Pathogenesis of Extraarticular Manifestations in Rheumatoid Arthritis—A Comprehensive Review
Abstract
:1. Introduction
2. Cardiovascular Disease
2.1. Atherosclerosis
2.1.1. Endothelial Dysfunction
2.1.2. Lipoprotein Abnormalities
2.1.3. Role of Immune Cells
2.2. Non-Ischemic Heart Disease
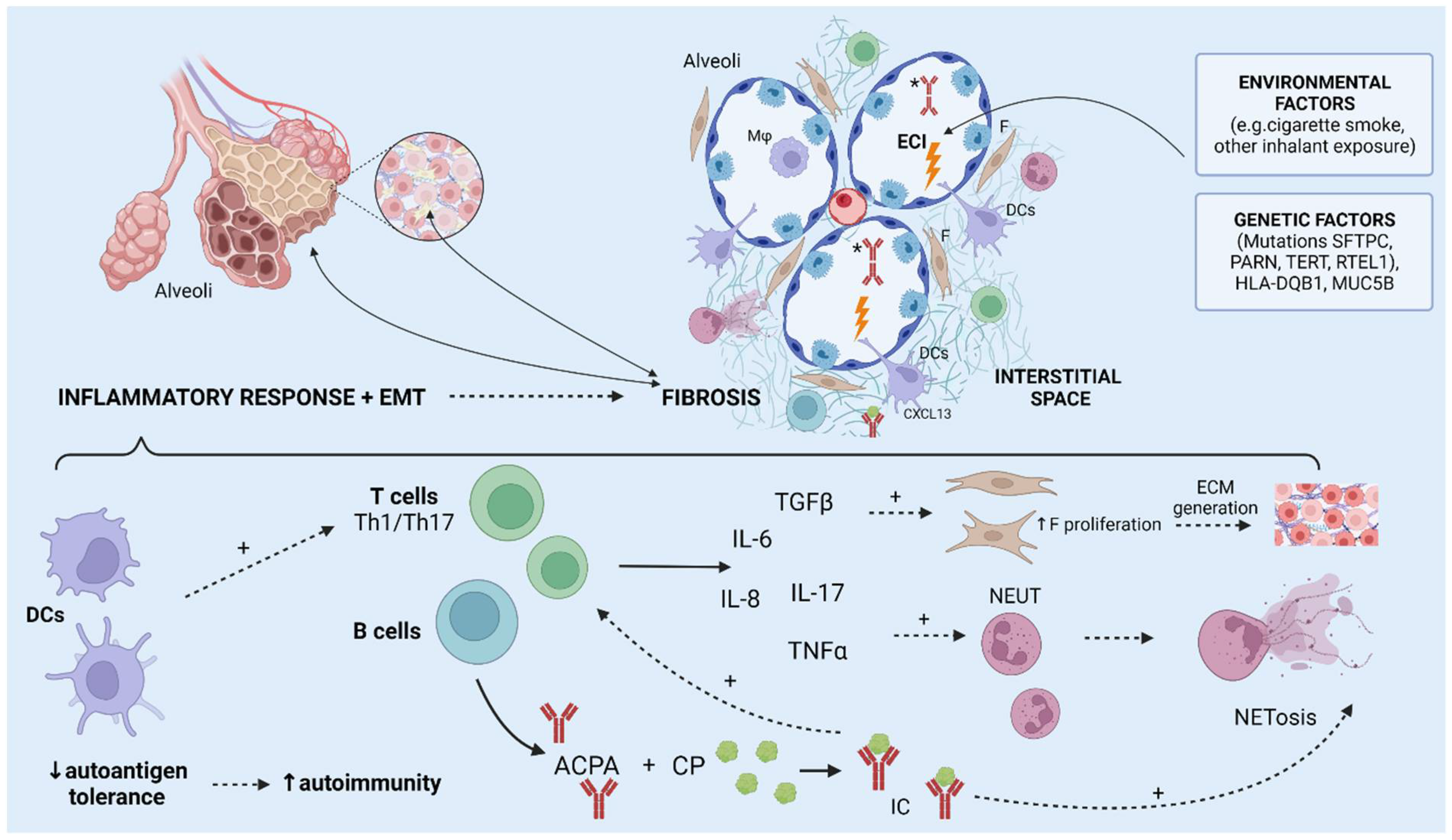
3. Pulmonary Manifestations
3.1. Interstitial Lung Disease
3.2. Bronchiectasis
4. Rheumatoid Nodules
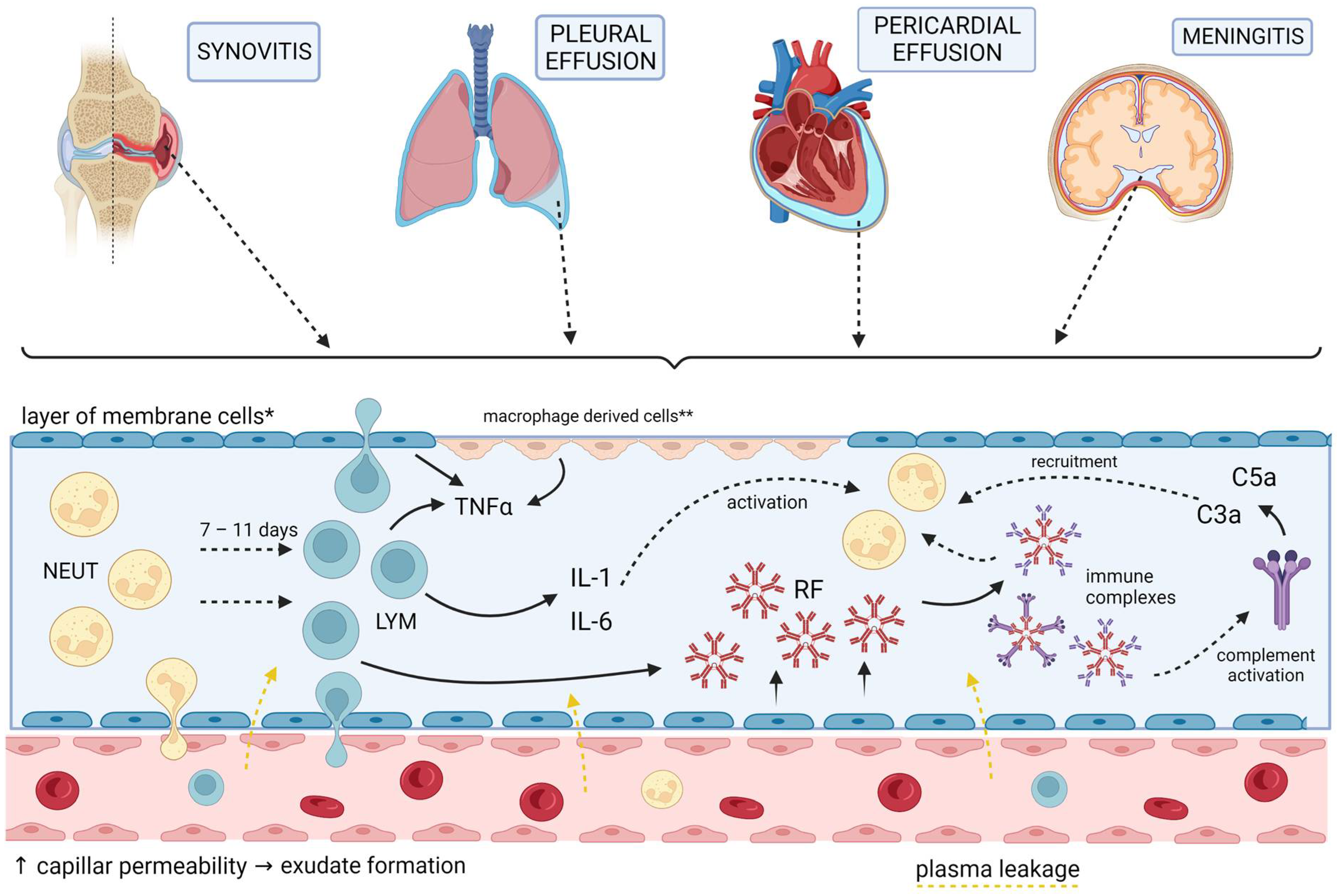
5. Membrane Involvement
6. Neurologic Manifestations
7. Ocular Involvement
8. Hematologic Manifestations
Felty Syndrome
9. Osteoporosis
10. Vasculitis
11. Renal Manifestations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid Arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Van Delft, M.A.M.; Huizinga, T.W.J. An Overview of Autoantibodies in Rheumatoid Arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, M.; Cojocaru, I.; Silosi, I.; Vrabie, C.; Tanasescu, R. Extra-Articular Manifestations in Rheumatoid Arthritis. Maedica (Bucur.) 2010, 5, 286–291. [Google Scholar]
- Scott, D.L.; Smith, C.; Kingsley, G. Joint Damage and Disability in Rheumatoid Arthritis: An Updated Systematic Review. Clin. Exp. Rheumatol. 2003, 21, S20–S27. [Google Scholar] [PubMed]
- Patel R, A.H. Felty Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546693/ (accessed on 20 February 2023).
