertensive patients may influence nephrosclerosis [118]. Serum uric acid was also suggested to be involved in CKD in a large cohort study [119]. In addition, a prospective observational cohort of normotensive healthy individuals with normal renal function showed an association between elevated serum uric acid levels and renal impairment [120]. A meta-analysis by Luo et al. also reported that elevated serum uric acid levels were associated with an increased risk of cardiovascular mortality in patients with CKD [121]. Moreover, Kuwabara et al. reported that, among healthy subjects, each 1 mg/dL decrease in serum uric acid in men was associated with a 23% increase in the protective effect against CKD, and each 1 mg/dL decrease in serum uric acid in women was associated with a 42% increase in the prevention of CKD [70].
Meanwhile, a 10-year follow-up study of routine health check-up participants by Mori et al. showed a U-shaped relationship between uric acid levels and the risk of CKD in women [122]. In that study, the risk of CKD in men increased with higher levels of uric acid but not with lower levels. Therefore, only in women was a low uric acid level a significant risk factor for CKD. A large cross-sectional study by Kuwabara et al. showed that patients with hypouricemia had a 9-fold higher incidence of kidney disease than patients with no hypouricemia [70]. That study also examined the cumulative incidence of cardiometabolic disease over a 5-year period in patients with hypouricemia and normouricemia (3–5 mg/dL in men and 2–4 mg/dL in women) and indicated that hypouricemia was a risk factor for the development of hypertension and CKD only in women [70]. Renal hypouricemia is a disease associated with the increased excretion of uric acid in the kidneys. Patients with renal hypouricemia can develop urolithiasis and exercise-induced acute kidney injury (EIAKI) [123]. EIAKI is considered a transient acute kidney injury that does not cause severe kidney dysfunction over an extended period.
Whether interventions with uric acid-lowering drugs would reduce renal dysfunction is unclear. In recent years, two clinical studies on patients with asymptomatic hyperuricemia without gout have been reported in Japan [124,125]. In the FEATHER Study, febuxostat, an XOR inhibitor, did not improve renal function compared with placebo in patients with CKD and hyperuricemia; however, subgroup analyses showed that it significantly reduced renal function decline in patients without proteinuria and in patients with serum creatinine levels below the median [124]. The FREED Study demonstrated that febuxostat significantly reduced brain, cardiovascular, and renal events compared with conventional therapy with lifestyle modifications in patients with asymptomatic hyperuricemia aged 65 years and older [125].
2.6. Cardiovascular Disease
Longitudinal data from the NHANES showed that all-cause mortality and cardiovascular mortality were associated with serum uric acid levels in both men and women at 10 years of follow-up [126]. Serum uric acid levels may be associated with cardiovascular disease and prognosis, but their role remains controversial. The Framingham Heart Study showed that serum uric acid levels were not causally related to the development of coronary heart disease, death from cardiovascular disease, or death from all causes [127]. Some studies have shown that serum uric acid levels are a strong predictor of mortality from cardiovascular diseases [128,129,130]. Furthermore, it has been reported that, in humans, treatment with high doses of allopurinol improves endothelial cell function not by lowering plasma uric acid levels but by lowering oxidative stress in the blood vessels [131].
Meanwhile, there is a J-shaped relationship between serum uric acid and cardiovascular disease, and attention has recently been paid to the idea that both low and high uric acid levels are associated with a greater risk of developing cardiovascular events [132]. Vascular endothelial dysfunction due to decreased antioxidant activity associated with uric acid levels has been suggested as a factor contributing to an increased risk of cardiovascular events caused by hypouricemia [133]. The PIUMA study showed that serum uric acid levels lower than 4.5 mg/dL in men and 3.2 mg/dL in women with essential hypertension increased the incidence of cardiovascular disease and its-related deaths [74]. That is, it was suggested that there is an inverse relationship between serum uric acid levels and the incidence of cardiovascular disease among subjects with serum uric acid levels below 4.5 mg/dL in men and 3.2 mg/dL in women [74,75,76].
2.7. COVID-19
Some previous studies showed associations between high [134,135] and low uric acid levels [136,137,138] with COVID-19 severity. For example, Fukushima et al. investigated the relationship between uric acid levels and COVID-19 severity in a Japanese cohort and reported that uric acid levels of 7.6 mg/dL or higher and uric acid levels of 2.5 mg or lower were associated with COVID-19 severity. This indicated a U-shaped relationship between both low and high serum uric acid levels and COVID-19 severity [139].