- Myasoedova, E.; Crowson, C.S.; Turesson, C.; Gabriel, S.E.; Matteson, E.L. Incidence of Extraarticular Rheumatoid Arthritis in Olmsted County, Minnesota, in 1995–2007 versus 1985–1994: A Population-Based Study. J. Rheumatol. 2011, 38, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Calgüneri, M.; Ureten, K.; Akif Oztürk, M.; Onat, A.M.; Ertenli, I.; Kiraz, S.; Akdogan, A. Extra-Articular Manifestations of Rheumatoid Arthritis: Results of a University Hospital of 526 Patients in Turkey. Clin. Exp. Rheumatol. 2006, 24, 305–308. [Google Scholar]
- Turesson, C.; O’Fallon, W.M.; Crowson, C.S.; Gabriel, S.E.; Matteson, E.L. Extra-Articular Disease Manifestations in Rheumatoid Arthritis: Incidence Trends and Risk Factors over 46 Years. Ann. Rheum. Dis. 2003, 62, 722–727. [Google Scholar] [CrossRef]
- Turesson, C.; Jacobsson, L.T.H. Epidemiology of Extra-Articular Manifestations in Rheumatoid Arthritis. Scand. J. Rheumatol. 2004, 33, 65–72. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Wang, T.; Li, Y.; Wei, M. Influence Factors of Extra-Articular Manifestations in Rheumatoid Arthritis. Open Med. 2020, 15, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, M.A.; Salvarani, C.; Macchioni, P.; Montecucco, C.; Fossaluzza, V.; Mascia, M.T.; Punzi, L.; Davoli, C.; Filippini, D.; Numo, R. Extra-Articular Manifestations in 587 Italian Patients with Rheumatoid Arthritis. Rheumatol. Int. 2000, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E.; Crowson, C.S.; Kremers, H.M.; Doran, M.F.; Turesson, C.; O’Fallon, W.M.; Matteson, E.L. Survival in Rheumatoid Arthritis: A Population-Based Analysis of Trends over 40 Years. Arthritis Rheum. 2003, 48, 54–58. [Google Scholar] [CrossRef]
- Hochberg, M.; Gravallese, E.M.; Silman, A.J.; Smolen, J.S. Rheumatology, 7th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 747–860. [Google Scholar]
- Wang, D.; Zhang, J.; Lau, J.; Wang, S.; Taneja, V.; Matteson, E.L.; Vassallo, R. Mechanisms of Lung Disease Development in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2019, 15, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, M.; Cojocaru, I.M.; Chicoş, B. New Insight into the Rheumatoid Vasculitis. Rom. J. Intern. Med. 2015, 53, 128–132. [Google Scholar] [CrossRef]
- Trabelsi, M.; Romand, X.; Gilson, M.; Vaillant, M.; Guerne, P.-A.; Hayem, G.; Bertolini, E.; Baillet, A.; Gaudin, P. Rheumatoid Meningitis a Rare Extra-Articular Manifestation of Rheumatoid Arthritis: Report of 6 Cases and Literature Review. J. Clin. Med. 2020, 9, 1625. [Google Scholar] [CrossRef]
- Giles, J.T. Extra-articular Manifestations and Comorbidity in Rheumatoid Arthritis: Potential Impact of Pre-Rheumatoid Arthritis Prevention. Clin. Ther. 2019, 41, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Kerola, A.M.; Kazemi, A.; Rollefstad, S.; Lillegraven, S.; Sexton, J.; Wibetoe, G.; Haavardsholm, E.A.; Kvien, T.K.; Semb, A.G. All-Cause and Cause-Specific Mortality in Rheumatoid Arthritis, Psoriatic Arthritis and Axial Spondyloarthritis: A Nationwide Registry Study. Rheumatology 2022, 61, 4656–4666. [Google Scholar] [CrossRef] [PubMed]
- van den Hoek, J.; Boshuizen, H.C.; Roorda, L.D.; Tijhuis, G.J.; Nurmohamed, M.T.; van den Bos, G.A.M.; Dekker, J. Mortality in Patients with Rheumatoid Arthritis: A 15-Year Prospective Cohort Study. Rheumatol. Int. 2017, 37, 487–493. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of Incident Cardiovascular Events in Patients with Rheumatoid Arthritis: A Meta-Analysis of Observational Studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of Cardiovascular Mortality in Patients with Rheumatoid Arthritis: A Meta-Analysis of Observational Studies. Arthritis Care Res. 2008, 59, 1690–1697. [Google Scholar] [CrossRef]
- Del Rincón, I.; Williams, K.; Stern, M.P.; Freeman, G.L.; Escalante, A. High Incidence of Cardiovascular Events in a Rheumatoid Arthritis Cohort Not Explained by Traditional Cardiac Risk Factors. Arthritis Rheum. 2001, 44, 2737–2745. [Google Scholar]
- Moriya, J. Critical Roles of Inflammation in Atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; MacNamara, K.C. Atherosclerosis Is a Major Human Killer and Non-Resolving Inflammation Is a Prime Suspect. Cardiovasc. Res. 2021, 117, 2563–2574. [Google Scholar] [CrossRef]
- Aziz, M.; Yadav, K. Atherosclerosis: An Extra Articular Manifestation of Rheumatoid Arthritis. Ann. Clin. Lab. Res. 2016, 4, 4. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Neumann, P.; Gertzberg, N.; Johnson, A. TNF-α Induces a Decrease in ENOS Promoter Activity. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 286, L452–L459. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Heusch, G.; Schulz, R. TNFα in Atherosclerosis, Myocardial Ischemia/Reperfusion and Heart Failure. Pharmacol. Ther. 2010, 127, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Zafari, P.; Zarifian, A.; Alizadeh-Navaei, R.; Taghadosi, M.; Rafiei, A.; Samimi, Z.; Niksolat, F. Asymmetric and Symmetric Dimethylarginine Concentration as an Indicator of Cardiovascular Diseases in Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis of Case-Control Studies. Clin. Rheumatol. 2020, 39, 127–134. [Google Scholar] [CrossRef]
- Böger, R.H.; Vallance, P.; Cooke, J.P. Asymmetric Dimethylarginine (ADMA): A Key Regulator of Nitric Oxide Synthase. Atheroscler. Suppl. 2003, 4, 1–3. [Google Scholar] [CrossRef]
- Di Franco, M.; Lucchino, B.; Conti, F.; Valesini, G.; Spinelli, F.R. Asymmetric Dimethyl Arginine as a Biomarker of Atherosclerosis in Rheumatoid Arthritis. Mediat. Inflamm. 2018, 2018, 3897295. [Google Scholar] [CrossRef]
- Akhmedov, A.; Crucet, M.; Simic, B.; Kraler, S.; Bonetti, N.R.; Ospelt, C.; Distler, O.; Ciurea, A.; Liberale, L.; Jauhiainen, M.; et al. TNFα Induces Endothelial Dysfunction in Rheumatoid Arthritis via LOX-1 and Arginase 2: Reversal by Monoclonal TNFα Antibodies. Cardiovasc. Res. 2022, 118, 254–266. [Google Scholar] [CrossRef]
- Shin, W.; Berkowitz, D.E.; Ryoo, S. Increased Arginase II Activity Contributes to Endothelial Dysfunction through Endothelial Nitric Oxide Synthase Uncoupling in Aged Mice. Exp. Mol. Med. 2012, 44, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ming, X.-F. Functions of Arginase Isoforms in Macrophage Inflammatory Responses: Impact on Cardiovascular Diseases and Metabolic Disorders. Front. Immunol. 2014, 5, 533. [Google Scholar] [CrossRef] [PubMed]
- Atehortúa, L.; Rojas, M.; Vásquez, G.; Muñoz-Vahos, C.H.; Vanegas-García, A.; Posada-Duque, R.A.; Castaño, D. Endothelial Activation and Injury by Microparticles in Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis. Arthritis Res. Ther. 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Bergh, N.; Ulfhammer, E.; Glise, K.; Jern, S.; Karlsson, L. Influence of TNF-α and Biomechanical Stress on Endothelial Anti- and Prothrombotic Genes. Biochem. Biophys. Res. Commun. 2009, 385, 314–318. [Google Scholar] [CrossRef]