3. Conclusions
In addition to causing gout, hyperuricemia has been implicated in various cardiovascular and renal diseases, such as hypertension, arteriosclerosis, kidney disease, and cardiovascular disease. In the 1900s, it was believed that uric acid was not a true risk factor for developing cardiovascular or renal diseases [140]; consequently, uric acid levels were not assessed during routine blood tests. Additionally, routine assessment of uric acid levels was not recommended for patients with cardiovascular or kidney disease, and this level was eliminated as a risk factor from the lists provided by most cardiovascular and kidney societies. Therefore, uric acid was ultimately disregarded as a risk factor for kidney diseases [4]. However, in the late 1990s, uric acid was re-examined as a risk factor for cardiovascular and renal diseases, and in recent years, there has been growing interest in investigating the role of uric acid owing to the increased prevalence of hyperuricemia worldwide. The dual properties of uric acid create confusion regarding its effects on various diseases. Soluble uric acid is not an inert substance and has been found to possess a number of biological properties [141]. It has become clear that uric acid has two contradictory properties: on the one hand, it acts as an antioxidant, while on the other hand, it acts as an oxidant. Thus, although it was previously thought that high uric acid levels would have negative effects on hypertension and cardiovascular diseases, it is also important to consider the effects of low uric acid levels. Although many clinical trials have been conducted on uric acid, the causal relationship between uric acid and hypertension or cardiovascular disease remains unclear, probably because of its biological properties. Therefore, if we propose the concept of “dysuricemia,” which encompasses both hyperuricemia and hypouricemia and consider the involvement of this condition in various diseases, we believe that further research on uric acid will be enhanced. In conclusion, we suggest that future clinical studies focusing not only on high uric acid levels but also on low uric acid levels are needed.
Author Contributions
Writing-original draft preparation, N.O.; writing-review and editing, M.O., E.M., A.M., T.F., N.A. and I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sector.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, M.R.; Moe, O.W. Uric Acid Nephrolithiasis: A Systemic Metabolic Disorder. Clin. Rev. Bone Miner. Metab. 2011, 9, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Garrod, A.B. Observations on certain pathological conditions of the blood and urine, in gout, rheumatism, and Bright’s disease. Med. Chir. Trans. 1848, 31, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.H. Requiem for gouty nephropathy. Kidney Int. 1986, 30, 280–287. [Google Scholar] [CrossRef]
- Duffy, W.B.; Senekjian, H.O.; Knight, T.F.; Weinman, E.J. Management of asymptomatic hyperuricemia. JAMA 1981, 246, 2215–2216. [Google Scholar] [CrossRef]
- Masuo, K.; Kawaguchi, H.; Mikami, H.; Ogihara, T.; Tuck, M.L. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003, 42, 474–480. [Google Scholar] [CrossRef]
- Alper, A.B.J.; Chen, W.; Yau, L.; Srinivasan, S.R.; Berenson, G.S.; Hamm, L.L. Childhood uric acid predicts adult blood pressure: The Bogalusa Heart Study. Hypertension 2005, 45, 34–38. [Google Scholar] [CrossRef]
- Nakanishi, N.; Okamoto, M.; Yoshida, H.; Matsuo, Y.; Suzuki, K.; Tatara, K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur. J. Epidemiol. 2003, 18, 523–530. [Google Scholar] [CrossRef]
- Choi, H.K.; Ford, E.S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am. J. Med. 2007, 120, 442–447. [Google Scholar] [CrossRef]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M.B. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, X.; Jiang, L.; Mao, S.; Yin, X.; Guo, L. Hyperuricemia and coronary heart disease mortality: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2016, 16, 207. [Google Scholar] [CrossRef]
- Roddy, E.; Zhang, W.; Doherty, M. Is gout associated with reduced quality of life? A case-control study. Rheumatology 2007, 46, 1441–1444. [Google Scholar] [CrossRef]