- Rajan, S.; Ye, J.; Bai, S.; Huang, F.; Guo, Y.-L. NF-KappaB, but Not P38 MAP Kinase, Is Required for TNF-Alpha-Induced Expression of Cell Adhesion Molecules in Endothelial Cells. J. Cell. Biochem. 2008, 105, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Connell, M.C.; MacEwan, D.J. TNFR1-Induced NF-ΚB, but Not ERK, P38MAPK or JNK Activation, Mediates TNF-Induced ICAM-1 and VCAM-1 Expression on Endothelial Cells. Cell Signal. 2007, 19, 1238–1248. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Brunzell, J.D.; Ayyobi, A.F. Dyslipidemia in the Metabolic Syndrome and Type 2 Diabetes Mellitus. Am. J. Med. 2003, 115, 24–28. [Google Scholar] [CrossRef]
- Zegkos, T.; Kitas, G.; Dimitroulas, T. Cardiovascular Risk in Rheumatoid Arthritis: Assessment, Management and next Steps. Ther. Adv. Musculoskelet. Dis. 2016, 8, 86–101. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Zengin, G.; Brisc, C.; Brisc, M.C.; Munteanu, M.A.; Nistor-Cseppento, D.C.; Bungau, S. The Lipid Paradox as a Metabolic Checkpoint and Its Therapeutic Significance in Ameliorating the Associated Cardiovascular Risks in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2020, 21, 9505. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid Paradox in Rheumatoid Arthritis: The Impact of Serum Lipid Measures and Systemic Inflammation on the Risk of Cardiovascular Disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Chen, C.-H.; Chen, Y.-M.; Hsieh, T.-Y.; Li, J.-P.; Shen, M.-Y.; Lan, J.-L.; Chen, D.-Y. Association between Negatively Charged Low-Density Lipoprotein L5 and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients. J. Clin. Med. 2019, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-K.; Chen, P.-K.; Lan, J.-L.; Chang, S.-H.; Hsieh, T.-Y.; Liao, P.-J.; Chen, C.-H.; Chen, D.-Y. Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2020, 21, 5883. [Google Scholar] [CrossRef] [PubMed]
- Barter, P. The Role of HDL-Cholesterol in Preventing Atherosclerotic Disease. Eur. Heart J. Suppl. 2005, 7, F4–F8. [Google Scholar] [CrossRef]
- Vyletelová, V.; Nováková, M.; Pašková, Ľ. Alterations of HDL’s to piHDL’s Proteome in Patients with Chronic Inflammatory Diseases, and HDL-Targeted Therapies. Pharmaceuticals 2022, 15, 1278. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Watanabe, J.; Lee, Y.Y.; Furst, D.E.; Amjadi, S.; Elashoff, D.; Park, G.; McMahon, M.; Paulus, H.E.; Fogelman, A.M.; et al. Abnormal Function of High-Density Lipoprotein Is Associated with Poor Disease Control and an Altered Protein Cargo in Rheumatoid Arthritis. Arthritis Rheum. 2009, 60, 2870–2879. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Lee, Y.Y.; Grijalva, V.; Amjadi, S.; FitzGerald, J.; Ranganath, V.K.; Taylor, M.; McMahon, M.; Paulus, H.E.; Reddy, S.T. Cholesterol Efflux by High Density Lipoproteins Is Impaired in Patients with Active Rheumatoid Arthritis. Ann. Rheum. Dis. 2012, 71, 1157–1162. [Google Scholar] [CrossRef]
- Tejera-Segura, B.; Macía-Díaz, M.; Machado, J.D.; de Vera-González, A.; García-Dopico, J.A.; Olmos, J.M.; Hernández, J.L.; Díaz-González, F.; González-Gay, M.A.; Ferraz-Amaro, I. HDL Cholesterol Efflux Capacity in Rheumatoid Arthritis Patients: Contributing Factors and Relationship with Subclinical Atherosclerosis. Arthritis Res. Ther. 2017, 19, 113. [Google Scholar] [CrossRef]
- Zheng, L.; Nukuna, B.; Brennan, M.-L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thomson, L.; Fox, P.L.; et al. Apolipoprotein A-I Is a Selective Target for Myeloperoxidase-Catalyzed Oxidation and Functional Impairment in Subjects with Cardiovascular Disease. J. Clin. Investig. 2004, 114, 529–541. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Pérez-Álvarez, Á.I.; Robinson, G.A.; Alonso-Castro, S.; Amigó, N.; Atzeni, F.; Suárez, A. Humoral Responses against HDL Particles Are Linked to Lipoprotein Traits, Atherosclerosis Occurrence, Inflammation and Pathogenic Pathways during the Earliest Stages of Arthritis. medRxiv 2022. [Google Scholar] [CrossRef]
- Ozaki, Y.; Imanishi, T.; Taruya, A.; Aoki, H.; Masuno, T.; Shiono, Y.; Komukai, K.; Tanimoto, T.; Kitabata, H.; Akasaka, T. Circulating CD14+CD16+ Monocyte Subsets as Biomarkers of the Severity of Coronary Artery Disease in Patients with Stable Angina Pectoris. Circ. J. 2012, 76, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Winchester, R.; Giles, J.T.; Nativ, S.; Downer, K.; Zhang, H.-Z.; Bag-Ozbek, A.; Zartoshti, A.; Bokhari, S.; Bathon, J.M. Association of Elevations of Specific T Cell and Monocyte Subpopulations in Rheumatoid Arthritis with Subclinical Coronary Artery Atherosclerosis. Arthritis Rheumatol. 2016, 68, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Dragoljevic, D.; Kraakman, M.J.; Nagareddy, P.R.; Ngo, D.; Shihata, W.; Kammoun, H.L.; Whillas, A.; Lee, M.K.S.; Al-Sharea, A.; Pernes, G.; et al. Defective Cholesterol Metabolism in Haematopoietic Stem Cells Promotes Monocyte-Driven Atherosclerosis in Rheumatoid Arthritis. Eur. Heart J. 2018, 39, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Ott, I.; Marx, N.; Luther, T.; Kenngott, S.; Gawaz, M.; Kotzsch, M.; Schömig, A. Effect of Human Recombinant Interleukin-6 and Interleukin-8 on Monocyte Procoagulant Activity. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3399–3405. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef]
- Luchtefeld, M.; Schunkert, H.; Stoll, M.; Selle, T.; Lorier, R.; Grote, K.; Sagebiel, C.; Jagavelu, K.; Tietge, U.J.F.; Assmus, U.; et al. Signal Transducer of Inflammation Gp130 Modulates Atherosclerosis in Mice and Man. J. Exp. Med. 2007, 204, 1935–1944. [Google Scholar] [CrossRef]
- Rammos, G.; Kondomerkos, D. The Role of CD4+CD28(Null) T-Lymphocytes and Statins in Rheumatoid Arthritis and Unstable Atherosclerotic Plaque. Hell. J. Cardiol. 2007, 48, 165–174. [Google Scholar]
- Liuzzo, G.; Goronzy, J.J.; Yang, H.; Kopecky, S.L.; Holmes, D.R.; Frye, R.L.; Weyand, C.M. Monoclonal T-Cell Proliferation and Plaque Instability in Acute Coronary Syndromes. Circulation 2000, 101, 2883–2888. [Google Scholar] [CrossRef]
- Martens, P.B.; Goronzy, J.J.; Schaid, D.; Weyand, C.M. Expansion of Unusual CD4+ T Cells in Severe Rheumatoid Arthritis. Arthritis Rheum. 1997, 40, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Namekawa, T.; Wagner, U.G.; Goronzy, J.J.; Weyand, C.M. Functional Subsets of CD4 T Cells in Rheumatoid Synovitis. Arthritis Rheum. 1998, 41, 2108–2116. [Google Scholar] [CrossRef]
- Schmidt, D.; Martens, P.B.; Weyand, C.M.; Goronzy, J.J. The Repertoire of CD4+ CD28− T Cells in Rheumatoid Arthritis. Mol. Med. 1996, 2, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Gerli, R.; Schillaci, G.; Giordano, A.; Bocci, E.B.; Bistoni, O.; Vaudo, G.; Marchesi, S.; Pirro, M.; Ragni, F.; Shoenfeld, Y.; et al. CD4+CD28− T Lymphocytes Contribute to Early Atherosclerotic Damage in Rheumatoid Arthritis Patients. Circulation 2004, 109, 2744–2748. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, C.; Ruiz-Limón, P.; Aguirre, M.A.; Jiménez-Gómez, Y.; Arias-de la Rosa, I.; Ábalos-Aguilera, M.C.; Rodriguez-Ariza, A.; Castro-Villegas, M.C.; Ortega-Castro, R.; Segui, P.; et al. Diagnostic Potential of NETosis-Derived Products for Disease Activity, Atherosclerosis and Therapeutic Effectiveness in Rheumatoid Arthritis Patients. J. Autoimmun. 2017, 82, 31–40. [Google Scholar] [CrossRef]