- Tatlock, S.; Rüdell, K.; Panter, C.; Arbuckle, R.; Harrold, L.R.; Taylor, W.J.; Symonds, T. What Outcomes are Important for Gout Patients? In-Depth Qualitative Research into the Gout Patient Experience to Determine Optimal Endpoints for Evaluating Therapeutic Interventions. Patient 2017, 10, 65–79. [Google Scholar] [CrossRef]
- Otani, N.; Ouchi, M.; Misawa, K.; Hisatome, I.; Anzai, N. Hypouricemia and Urate Transporters. Biomedicines 2022, 10, 652. [Google Scholar] [CrossRef]
- Wakasugi, M.; Kazama, J.J.; Narita, I.; Konta, T.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Asahi, K.; et al. Association between hypouricemia and reduced kidney function: A cross-sectional population-based study in Japan. Am. J. Nephrol. 2015, 41, 138–146. [Google Scholar] [CrossRef]
- Gresser, U.; Gathof, B.; Zöllner, N. Uric acid levels in southern Germany in 1989. A comparison with studies from 1962, 1971, and 1984. Klin. Wochenschr. 1990, 68, 1222–1228. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Coiffier, B.; Altman, A.; Pui, C.; Younes, A.; Cairo, M.S. Guidelines for the management of pediatric and adult tumor lysis syndrome: An evidence-based review. J. Clin. Oncol. 2008, 26, 2767–2778. [Google Scholar] [CrossRef]
- Stewart, D.J.; Langlois, V.; Noone, D. Hyperuricemia and Hypertension: Links and Risks. Integr. Blood Press. Control 2019, 12, 43–62. [Google Scholar] [CrossRef]
- Fathallah-Shaykh, S.A.; Cramer, M.T. Uric acid and the kidney. Pediatr. Nephrol. 2014, 29, 999–1008. [Google Scholar] [CrossRef]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Ouchi, M.; Kudo, H.; Tsuruoka, S.; Hisatome, I.; Anzai, N. Recent approaches to gout drug discovery: An update. Expert Opin. Drug Discov. 2020, 15, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef]
- Nakayama, A.; Matsuo, H.; Nakaoka, H.; Nakamura, T.; Nakashima, H.; Takada, Y.; Oikawa, Y.; Takada, T.; Sakiyama, M.; Shimizu, S.; et al. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci. Rep. 2014, 4, 5227. [Google Scholar] [CrossRef]
- Stiburkova, B.; Pavelcova, K.; Pavlikova, M.; Ješina, P.; Pavelka, K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Res. Ther. 2019, 21, 77. [Google Scholar] [CrossRef]
- Nakayama, A.; Nakatochi, M.; Kawamura, Y.; Yamamoto, K.; Nakaoka, H.; Shimizu, S.; Higashino, T.; Koyama, T.; Hishida, A.; Kuriki, K.; et al. Subtype-specific gout susceptibility loci and enrichment of selection pressure on ABCG2 and ALDH2 identified by subtype genome-wide meta-analyses of clinically defined gout patients. Ann. Rheum. Dis. 2020, 79, 657–665. [Google Scholar] [CrossRef]
- Jutabha, P.; Anzai, N.; Kitamura, K.; Taniguchi, A.; Kaneko, S.; Yan, K.; Yamada, H.; Shimada, H.; Kimura, T.; Katada, T.; et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J. Biol. Chem. 2010, 285, 35123–35132. [Google Scholar] [CrossRef]
- Otani, N.; Ouchi, M.; Hayashi, K.; Jutabha, P.; Anzai, N. Roles of organic anion transporters (OATs) in renal proximal tubules and their localization. Anat. Sci. Int. 2017, 92, 200–206. [Google Scholar] [CrossRef]
- Iwanaga, T.; Sato, M.; Maeda, T.; Ogihara, T.; Tamai, I. Concentration-dependent mode of interaction of angiotensin II receptor blockers with uric acid transporter. J. Pharmacol. Exp. Ther. 2007, 320, 211–217. [Google Scholar] [CrossRef]
- Hori, T.; Ouchi, M.; Otani, N.; Nohara, M.; Morita, A.; Otsuka, Y.; Jutabha, P.; Shibasaki, I.; Matsushita, Y.; Fujita, T.; et al. The uricosuric effects of dihydropyridine calcium channel blockers in vivo using urate under-excretion animal models. J. Pharmacol. Sci. 2018, 136, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Bahn, A.; Hagos, Y.; Reuter, S.; Balen, D.; Brzica, H.; Krick, W.; Burckhardt, B.C.; Sabolic, I.; Burckhardt, G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J. Biol. Chem. 2008, 283, 16332–16341. [Google Scholar] [CrossRef]
- Yu, L.; Lv, J.; Zhou, X.; Zhu, L.; Hou, P.; Zhang, H. Abnormal expression and dysfunction of novel SGLT2 mutations identified in familial renal glucosuria patients. Hum. Genet. 2011, 129, 335–344. [Google Scholar] [CrossRef]
- Mogensen, U.M.; Køber, L.; Jhund, P.S.; Desai, A.S.; Senni, M.; Kristensen, S.L.; Dukát, A.; Chen, C.; Ramires, F.; Lefkowitz, M.P.; et al. Sacubitril/valsartan reduces serum uric acid concentration, an independent predictor of adverse outcomes in PARADIGM-HF. Eur. J. Heart Fail. 2018, 20, 514–522. [Google Scholar] [CrossRef]
- Hayashi, S.; Fujiwara, S.; Noguchi, T. Evolution of urate-degrading enzymes in animal peroxisomes. Cell Biochem. Biophys. 2000, 32, 123–129. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Crotty, G.F.; Ascherio, A.; Schwarzschild, M.A. Targeting urate to reduce oxidative stress in Parkinson disease. Exp. Neurol. 2017, 298, 210–224. [Google Scholar] [CrossRef]
- Gersch, C.; Palii, S.P.; Imaram, W.; Kim, K.M.; Karumanchi, S.A.; Angerhofer, A.; Johnson, R.J.; Henderson, G.N. Reactions of peroxynitrite with uric acid: Formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress. Nucleosides Nucleotides Nucleic Acids 2009, 28, 118–149. [Google Scholar] [CrossRef] [PubMed]
- Rosin, M.P.; San, R.H.; Stich, H.F. Mutagenic activity of ascorbate in mammalian cell cultures. Cancer Lett. 1980, 8, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.; Russell, R.M.; Krinsky, N.I.; Aldini, G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch. Biochem. Biophys. 2004, 430, 97–103. [Google Scholar] [CrossRef]
- Nieto, F.J.; Iribarren, C.; Gross, M.D.; Comstock, G.W.; Cutler, R.G. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis? Atherosclerosis 2000, 148, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Robinson, K.M.; Morré, J.T.; Beckman, J.S. Triuret: A novel product of peroxynitrite-mediated oxidation of urate. Arch. Biochem. Biophys. 2004, 423, 213–217. [Google Scholar] [CrossRef]
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: Implications for uncoupling endothelial nitric oxide synthase. Biochem. Pharmacol. 2005, 70, 343–354. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2007, 293, 584–596. [Google Scholar] [CrossRef]
- Muraoka, S.; Miura, T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol. Toxicol. 2003, 93, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Stenvinkel, P.; Jensen, T.; Lanaspa, M.A.; Roncal, C.; Song, Z.; Bankir, L.; Sánchez-Lozada, L.G. Metabolic and Kidney Diseases in the Setting of Climate Change, Water Shortage, and Survival Factors. J. Am. Soc. Nephrol. 2016, 27, 2247–2256. [Google Scholar] [CrossRef]
- Sutin, A.R.; Cutler, R.G.; Camandola, S.; Uda, M.; Feldman, N.H.; Cucca, F.; Zonderman, A.B.; Mattson, M.P.; Ferrucci, L.; Schlessinger, D.; et al. Impulsivity is associated with uric acid: Evidence from humans and mice. Biol. Psychiatry 2014, 75, 31–37. [Google Scholar] [CrossRef]
- Watanabe, S.; Kang, D.; Feng, L.; Nakagawa, T.; Kanellis, J.; Lan, H.; Mazzali, M.; Johnson, R.J. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 2002, 40, 355–360. [Google Scholar] [CrossRef]
- Challet, E.; le Maho, Y.; Robin, J.P.; Malan, A.; Cherel, Y. Involvement of corticosterone in the fasting-induced rise in protein utilization and locomotor activity. Pharmacol. Biochem. Behav. 1995, 50, 405–412. [Google Scholar] [CrossRef]
- Orowan, E. The origin of man. Nature 1955, 175, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Stetten, D.J.; Hearon, J.Z. Intellectual level measured by army classification battery and serum uric acid concentration. Science 1959, 129, 1737. [Google Scholar] [CrossRef]
- Bloch, S.; Brackenridge, C.J. Psychological, performance and biochemical factors in medical students under examination stress. J. Psychosom. Res. 1972, 16, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Evans, J.E.; Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003, 425, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Galusha, S.A.; Rock, K.L. Cutting edge: Elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J. Immunol. 2006, 176, 3905–3908. [Google Scholar] [CrossRef]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez Lozada, L.G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Zharikov, S.; Krotova, K.; Hu, H.; Baylis, C.; Johnson, R.J.; Block, E.R.; Patel, J. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am. J. Physiol. Cell Physiol. 2008, 295, 1183–1190. [Google Scholar] [CrossRef]