- Ruiz-Limón, P.; Ortega, R.; Arias de la Rosa, I.; Abalos-Aguilera, M.d.C.; Perez-Sanchez, C.; Jimenez-Gomez, Y.; Peralbo-Santaella, E.; Font, P.; Ruiz-Vilches, D.; Ferrin, G.; et al. Tocilizumab Improves the Proatherothrombotic Profile of Rheumatoid Arthritis Patients Modulating Endothelial Dysfunction, NETosis, and Inflammation. Transl. Res. 2017, 183, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Mantel, Ä.; Holmqvist, M.; Andersson, D.C.; Lund, L.H.; Askling, J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1275–1285. [Google Scholar] [CrossRef]
- Błyszczuk, P.; Szekanecz, Z. Pathogenesis of Ischaemic and Non-Ischaemic Heart Diseases in Rheumatoid Arthritis. RMD Open 2020, 6, e001032. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Karabela, G.; Stavropoulos, E.; Gialafos, E.; Sfendouraki, E.; Kyrou, L.; Kolovou, G. Imaging Patterns of Heart Failure in Rheumatoid Arthritis Evaluated by Cardiovascular Magnetic Resonance. Int. J. Cardiol. 2013, 168, 4333–4335. [Google Scholar] [CrossRef]
- Amigues, I.; Tugcu, A.; Russo, C.; Giles, J.T.; Morgenstein, R.; Zartoshti, A.; Schulze, C.; Flores, R.; Bokhari, S.; Bathon, J.M. Myocardial Inflammation, Measured Using 18-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography, Is Associated with Disease Activity in Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 496–506. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kribbs, S.B.; Clubb, F.J.; Michael, L.H.; Didenko, V.V.; Hornsby, P.J.; Seta, Y.; Oral, H.; Spinale, F.G.; Mann, D.L. Pathophysiologically Relevant Concentrations of Tumor Necrosis Factor-α Promote Progressive Left Ventricular Dysfunction and Remodeling in Rats. Circulation 1998, 97, 1382–1391. [Google Scholar] [CrossRef]
- Oral, H.; Dorn, G.W., II; Mann, D.L. Sphingosine Mediates the Immediate Negative Inotropic Effects of Tumor Necrosis Factor-Alpha; in the Adult Mammalian Cardiac Myocyte. J. Biol. Chem. 1997, 272, 4836–4842. [Google Scholar] [CrossRef]
- Gulick, T.; Chung, M.K.; Pieper, S.J.; Lange, L.G.; Schreiner, G.F. Interleukin 1 and Tumor Necrosis Factor Inhibit Cardiac Myocyte Beta-Adrenergic Responsiveness. Proc. Natl. Acad. Sci. USA 1989, 86, 6753–6757. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.N.; Kremastinos, D.T. Inhibition of Interleukin-1 by Anakinra Improves Vascular and Left Ventricular Function in Patients with Rheumatoid Arthritis. Circulation 2008, 117, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Raleigh, J.M.V.; Abbate, A. Targeting Interleukin-1 in Heart Failure and Inflammatory Heart Disease. Curr. Heart Fail. Rep. 2015, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Griffin, J.; Bathon, J.M. Myocardial Dysfunction and Heart Failure in Rheumatoid Arthritis. Arthritis Rheumatol. 2022, 74, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Geraldino-Pardilla, L.; Russo, C.; Sokolove, J.; Robinson, W.H.; Zartoshti, A.; Van Eyk, J.; Fert-Bober, J.; Lima, J.; Giles, J.T.; Bathon, J.M. Association of Anti-Citrullinated Protein or Peptide Antibodies with Left Ventricular Structure and Function in Rheumatoid Arthritis. Rheumatology 2017, 56, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Fert-Bober, J.; Park, J.K.; Bingham, C.O.; Andrade, F.; Fox-Talbot, K.; Pappas, D.; Rosen, A.; van Eyk, J.; Bathon, J.M.; et al. Myocardial Citrullination in Rheumatoid Arthritis: A Correlative Histopathologic Study. Arthritis Res. Ther. 2012, 14, R39. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Yang, H.-Y.; Lai, J.-H. Anti-Citrullinated Protein Antibodies in Patients with Rheumatoid Arthritis: Biological Effects and Mechanisms of Immunopathogenesis. Int. J. Mol. Sci. 2020, 21, 4015. [Google Scholar] [CrossRef] [PubMed]
- Yunt, Z.X.; Solomon, J.J. Lung Disease in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2015, 41, 225–236. [Google Scholar] [CrossRef]
- Mori, S.; Cho, I.; Koga, Y.; Sugimoto, M. Comparison of Pulmonary Abnormalities on High-Resolution Computed Tomography in Patients with Early versus Longstanding Rheumatoid Arthritis. J. Rheumatol. 2008, 35, 1513–1521. [Google Scholar]
- Bongartz, T.; Nannini, C.; Medina-Velasquez, Y.F.; Achenbach, S.J.; Crowson, C.S.; Ryu, J.H.; Vassallo, R.; Gabriel, S.E.; Matteson, E.L. Incidence and Mortality of Interstitial Lung Disease in Rheumatoid Arthritis: A Population-Based Study. Arthritis Rheum. 2010, 62, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Wilsher, M.; Voight, L.; Milne, D.; Teh, M.; Good, N.; Kolbe, J.; Williams, M.; Pui, K.; Merriman, T.; Sidhu, K.; et al. Prevalence of Airway and Parenchymal Abnormalities in Newly Diagnosed Rheumatoid Arthritis. Respir. Med. 2012, 106, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.; Remy-Jardin, M.; Cortet, B. Airways Involvement in Rheumatoid Arthritis: Clinical, Functional, and HRCT Findings. Am. J. Respir. Crit. Care Med. 1998, 157, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Kadura, S.; Raghu, G. Rheumatoid Arthritis-Interstitial Lung Disease: Manifestations and Current Concepts in Pathogenesis and Management. Eur. Respir. Rev. 2021, 30, 210011. [Google Scholar] [CrossRef]
- Shaw, M.; Collins, B.F.; Ho, L.A.; Raghu, G. Rheumatoid Arthritis-Associated Lung Disease. Eur. Respir. Rev. 2015, 24, 1–16. [Google Scholar] [CrossRef]
- Lee, H.-K.; Kim, D.S.; Yoo, B.; Seo, J.B.; Rho, J.-Y.; Colby, T.V.; Kitaichi, M. Histopathologic Pattern and Clinical Features of Rheumatoid Arthritis-Associated Interstitial Lung Disease. Chest 2005, 127, 2019–2027. [Google Scholar] [CrossRef]
- Olson, A.L.; Swigris, J.J.; Sprunger, D.B.; Fischer, A.; Fernandez-Perez, E.R.; Solomon, J.; Murphy, J.; Cohen, M.; Raghu, G.; Brown, K.K. Rheumatoid Arthritis-Interstitial Lung Disease-Associated Mortality. Am. J. Respir. Crit. Care Med. 2011, 183, 372–378. [Google Scholar] [CrossRef]
- Paulin, F.; Doyle, T.; Fletcher, E.; Ascherman, D.; Rosas, I. Rheumatoid Arthritis-Associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis: Shared Mechanistic and Phenotypic Traits Suggest Overlapping Disease Mechanisms. Rev. Investig. Clin. 2015, 67, 280–286. [Google Scholar]
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid Arthritis and the Mucosal Origins Hypothesis: Protection Turns to Destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557. [Google Scholar] [CrossRef]
- Willis, V.C.; Demoruelle, M.K.; Derber, L.A.; Chartier-Logan, C.J.; Parish, M.C.; Pedraza, I.F.; Weisman, M.H.; Norris, J.M.; Holers, V.M.; Deane, K.D. Sputum Autoantibodies in Patients with Established Rheumatoid Arthritis and Subjects at Risk of Future Clinically Apparent Disease. Arthritis Rheum. 2013, 65, 2545–2554. [Google Scholar] [CrossRef]
- Reynisdottir, G.; Olsen, H.; Joshua, V.; Engström, M.; Forsslund, H.; Karimi, R.; Sköld, C.M.; Nyren, S.; Eklund, A.; Grunewald, J.; et al. Signs of Immune Activation and Local Inflammation Are Present in the Bronchial Tissue of Patients with Untreated Early Rheumatoid Arthritis. Ann. Rheum. Dis. 2016, 75, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Juge, P.A.; Borie, R.; Kannengiesser, C.; Gazal, S.; Revy, P.; Wemeau-Stervinou, L.; Debray, M.P.; Ottaviani, S.; Marchand-Adam, S.; Nathan, N.; et al. AB0007 Shared Genetic Predisposition in Rheumatoid Arthritis–Interstitial Lung Disease and Familial Pulmonary Fibrosis. Ann. Rheum. Dis. 2017, 76, 1049. [Google Scholar] [CrossRef]
- Oka, S.; Furukawa, H.; Shimada, K.; Sugii, S.; Hashimoto, A.; Komiya, A.; Fukui, N.; Suda, A.; Tsunoda, S.; Ito, S.; et al. Association of Human Leukocyte Antigen Alleles with Chronic Lung Diseases in Rheumatoid Arthritis. Rheumatology 2016, 55, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Audiger, C.; Rahman, M.J.; Yun, T.J.; Tarbell, K.V.; Lesage, S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J. Immunol. 2017, 198, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.; Nogueira, L.; Laurent, L.; Iobagiu, C.; Vincent, C.; Sebbag, M.; Serre, G. Induction of Macrophage Secretion of Tumor Necrosis Factor Alpha through Fcgamma Receptor IIa Engagement by Rheumatoid Arthritis-Specific Autoantibodies to Citrullinated Proteins Complexed with Fibrinogen. Arthritis Rheum. 2008, 58, 678–688. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Wang, L.; Wang, S.; Roden, A.C.; Zhao, H.; Li, X.; Prakash, Y.S.; Matteson, E.L.; Tschumperlin, D.J.; et al. Profibrotic Effect of IL-17A and Elevated IL-17RA in Idiopathic Pulmonary Fibrosis and Rheumatoid Arthritis-Associated Lung Disease Support a Direct Role for IL-17A/IL-17RA in Human Fibrotic Interstitial Lung Disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L487–L497. [Google Scholar] [CrossRef]
- Broekelmann, T.J.; Limper, A.H.; Colby, T.V.; McDonald, J.A. Transforming Growth Factor Beta 1 Is Present at Sites of Extracellular Matrix Gene Expression in Human Pulmonary Fibrosis. Proc. Natl. Acad. Sci. USA 1991, 88, 6642–6646. [Google Scholar] [CrossRef]
- Kazantseva, M.G.; Highton, J.; Stamp, L.K.; Hessian, P.A. Dendritic Cells Provide a Potential Link between Smoking and Inflammation in Rheumatoid Arthritis. Arthritis Res. Ther. 2012, 14, R208. [Google Scholar] [CrossRef]
- Lee, J.; Luria, A.; Rhodes, C.; Raghu, H.; Lingampalli, N.; Sharpe, O.; Rada, B.; Sohn, D.H.; Robinson, W.H.; Sokolove, J. Nicotine Drives Neutrophil Extracellular Traps Formation and Accelerates Collagen-Induced Arthritis. Rheumatology 2017, 56, 644–653. [Google Scholar] [CrossRef]
- Lee, K.H.; Kronbichler, A.; Park, D.D.-Y.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil Extracellular Traps (NETs) in Autoimmune Diseases: A Comprehensive Review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, R.; Walters, P.R.; Lamont, J.; Kottom, T.J.; Yi, E.S.; Limper, A.H. Cigarette Smoke Promotes Dendritic Cell Accumulation in COPD; a Lung Tissue Research Consortium Study. Respir. Res. 2010, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; You, W.J.; Liu, X.Q.; Xue, S.; Qin, H.; Jiang, H.D. Chronic Microaspiration of Bile Acids Induces Lung Fibrosis through Multiple Mechanisms in Rats. Clin. Sci. (Lond.) 2017, 131, 951–963. [Google Scholar] [CrossRef]
- Jensen, K.; Nizamutdinov, D.; Guerrier, M.; Afroze, S.; Dostal, D.; Glaser, S. General Mechanisms of Nicotine-Induced Fibrogenesis. FASEB J. 2012, 26, 4778–4787. [Google Scholar] [CrossRef]
- Zou, W.; Zou, Y.; Zhao, Z.; Li, B.; Ran, P. Nicotine-Induced Epithelial-Mesenchymal Transition via Wnt/β-Catenin Signaling in Human Airway Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L199–L209. [Google Scholar] [CrossRef]
- Duarte, A.C.; Porter, J.; Leandro, M.J. Bronchiectasis in Rheumatoid Arthritis. A Clinical Appraisial. Jt. Bone Spine 2020, 87, 419–424. [Google Scholar] [CrossRef]
- Geri, G.; Dadoun, S.; Bui, T.; Del Castillo Pinol, N.; Paternotte, S.; Dougados, M.; Gossec, L. Risk of Infections in Bronchiectasis during Disease-Modifying Treatment and Biologics for Rheumatic Diseases. BMC Infect. Dis. 2011, 11, 304. [Google Scholar] [CrossRef]
- Cole, P.J. Inflammation: A Two-Edged Sword—The Model of Bronchiectasis. Eur. J. Respir. Dis. Suppl. 1986, 147, 6–15. [Google Scholar] [PubMed]
- Wilczynska, M.M.; Condliffe, A.M.; McKeon, D.J. Coexistence of Bronchiectasis and Rheumatoid Arthritis: Revisited. Respir. Care 2013, 58, 694. [Google Scholar] [CrossRef]
- Menéndez, R.; Méndez, R.; Amara-Elori, I.; Reyes, S.; Montull, B.; Feced, L.; Alonso, R.; Amaro, R.; Alcaraz, V.; Fernandez-Barat, L.; et al. Systemic Inflammation during and after Bronchiectasis Exacerbations: Impact of Pseudomonas Aeruginosa. J. Clin. Med. 2020, 9, 2631. [Google Scholar] [CrossRef]
- Ayhan, G.; Tas, D.; Yilmaz, I.; Okutan, O.; Demirer, E.; Ayten, O.; Kartaloglu, Z. Relation between Inflammatory Cytokine Levels in Serum and Bronchoalveolar Lavage Fluid and Gene Polymorphism in Young Adult Patients with Bronchiectasis. J. Thorac. Dis. 2014, 6, 684–693. [Google Scholar] [CrossRef]
- Demoruelle, M.K.; Weisman, M.H.; Simonian, P.L.; Lynch, D.A.; Sachs, P.B.; Pedraza, I.F.; Harrington, A.R.; Kolfenbach, J.R.; Striebich, C.C.; Pham, Q.N.; et al. Brief Report: Airways Abnormalities and Rheumatoid Arthritis–Related Autoantibodies in Subjects without Arthritis: Early Injury or Initiating Site of Autoimmunity? Arthritis Rheum. 2012, 64, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Quirke, A.-M.; Perry, E.; Cartwright, A.; Kelly, C.; De Soyza, A.; Eggleton, P.; Hutchinson, D.; Venables, P.J. Bronchiectasis Is a Model for Chronic Bacterial Infection Inducing Autoimmunity in Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 2335–2342. [Google Scholar] [CrossRef]
- García-Patos, V. Rheumatoid Nodule. Semin. Cutan. Med. Surg. 2007, 26, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, J.S.; Lienesch, D.W. Rheumatoid Nodules. Dermatol. Clin. 2015, 33, 361–371. [Google Scholar] [CrossRef]
- Habib, H.M.; Eisa, A.A.; Arafat, W.R.; Marie, M.A. Pulmonary Involvement in Early Rheumatoid Arthritis Patients. Clin. Rheumatol. 2011, 30, 217–221. [Google Scholar] [CrossRef]
- Yousem, S.A.; Colby, T.V.; Carrington, C.B. Lung Biopsy in Rheumatoid Arthritis. Am. Rev. Respir. Dis. 1985, 131, 770–777. [Google Scholar]
- Toussirot, E.; Berthelot, J.M.; Pertuiset, E.; Bouvard, B.; Gaudin, P.; Wendling, D.; le Noach, J.; Lohse, A.; Lecuyer, E.; Cri, L. Pulmonary Nodulosis and Aseptic Granulomatous Lung Disease Occurring in Patients with Rheumatoid Arthritis Receiving Tumor Necrosis Factor-Alpha-Blocking Agent: A Case Series. J. Rheumatol. 2009, 36, 2421–2427. [Google Scholar] [CrossRef]