- Gersch, C.; Palii, S.P.; Kim, K.M.; Angerhofer, A.; Johnson, R.J.; Henderson, G.N. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 2008, 27, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Feig, D.I.; Herrera-Acosta, J.; Kang, D. Resurrection of uric acid as a causal risk factor in essential hypertension. Hypertension 2005, 45, 18–20. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Kelley, E.E.; Nakagawa, T.; Madero, M.; Feig, D.I.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric Acid and Hypertension: An Update With Recommendations. Am. J. Hypertens. 2020, 33, 583–594. [Google Scholar] [CrossRef]
- Feig, D.I.; Madero, M.; Jalal, D.I.; Sanchez-Lozada, L.G.; Johnson, R.J. Uric acid and the origins of hypertension. J. Pediatr. 2013, 162, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Niwa, K.; Hisatome, I.; Nakagawa, T.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Bjornstad, P.; Jensen, T.; Sato, Y.; Milagres, T.; et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension 2017, 69, 1036–1044. [Google Scholar] [CrossRef]
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef]
- Johnson, R.J.; Rodriguez-Iturbe, B.; Kang, D.; Feig, D.I.; Herrera-Acosta, J. A unifying pathway for essential hypertension. Am. J. Hypertens. 2005, 18, 431–440. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef]
- Feig, D.I.; Johnson, R.J. Hyperuricemia in childhood primary hypertension. Hypertension 2003, 42, 247–252. [Google Scholar] [CrossRef]
- Kuwabara, M.; Hisatome, I.; Roncal-Jimenez, C.A.; Niwa, K.; Andres-Hernando, A.; Jensen, T.; Bjornstad, P.; Milagres, T.; Cicerchi, C.; Song, Z.; et al. Increased Serum Sodium and Serum Osmolarity Are Independent Risk Factors for Developing Chronic Kidney Disease; 5 Year Cohort Study. PLoS ONE 2017, 12, e0169137. [Google Scholar] [CrossRef] [PubMed]
- Soletsky, B.; Feig, D.I. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 2012, 60, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Soletsky, B.; Johnson, R.J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA 2008, 300, 924–932. [Google Scholar] [CrossRef]
- Tani, S.; Nagao, K.; Hirayama, A. Effect of Febuxostat, a Xanthine Oxidase Inhibitor, on Cardiovascular Risk in Hyperuricemic Patients with Hypertension: A Prospective, Open-label, Pilot Study. Clin. Drug Investig. 2015, 35, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Schillaci, G.; Reboldi, G.; Santeusanio, F.; Porcellati, C.; Brunetti, P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000, 36, 1072–1078. [Google Scholar] [CrossRef]
- De Leeuw, P.W.; Thijs, L.; Birkenhäger, W.H.; Voyaki, S.M.; Efstratopoulos, A.D.; Fagard, R.H.; Leonetti, G.; Nachev, C.; Petrie, J.C.; Rodicio, J.L.; et al. Prognostic significance of renal function in elderly patients with isolated systolic hypertension: Results from the Syst-Eur trial. J. Am. Soc. Nephrol. 2002, 13, 2213–2222. [Google Scholar] [CrossRef]
- Mazza, A.; Zamboni, S.; Rizzato, E.; Pessina, A.C.; Tikhonoff, V.; Schiavon, L.; Casiglia, E. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The CArdiovascular STudy in the ELderly (CASTEL). Acta Diabetol. 2007, 44, 99–105. [Google Scholar] [CrossRef]
- Kawasoe, S.; Kubozono, T.; Ojima, S.; Kawabata, T.; Miyahara, H.; Tokushige, K.; Ohishi, M. J-shaped curve for the association between serum uric acid levels and the prevalence of blood pressure abnormalities. Hypertens. Res. 2021, 44, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann. Med. 2012, 44 (Suppl. S1), 2–16. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. The concept of metabolic syndrome: Contribution of visceral fat accumulation and its molecular mechanism. J. Atheroscler. Thromb. 2011, 18, 629–639. [Google Scholar] [CrossRef]
- Furuhashi, M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 2020, 319, 827–834. [Google Scholar] [CrossRef]
- Hu, X.; Rong, S.; Wang, Q.; Sun, T.; Bao, W.; Chen, L.; Liu, L. Association between plasma uric acid and insulin resistance in type 2 diabetes: A Mendelian randomization analysis. Diabetes Res. Clin. Pract. 2021, 171, 108542. [Google Scholar] [CrossRef]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sautin, Y.Y.; Oliver, W.J.; Roncal, C.; Mu, W.; Gabriela Sanchez-Lozada, L.; Rodriguez-Iturbe, B.; Nakagawa, T.; Benner, S.A. Lessons from comparative physiology: Could uric acid represent a physiologic alarm signal gone awry in western society? J. Comp. Physiol. B 2009, 179, 67–76. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Choi, H.K.; De Vera, M.A.; Krishnan, E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology 2008, 47, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Fortmann, S.P.; Palaniappan, L.; Duncan, B.B.; Schmidt, M.I.; Chambless, L.E. Risk factors for progression to incident hyperinsulinemia: The Atherosclerosis Risk in Communities Study, 1987–1998. Am. J. Epidemiol. 2003, 158, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, L.; Laaksonen, D.E.; Lindström, J.; Eriksson, J.G.; Keinänen-Kiukaanniemi, S.; Ilanne-Parikka, P.; Aunola, S.; Hämäläinen, H.; Tuomilehto, J.; Uusitupa, M. Serum uric acid as a harbinger of metabolic outcome in subjects with impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care 2006, 29, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.; Chen, Y.D.; Hollenbeck, C.B.; Reaven, G.M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991, 266, 3008–3011. [Google Scholar] [CrossRef]
- Choi, H.K.; Mount, D.B.; Reginato, A.M.; American College of Physicians; American Physiological Society. Pathogenesis of gout. Ann. Intern. Med. 2005, 143, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Quiñones Galvan, A.; Natali, A.; Baldi, S.; Frascerra, S.; Sanna, G.; Ciociaro, D.; Ferrannini, E. Effect of insulin on uric acid excretion in humans. Am. J. Physiol. 1995, 268, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Ford, E.S. Haemoglobin A1c, fasting glucose, serum C-peptide and insulin resistance in relation to serum uric acid levels—The Third National Health and Nutrition Examination Survey. Rheumatology 2008, 47, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Škrtić, M.; Yang, G.K.; Yip, P.M.; Perkins, B.A.; Cherney, D.Z.I. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am. J. Physiol. Renal Physiol. 2015, 308, 77–88. [Google Scholar] [CrossRef]
- Ouchi, M.; Oba, K.; Kaku, K.; Suganami, H.; Yoshida, A.; Fukunaka, Y.; Jutabha, P.; Morita, A.; Otani, N.; Hayashi, K.; et al. Uric acid lowering in relation to HbA1c reductions with the SGLT2 inhibitor tofogliflozin. Diabetes Obes. Metab. 2018, 20, 1061–1065. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Tian, D.; Xia, P.; Zheng, H.; Wang, L.; Chen, L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2018, 20, 458–462. [Google Scholar] [CrossRef]
- Chino, Y.; Samukawa, Y.; Sakai, S.; Nakai, Y.; Yamaguchi, J.; Nakanishi, T.; Tamai, I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm. Drug Dispos. 2014, 35, 391–404. [Google Scholar] [CrossRef]
- Chaudhary, N.S.; Bridges, S.L.J.; Saag, K.G.; Rahn, E.J.; Curtis, J.R.; Gaffo, A.; Limdi, N.A.; Levitan, E.B.; Singh, J.A.; Colantonio, L.D.; et al. Severity of Hypertension Mediates the Association of Hyperuricemia With Stroke in the REGARDS Case Cohort Study. Hypertension 2020, 75, 246–256. [Google Scholar] [CrossRef]
- Kamei, K.; Konta, T.; Hirayama, A.; Ichikawa, K.; Kubota, I.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; et al. Associations between serum uric acid levels and the incidence of nonfatal stroke: A nationwide community-based cohort study. Clin. Exp. Nephrol. 2017, 21, 497–503. [Google Scholar] [CrossRef]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J.J. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 2012, 4, 1399–1440. [Google Scholar] [CrossRef]
- Weisskopf, M.G.; O’Reilly, E.; Chen, H.; Schwarzschild, M.A.; Ascherio, A. Plasma urate and risk of Parkinson’s disease. Am. J. Epidemiol. 2007, 166, 561–567. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Wang, J.; Qian, W.; Xiao, H. Low serum uric acid levels in patients with multiple sclerosis and neuromyelitis optica: An updated meta-analysis. Mult. Scler. Relat. Disord. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Lee, S.; Han, K.; Kwon, H. Association of Body Mass Index and Fracture Risk Varied by Affected Bones in Patients with Diabetes: A Nationwide Cohort Study. Diabetes Metab. J. 2023, 47, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Euser, S.M.; Hofman, A.; Westendorp, R.G.J.; Breteler, M.M.B. Serum uric acid and cognitive function and dementia. Brain 2009, 132, 377–382. [Google Scholar] [CrossRef]
- Lu, N.; Dubreuil, M.; Zhang, Y.; Neogi, T.; Rai, S.K.; Ascherio, A.; Hernán, M.A.; Choi, H.K. Gout and the risk of Alzheimer’s disease: A population-based, BMI-matched cohort study. Ann. Rheum. Dis. 2016, 75, 547–551. [Google Scholar] [CrossRef]