- Sagdeo, P.; Gattimallanahali, Y.; Kakade, G.; Canchi, B. Rheumatoid Lung Nodule. BMJ Case Rep. 2015, 2015, bcr2015213083. [Google Scholar] [CrossRef] [PubMed]
- Highton, J.; Hung, N.; Hessian, P.; Wilsher, M. Pulmonary Rheumatoid Nodules Demonstrating Features Usually Associated with Rheumatoid Synovial Membrane. Rheumatology 2007, 46, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Rasker, J.J.; Kuipers, F.C. Are Rheumatoid Nodules Caused by Vasculitis? A Study of 13 Early Cases. Ann. Rheum. Dis. 1983, 42, 384. [Google Scholar] [CrossRef] [PubMed]
- Hessian, P.A.; Highton, J.; Kean, A.; Sun, C.K.; Chin, M. Cytokine Profile of the Rheumatoid Nodule Suggests That It Is a Th1 Granuloma. Arthritis Rheum. 2003, 48, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Avnon, L.S.; Abu-Shakra, M.; Flusser, D.; Heimer, D.; Sion-Vardy, N. Pleural Effusion Associated with Rheumatoid Arthritis: What Cell Predominance to Anticipate? Rheumatol. Int. 2007, 27, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Magaki, S.; Chang, E.; Hammond, R.R.; Yang, I.; Mackenzie, I.R.A.; Chou, B.T.; Choi, S.I.; Jen, J.C.; Pope, W.B.; Bell, D.A.; et al. Two Cases of Rheumatoid Meningitis. Neuropathology 2016, 36, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kumon, Y.; Kataoka, H.; Matsumura, Y.; Takeuchi, H.; Doi, Y. Asymptomatic Pericardial Effusion in Patients with Rheumatoid Arthritis. Cardiology 2008, 110, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Mankad, R.; Ball, C.A.; Myasoedova, E.; Matteson, E.L. Non-Atherosclerotic Cardiac Manifestations of Rheumatoid Arthritis. In Handbook of Cardiovascular Disease Management in Rheumatoid Arthritis; Semb, A.G., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–38. ISBN 978-3-319-26782-1. [Google Scholar]
- Halla, J.T.; Schrohenloher, R.E.; Koopman, W.J. Local Immune Responses in Certain Extra-Articular Manifestations of Rheumatoid Arthritis. Ann. Rheum. Dis. 1992, 51, 698–701. [Google Scholar] [CrossRef]
- Hara, K.S.; Ballard, D.J.; Ilstrup, D.M.; Connolly, D.C.; Vollertsen, R.S. Rheumatoid Pericarditis: Clinical Features and Survival. Medicine 1990, 69, 81–91. [Google Scholar] [CrossRef]
- Komarla, A.; Yu, G.H.; Shahane, A. Pleural Effusion, Pneumothorax, and Lung Entrapment in Rheumatoid Arthritis. J. Clin. Rheumatol. 2015, 21, 211–215. [Google Scholar] [CrossRef]
- Ozaki, Y.; Tanaka, A.; Shimamoto, K.; Amuro, H.; Kawakami, K.; Son, Y.; Ito, T.; Wada, T.; Nomura, S. A Case of Rheumatoid Pericarditis Associated with a High IL-6 Titer in the Pericardial Fluid and Tocilizumab Treatment. Mod. Rheumatol. 2011, 21, 302–304. [Google Scholar] [CrossRef]
- Schatz, A.; Trankle, C.; Yassen, A.; Chipko, C.; Rajab, M.; Abouzaki, N.; Abbate, A. Resolution of Pericardial Constriction with Anakinra in a Patient with Effusive-Constrictive Pericarditis Secondary to Rheumatoid Arthritis. Int. J. Cardiol. 2016, 223, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Ristić, A.D.; Pankuweit, S.; Maksimović, R.; Moosdorf, R.; Maisch, B. Pericardial Cytokines in Neoplastic, Autoreactive, and Viral Pericarditis. Heart Fail. Rev. 2013, 18, 345–353. [Google Scholar] [CrossRef]
- McClelland, T.J.; Penfold, R.; Kluzek, S.; Nagra, N.S. A Large Chronic Pericardial Effusion in an Ultramarathon Runner with Anti-CCP Positive Rheumatoid Arthritis. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hoshi, K.; Sekijima, Y.; Matsuda, M.; Hashimoto, T.; Otani, M.; Suzuki, A.; Ikeda, S. Rheumatoid Meningitis: An Autopsy Report and Review of the Literature. Clin. Rheumatol. 2003, 22, 475–480. [Google Scholar] [CrossRef]
- Nihat, A.; Chinthapalli, K.; Bridges, L.; Johns, P.; Sofat, N.; Moynihan, B. Rheumatoid Meningitis. Pract. Neurol. 2016, 16, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Markenson, J.A.; McDougal, J.S.; Tsairis, P.; Lockshin, M.D.; Christian, C.L. Rheumatoid Meningitis: A Localized Immune Process. Ann. Intern. Med. 1979, 90, 786–789. [Google Scholar] [CrossRef] [PubMed]
- DeQuattro, K.; Imboden, J.B. Neurologic Manifestations of Rheumatoid Arthritis. Rheum. Dis. Clin. 2017, 43, 561–571. [Google Scholar] [CrossRef]
- Ramos-Remus, C.; Duran-Barragan, S.; Castillo-Ortiz, J.D. Beyond the Joints. Clin. Rheumatol. 2012, 31, 1–12. [Google Scholar] [CrossRef]
- Turk, M.A.; Hayworth, J.L.; Nevskaya, T.; Pope, J.E. Ocular Manifestations in Rheumatoid Arthritis, Connective Tissue Disease, and Vasculitis: A Systematic Review and Metaanalysis. J. Rheumatol. 2021, 48, 25–34. [Google Scholar] [CrossRef]
- Dammacco, R.; Guerriero, S.; Alessio, G.; Dammacco, F. Natural and Iatrogenic Ocular Manifestations of Rheumatoid Arthritis: A Systematic Review. Int. Ophthalmol. 2022, 42, 689–711. [Google Scholar] [CrossRef]
- Marsovszky, L.; Resch, M.D.; Németh, J.; Toldi, G.; Medgyesi, E.; Kovács, L.; Balog, A. In Vivo Confocal Microscopic Evaluation of Corneal Langerhans Cell Density, and Distribution and Evaluation of Dry Eye in Rheumatoid Arthritis. Innate Immun. 2012, 19, 348–354. [Google Scholar] [CrossRef]
- Bitirgen, G.; Kucuk, A.; Ergun, M.C.; Satirtav, G.; Malik, R.A. Corneal Nerve Loss and Increased Langerhans Cells Are Associated with Disease Severity in Patients with Rheumatoid Arthritis. Eye 2023. Published online. [Google Scholar] [CrossRef]
- Villani, E.; Galimberti, D.; Del Papa, N.; Nucci, P.; Ratiglia, R. Inflammation in Dry Eye Associated with Rheumatoid Arthritis: Cytokine and in Vivo Confocal Microscopy Study. Innate Immun. 2013, 19, 420–427. [Google Scholar] [CrossRef]
- Kang, M.H.; Kim, M.K.; Lee, H.J.; Lee, H.I.; Wee, W.R.; Lee, J.H. Interleukin-17 in Various Ocular Surface Inflammatory Diseases. J. Korean Med. Sci. 2011, 26, 938–944. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D., III; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 Disrupts Corneal Barrier Following Desiccating Stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Elsheikh, A.; Alweis, R. Is This a Worrisome Red Eye? Episcleritis in the Primary Care Setting. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Bhamra, M.S.; Gondal, I.; Amarnani, A.; Betesh, S.; Zhyvotovska, A.; Scott, W.; Rodriguez-Alvarez, M.; Lazzaro, D.R.; McFarlane, I.M. Ocular Manifestations of Rheumatoid Arthritis: Implications of Recent Clinical Trials. Int. J. Clin. Res. Trials 2019, 4, 139. [Google Scholar] [CrossRef]
- Tong, L.; Thumboo, J.; Tan, Y.K.; Wong, T.-Y.; Albani, S. The Eye: A Window of Opportunity in Rheumatoid Arthritis? Nat. Rev. Rheumatol. 2014, 10, 552–560. [Google Scholar] [CrossRef]
- Nishio, Y.; Taniguchi, H.; Takeda, A.; Hori, J. Immunopathological Analysis of a Mouse Model of Arthritis-Associated Scleritis and Implications for Molecular Targeted Therapy for Severe Scleritis. Int. J. Mol. Sci. 2022, 23, 341. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, W.; Wu, J.; Zhang, H.; Zhou, H. Peripheral Ulcerative Keratitis Associated with Autoimmune Disease: Pathogenesis and Treatment. J. Ophthalmol. 2017, 2017, 7298026. [Google Scholar] [CrossRef]