- Khan, A.A.; Quinn, T.J.; Hewitt, J.; Fan, Y.; Dawson, J. Serum uric acid level and association with cognitive impairment and dementia: Systematic review and meta-analysis. AGE 2016, 38, 16. [Google Scholar] [CrossRef]
- Lorenzi, T.M.; Borba, D.L.; Dutra, G.; Lara, D.R. Association of serum uric acid levels with emotional and affective temperaments. J. Affect. Disord. 2010, 121, 161–164. [Google Scholar] [CrossRef]
- Mrug, S.; Mrug, M. Uric acid excretion predicts increased aggression in urban adolescents. Physiol. Behav. 2016, 163, 144–148. [Google Scholar] [CrossRef]
- Fu, R.; Chen, C.; Jinnah, H.A. Genotypic and phenotypic spectrum in attenuated variants of Lesch-Nyhan disease. Mol. Genet. Metab. 2014, 112, 280–285. [Google Scholar] [CrossRef]
- Lara, D.R.; Cruz, M.R.S.; Xavier, F.; Souza, D.O.; Moriguchi, E.H. Allopurinol for the treatment of aggressive behaviour in patients with dementia. Int. Clin. Psychopharmacol. 2003, 18, 53–55. [Google Scholar] [CrossRef]
- Göttle, M.; Prudente, C.N.; Fu, R.; Sutcliffe, D.; Pang, H.; Cooper, D.; Veledar, E.; Glass, J.D.; Gearing, M.; Visser, J.E.; et al. Loss of dopamine phenotype among midbrain neurons in Lesch-Nyhan disease. Ann. Neurol. 2014, 76, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Crocamo, C.; Dakanalis, A.; Brosio, E.; Miotto, A.; Capuzzi, E.; Clerici, M.; Carrà, G. Purinergic system dysfunctions in subjects with bipolar disorder: A comparative cross-sectional study. Compr. Psychiatry 2017, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Trotta, G.; Crocamo, C.; Malerba, M.R.; Clerici, M.; Carrà, G. Antioxidant uric acid in treated and untreated subjects with major depressive disorder: A meta-analysis and meta-regression. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 119–127. [Google Scholar] [CrossRef]
- Sekine, M.; Okamoto, K.; Ichida, K. Association of Mutations Identified in Xanthinuria with the Function and Inhibition Mechanism of Xanthine Oxidoreductase. Biomedicines 2021, 9, 1723. [Google Scholar] [CrossRef]
- Nakayama, A.; Matsuo, H.; Ohtahara, A.; Ogino, K.; Hakoda, M.; Hamada, T.; Hosoyamada, M.; Yamaguchi, S.; Hisatome, I.; Ichida, K.; et al. Clinical practice guideline for renal hypouricemia (1st edition). Hum. Cell 2019, 32, 83–87. [Google Scholar] [CrossRef]
- Hakoda, M.; Ichida, K. Genetic Basis of the Epidemiological Features and Clinical Significance of Renal Hypouricemia. Biomedicines 2022, 10, 1696. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef]
- Messerli, F.H.; Frohlich, E.D.; Dreslinski, G.R.; Suarez, D.H.; Aristimuno, G.G. Serum uric acid in essential hypertension: An indicator of renal vascular involvement. Ann. Intern. Med. 1980, 93, 817–821. [Google Scholar] [CrossRef]
- Jing, J.; Kielstein, J.T.; Schultheiss, U.T.; Sitter, T.; Titze, S.I.; Schaeffner, E.S.; McAdams-DeMarco, M.; Kronenberg, F.; Eckardt, K.; Köttgen, A.; et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: The German Chronic Kidney Disease (GCKD) study. Nephrol. Dial. Transplant. 2015, 30, 613–621. [Google Scholar] [CrossRef]
- Bellomo, G.; Venanzi, S.; Verdura, C.; Saronio, P.; Esposito, A.; Timio, M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am. J. Kidney Dis. 2010, 56, 264–272. [Google Scholar] [CrossRef]
- Luo, Q.; Xia, X.; Li, B.; Lin, Z.; Yu, X.; Huang, F. Serum uric acid and cardiovascular mortality in chronic kidney disease: A meta-analysis. BMC Nephrol. 2019, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Furuhashi, M.; Tanaka, M.; Numata, K.; Hisasue, T.; Hanawa, N.; Koyama, M.; Osanami, A.; Higashiura, Y.; Inyaku, M.; et al. U-shaped relationship between serum uric acid level and decline in renal function during a 10-year period in female subjects: BOREAS-CKD2. Hypertens. Res. 2021, 44, 107–116. [Google Scholar] [CrossRef]
- Ishikawa, I. Acute renal failure with severe loin pain and patchy renal ischemia after anaerobic exercise in patients with or without renal hypouricemia. Nephron 2002, 91, 559–570. [Google Scholar] [CrossRef]
- Kimura, K.; Hosoya, T.; Uchida, S.; Inaba, M.; Makino, H.; Maruyama, S.; Ito, S.; Yamamoto, T.; Tomino, Y.; Ohno, I.; et al. Febuxostat Therapy for Patients with Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am. J. Kidney Dis. 2018, 72, 798–810. [Google Scholar] [CrossRef]
- Kojima, S.; Matsui, K.; Hiramitsu, S.; Hisatome, I.; Waki, M.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Jinnouchi, H.; et al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur. Heart J. 2019, 40, 1778–1786. [Google Scholar] [CrossRef]
- Stack, A.G.; Hanley, A.; Casserly, L.F.; Cronin, C.J.; Abdalla, A.A.; Kiernan, T.J.; Murthy, B.V.R.; Hegarty, A.; Hannigan, A.; Nguyen, H.T. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM 2013, 106, 647–658. [Google Scholar] [CrossRef]
- Culleton, B.F.; Larson, M.G.; Kannel, W.B.; Levy, D. Serum uric acid and risk for cardiovascular disease and death: The Framingham Heart Study. Ann. Intern. Med. 1999, 131, 7–13. [Google Scholar] [CrossRef]
- Niskanen, L.K.; Laaksonen, D.E.; Nyyssönen, K.; Alfthan, G.; Lakka, H.; Lakka, T.A.; Salonen, J.T. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: A prospective cohort study. Arch. Intern. Med. 2004, 164, 1546–1551. [Google Scholar] [CrossRef]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef]
- Ioachimescu, A.G.; Brennan, D.M.; Hoar, B.M.; Hazen, S.L.; Hoogwerf, B.J. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: A preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008, 58, 623–630. [Google Scholar] [CrossRef]
- George, J.; Carr, E.; Davies, J.; Belch, J.J.F.; Struthers, A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 2006, 114, 2508–2516. [Google Scholar] [CrossRef]
- Kuwabara, M.; Hisatome, I.; Niwa, K.; Bjornstad, P.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Kanbay, M.; Johnson, R.J.; Lanaspa, M.A. The Optimal Range of Serum Uric Acid for Cardiometabolic Diseases: A 5-Year Japanese Cohort Study. J. Clin. Med. 2020, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, S.; Hisatome, I.; Kuwabara, M.; Niwa, K.; Maharani, N.; Kato, M.; Ogino, K.; Hamada, T.; Ninomiya, H.; Higashi, Y.; et al. Depletion of Uric Acid Due to SLC22A12 (URAT1) Loss-of-Function Mutation Causes Endothelial Dysfunction in Hypouricemia. Circ. J. 2015, 79, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, T.; Salehi, M.R.; Namaki, S.; Arabkheradmand, J.; Rostamian, A.; Rajabnia Chenary, M.; Ghaffarpour, S.; Kaboudanian Ardestani, S.; Edalatifard, M.; Naghizadeh, M.M.; et al. Interpretation of Hematological, Biochemical, and Immunological Findings of COVID-19 Disease: Biomarkers Associated with Severity and Mortality. Iran. J. Allergy Asthma Immunol. 2021, 20, 46–66. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, X.; Wei, Y.; Li, X.; Zheng, B.; Gong, Q.; Dong, L.; Zhong, J. Laboratory Predictors of COVID-19 Mortality: A Retrospective Analysis from Tongji Hospital in Wuhan. Mediators Inflamm. 2021, 2021, 6687412. [Google Scholar] [CrossRef] [PubMed]
- Dufour, I.; Werion, A.; Belkhir, L.; Wisniewska, A.; Perrot, M.; De Greef, J.; Schmit, G.; Yombi, J.C.; Wittebole, X.; Laterre, P.; et al. Serum uric acid, disease severity and outcomes in COVID-19. Crit. Care 2021, 25, 212. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Zhou, C.; Wang, Y.; Song, B.; Cheng, X.; Dong, Q.; Wang, L.; You, S.; Ba, Y. Uric acid as a prognostic factor and critical marker of COVID-19. Sci. Rep. 2021, 11, 17791. [Google Scholar] [CrossRef]
- Werion, A.; Belkhir, L.; Perrot, M.; Schmit, G.; Aydin, S.; Chen, Z.; Penaloza, A.; De Greef, J.; Yildiz, H.; Pothen, L.; et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020, 98, 1296–1307. [Google Scholar] [CrossRef]
- Fukushima, T.; Chubachi, S.; Namkoong, H.; Otake, S.; Nakagawara, K.; Tanaka, H.; Lee, H.; Morita, A.; Watase, M.; Kusumoto, T.; et al. U-shaped association between abnormal serum uric acid levels and COVID-19 severity: Reports from the Japan COVID-19 Task Force. Int. J. Infect. Dis. 2022, 122, 747–754. [Google Scholar] [CrossRef]
- Vaccarino, V.; Krumholz, H.M. Risk factors for cardiovascular disease: One down, many more to evaluate. Ann. Intern. Med. 1999, 131, 62–63. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kang, D.; Feig, D.; Sanchez-Lozada, L.G.; Srinivas, T.R.; Sautin, Y.; Ejaz, A.A.; Segal, M.; Johnson, R.J. Unearthing uric acid: An ancient factor with recently found significance in renal and cardiovascular disease. Kidney Int. 2006, 69, 1722–1725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).