- Bowman, S.J. Hematological Manifestations of Rheumatoid Arthritis. Scand. J. Rheumatol. 2002, 31, 251–259. [Google Scholar] [CrossRef]
- Papadaki, H.A.; Kritikos, H.D.; Valatas, V.; Boumpas, D.T.; Eliopoulos, G.D. Anemia of Chronic Disease in Rheumatoid Arthritis Is Associated with Increased Apoptosis of Bone Marrow Erythroid Cells: Improvement Following Anti-Tumor Necrosis Factor-α Antibody Therapy. Blood 2002, 100, 474–482. [Google Scholar] [CrossRef]
- Voulgari, P.V.; Kolios, G.; Papadopoulos, G.K.; Katsaraki, A.; Seferiadis, K.; Drosos, A.A. Role of Cytokines in the Pathogenesis of Anemia of Chronic Disease in Rheumatoid Arthritis. Clin. Immunol. 1999, 92, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, W.; Al-Rubaie, H.A.; Shihab, S. Studying Anemia of Chronic Disease and Iron Deficiency in Patients with Rheumatoid Arthritis by Iron Status and Circulating Hepcidin. Hematol. Rep. 2019, 11, 7708. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Molad, Y. Hematological Manifestations among Patients with Rheumatic Diseases. Acta Haematol. 2021, 144, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Balint, G.P.; Balint, P. V Felty’s Syndrome. Best Pract. Res. Clin. Rheumatol. 2004, 18, 631–645. [Google Scholar] [CrossRef]
- Burks, E.J.; Loughran, T.P. Pathogenesis of Neutropenia in Large Granular Lymphocyte Leukemia and Felty Syndrome. Blood Rev. 2006, 20, 245–266. [Google Scholar] [CrossRef]
- Liu, X.; Loughran, T.P.J. The Spectrum of Large Granular Lymphocyte Leukemia and Felty’s Syndrome. Curr. Opin. Hematol. 2011, 18, 254–259. [Google Scholar] [CrossRef]
- Bowman, S.J.; Sivakumaran, M.; Snowdendegs, N.; Bhavnani, M.; Hall, M.A.; Panayi, G.S.; Lanchbury, J.S. The Large Granular Lymphocyte Syndrome with Rheumatoid Arthritis. Immunogenetic Evidence for a Broader Definition of Felty’s Syndrome. Arthritis Rheum. 1994, 37, 1326–1330. [Google Scholar] [CrossRef]
- Savola, P.; Brück, O.; Olson, T.; Kelkka, T.; Kauppi, M.J.; Kovanen, P.E.; Kytölä, S.; Sokka-Isler, T.; Loughran, T.P.; Leirisalo-Repo, M.; et al. Somatic STAT3 Mutations in Felty Syndrome: An Implication for a Common Pathogenesis with Large Granular Lymphocyte Leukemia. Haematologica 2018, 103, 304–312. [Google Scholar] [CrossRef]
- Lamy, T.; Moignet, A.; Loughran, T.P., Jr. LGL Leukemia: From Pathogenesis to Treatment. Blood 2017, 129, 1082–1094. [Google Scholar] [CrossRef]
- Hellmich, B.; Csernok, E.; Schatz, H.; Gross, W.L.; Schnabel, A. Autoantibodies against Granulocyte Colony-Stimulating Factor in Felty’s Syndrome and Neutropenic Systemic Lupus Erythematosus. Arthritis Rheum. 2002, 46, 2384–2391. [Google Scholar] [CrossRef]
- Dwivedi, N.; Radic, M. Neutrophil Activation and B-Cell Stimulation in the Pathogenesis of Felty’s Syndrome. Pol. Arch. Med. Wewn. 2012, 122, 374–379. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ziff, M.; Hurd, E.R. Increased Endothelial Cell Adherence, Aggregation, and Superoxide Generation by Neutrophils Incubated in Systemic Lupus Erythematosus and Felty’s Syndrome Sera. Arthritis Rheum. 1982, 25, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, F.C.; Lafeber, G.J.; de Vries, E.; van Krieken, J.H.; Cats, A. Immune Complexes and the Pathogenesis of Neutropenia in Felty’s Syndrome. Ann. Rheum. Dis. 1986, 45, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Upadhyay, J.; Neeli, I.; Khan, S.; Pattanaik, D.; Myers, L.; Kirou, K.A.; Hellmich, B.; Knuckley, B.; Thompson, P.R.; et al. Felty’s Syndrome Autoantibodies Bind to Deiminated Histones and Neutrophil Extracellular Chromatin Traps. Arthritis Rheum. 2012, 64, 982–992. [Google Scholar] [CrossRef]
- van Krieken, J.H.J.M.; Breedveld, F.C.; te Velde, J. The Spleen in Felty’s Syndrome: A Histological, Morphometrical, and Immunohistochemical Study. Eur. J. Haematol. 1988, 40, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rashba, E.J.; Rowe, J.M.; Packman, C.H. Treatment of the Neutropenia of Felty Syndrome. Blood Rev. 1996, 10, 177–184. [Google Scholar] [CrossRef]
- Pietschmann, P.; Butylina, M.; Kerschan-Schindl, K.; Sipos, W. Mechanisms of Systemic Osteoporosis in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 8740. [Google Scholar] [CrossRef]
- Llorente, I.; García-Castañeda, N.; Valero, C.; González-Álvaro, I.; Castañeda, S. Osteoporosis in Rheumatoid Arthritis: Dangerous Liaisons. Front. Med. 2020, 7, 601618. [Google Scholar] [CrossRef]
- Qiu, J.; Lu, C.; Zhang, L.; Zhou, X.; Zou, H. Osteoporosis in Patients with Rheumatoid Arthritis Is Associated with Serum Immune Regulatory Cellular Factors. Clin. Rheumatol. 2022, 41, 2685–2693. [Google Scholar] [CrossRef]
- Fessler, J.; Husic, R.; Schwetz, V.; Lerchbaum, E.; Aberer, F.; Fasching, P.; Ficjan, A.; Obermayer-Pietsch, B.; Duftner, C.; Graninger, W.; et al. Senescent T-Cells Promote Bone Loss in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Kleyer, A.; Finzel, S.; Rech, J.; Manger, B.; Krieter, M.; Faustini, F.; Araujo, E.; Hueber, A.J.; Harre, U.; Engelke, K.; et al. Bone Loss before the Clinical Onset of Rheumatoid Arthritis in Subjects with Anticitrullinated Protein Antibodies. Ann. Rheum. Dis. 2014, 73, 854. [Google Scholar] [CrossRef]
- Hecht, C.; Englbrecht, M.; Rech, J.; Schmidt, S.; Araujo, E.; Engelke, K.; Finzel, S.; Schett, G. Additive Effect of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor on Bone Erosions in Patients with RA. Ann. Rheum. Dis. 2015, 74, 2151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, R.; Gu, Z.; Dong, C.; Guo, G.; Li, L. Effects of Glucocorticoids on Osteoporosis in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2020, 31, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Furuya, T.; Inoue, E.; Tanaka, E.; Ikari, K.; Yamanaka, H.; Harigai, M. Vitamin D Deficiency Is a Risk Factor for New Fractures in Japanese Postmenopausal Women with Rheumatoid Arthritis: Results from the IORRA Cohort Study. Arch. Osteoporos. 2021, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Oelzner, P.; Schwabe, A.; Lehmann, G.; Eidner, T.; Franke, S.; Wolf, G.; Hein, G. Significance of Risk Factors for Osteoporosis Is Dependent on Gender and Menopause in Rheumatoid Arthritis. Rheumatol. Int. 2008, 28, 1143–1150. [Google Scholar] [CrossRef]
- Stanmore, E.K.; Oldham, J.; Skelton, D.A.; O’Neill, T.; Pilling, M.; Campbell, A.J.; Todd, C. Risk Factors for Falls in Adults with Rheumatoid Arthritis: A Prospective Study. Arthritis Care Res. 2013, 65, 1251–1258. [Google Scholar] [CrossRef]
- Kishore, S.; Maher, L.; Majithia, V. Rheumatoid Vasculitis: A Diminishing Yet Devastating Menace. Curr. Rheumatol. Rep. 2017, 19, 39. [Google Scholar] [CrossRef]
- Voskuyl, A.E.; van Duinen, S.G.; Zwinderman, A.H.; Breedveld, F.C.; Hazes, J.M.W. The Diagnostic Value of Perivascular Infiltrates in Muscle Biopsy Specimens for the Assessment of Rheumatoid Vasculitis. Ann. Rheum. Dis. 1998, 57, 114. [Google Scholar] [CrossRef]
- Makol, A.; Matteson, E.L.; Warrington, K.J. Rheumatoid Vasculitis: An Update. Curr. Opin. Rheumatol. 2015, 27, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.D.; David-Vaudey, E.; Pai, M.; Lum, R.F.; Criswell, L.A. Particular HLA–DRB1 Shared Epitope Genotypes Are Strongly Associated with Rheumatoid Vasculitis. Arthritis Rheum. 2004, 50, 3476–3484. [Google Scholar] [CrossRef]
- Struthers, G.R.; Scott, D.L.; Delamere, J.P.; Sheppeard, H.; Kitt, M. Smoking and Rheumatoid Vasculitis. Rheumatol. Int. 1981, 1, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.G.; Bacon, P.A.; Allen, C.; Elson, C.J.; Wallington, T. IgG Rheumatoid Factor, Complement and Immune Complexes in Rheumatoid Synovitis and Vasculitis: Comparative and Serial Studies during Cytotoxic Therapy. Clin. Exp. Immunol. 1981, 43, 54–63. [Google Scholar] [PubMed]
- Elson, C.J.; Scott, D.G.; Blake, D.R.; Bacon, P.A.; Holt, P.D. Complement-Activating Rheumatoid-Factor-Containing Complexes in Patients with Rheumatoid Vasculitis. Ann. Rheum. Dis. 1983, 42, 147. [Google Scholar] [CrossRef]
- Flipo, R.M.; Cardon, T.; Copin, M.C.; Vandecandelaere, M.; Duquesnoy, B.; Janin, A. ICAM-1, E-Selectin, and TNF α Expression in Labial Salivary Glands of Patients with Rheumatoid Vasculitis. Ann. Rheum. Dis. 1997, 56, 41. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.-H.; Moore, B.E.; Nakajima, T.; Scholl, D.; Schaid, D.J.; Weyand, C.M.; Goronzy, J.J. Major Histocompatibility Complex Class I–Recognizing Receptors Are Disease Risk Genes in Rheumatoid Arthritis. J. Exp. Med. 2001, 193, 1159–1168. [Google Scholar] [CrossRef]
- Kapoor, T.; Bathon, J. Renal Manifestations of Rheumatoid Arthritis. Rheum. Dis. Clin. 2018, 44, 571–584. [Google Scholar] [CrossRef]
- Giles, J.T.; Simon, L.S.; Pope, J.; Paik, J.S.; Grabner, M.; Quebe, A.; Kannowski, C.L.; Salinas, C.A.; Curtis, J.R. Prevalence of Renal Impairment in a US Commercially Insured Rheumatoid Arthritis Population: A Retrospective Analysis. Rheumatol. Ther. 2021, 8, 1383–1391. [Google Scholar] [CrossRef]
- Daoussis, D.; Panoulas, V.F.; Antonopoulos, I.; John, H.; Toms, T.E.; Wong, P.; Nightingale, P.; Douglas, K.M.J.; Kitas, G.D. Cardiovascular Risk Factors and Not Disease Activity, Severity or Therapy Associate with Renal Dysfunction in Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2010, 69, 517. [Google Scholar] [CrossRef]
- Tokunaga, N.K.; Noda, R.; Kaneoka, H.; Ogahara, S.; Murata, T.; Hiratsuka, T.; Michinaga, I.; Naito, S. Association between HLA-DRB1*15 and Japanese Patients with Rheumatoid Arthritis Complicated by Renal Involvement. Nephron 1999, 81, 165–171. [Google Scholar] [CrossRef]
- Saccon, F.; Gatto, M.; Larosa, M.; Ometto, F.; Felicetti, M.; Padoan, R.; Zen, M. Diagnostic and Prognostic Role of Renal Histopathology in Rheumatic Diseases. Reumatismo 2018, 70, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Korpela, M.M.; Mustonen, J.T.; Teppo, A.; Helin, H.J.; Pasternack, A.I. Mesangial Glomerulonephritis as an Extra-Articular Manifestation of Rheumatoid Arthritis. Br. J. Rheumatol. 1997, 36, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Muraguchi, A.; Iwano, M.; Matsuda, T.; Hirayama, T.; Yamada, H.; Fujii, Y.; Dohi, K.; Ishikawa, H.; Ohmoto, Y. Involvement of IL-6 in Mesangial Proliferative Glomerulonephritis. J. Immunol. 1989, 143, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Yoshinaga, Y.; Yamasaki, Y.; Morita, Y.; Hashimoto, H.; Yamamura, M. Renal Involvement in Rheumatoid Arthritis: Analysis of Renal Biopsy Specimens from 100 Patients. Mod. Rheumatol. 2002, 12, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Ueno, M.; Nishi, S.; Shimada, H.; Hasegawa, H.; Watanabe, T.; Kuroda, T.; Sato, T.; Maruyama, Y.; Arakawa, M. Analysis of Renal Pathology and Drug History in 158 Japanese Patients with Rheumatoid Arthritis. Clin. Nephrol. 1998, 50, 154–160. [Google Scholar]
- Gois, M.; Carvalho, F.; Sousa, H.; Ferreira, A.C.; Sousa, J.; Nolasco, F. Renal Involvement in Rheumatoid Arthritis: Analysis of 53 Renal Biopsies. Port. J. Nephrol. Hypertens. 2017, 31, 25–30. [Google Scholar]

| Extraarticular Manifestation | Proposed Pathogenic Mechanisms |
|---|---|
| Atherosclerosis |
|
| Non-ischemic heart disease | |
| Interstitial lung disease | |
| Bronchiectasis |
|
| Rheumatoid nodules |
|
| Membrane involvement |
|
| Neurologic manifestations |
|
| Ocular involvement | |
| Anemia | |
| Felty syndrome | |
| Osteoporosis | |
| Vasculitis | |
| Renal manifestations |
| Condition/EAM | Mechanism Studied (Molecular/Cellular/Both) | Type of Study | Number of Patients/Controls | Points of Interest of Studies Used in This Review Article | References |
|---|---|---|---|---|---|
| Atherosclerosis | Molecular | In vitro, murine, review | N/A | NO, TNF, ADMA, LOX-1, IL-6 | [26,27,28,30,31,33,34,36,37,38,56] |
| Molecular | Systematic review | 582 */612 ** | ADMA | [29] | |
| Molecular | In vitro/murine/cross-sectional | 9 */6 ** | TNF-α, LOX1/NFκB/Arg2 | [32] | |
| Molecular | Prospective longitudinal | 64 */12 ** | L5 LDL-C | [44] | |
| Molecular | Cohort prospective | 93 */41 ** | L5 LDL-C | [45] | |
| Molecular | Cross-sectional | 40 */40 ** | HDL, MPO | [49] | |
| Molecular | Cross-sectional | 178 */223 ** | HDL, Cholesterol efflux | [50] | |
| Molecular | Cross-sectional/in vitro | 45 **/44 ** | HDL, MPO | [51] | |
| Molecular | Review | N/A | piHDL | [47] | |
| Molecular | Cohort/cross sectional | 132 * | piHDL | [48] | |
| Molecular | Cross-sectional | 82 */110 ** | HDL antibodies | [52] | |
| Cellular | Cross-sectional | 125 ** | CD14 + CD16+ monocytes | [53] | |
| Cellular | Cross sectional | 72 * | CD14 + CD16+ monocytes | [54] | |
| Molecular | Murine/cross-sectional | 12 */7 ** | Chol. efflux, HSPC monocyte | [55] | |
| Molecular | Murine/cross-sectional | 513 families ** | Gp 130 receptor | [58] | |
| Cellular | Cross-sectional | 87 */33 ** | CD4 + CD28null T-cell | [64] | |
| Both | Cohort/in vitro | 106 */40 ** | NETosis | [66] | |
| Molecular | Cohort/in vitro | 20* | NETosis, IL-6 | [67] | |
| NIHD | N/A | Cross-sectional | 45 */45 ** | MRI, LV morphology | [70] |
| N/A | Cross-sectional/longitudinal | 119 */27 ** | PET-CT, FDG uptake | [71] | |
| molecular | In vitro, murine, review | N/A | TNF-α, IL-1, β-adrenergic, ACPA | [72,73,76,77,80] | |
| molecular | Double-blind randomized | 23 */19 * | IL-1 | [75] | |
| molecular | Cross-sectional | 150 * | ACPAs | [78] | |
| molecular | Cross-sectional | 17 */15 ** | Citrullination | [79] | |
| ILD | N/A | Review | N/A | ILD patterns, inflammation origin theories | [14,90,91] |
| molecular | Cross-sectional | 14 * + 49 ± /21 ** | ACPAs, IgA ACPAs, | [92] | |
| both | Observational | 24 */84 ** | ACPAs, lymphocytes | [93] | |
| molecular | Case-control | 101 */1010 ** | HLA, genetic analysis | [94] | |
| molecular | Case-control/cross-sectional | 610 */773 * | HLA, genetic analysis | [95] | |
| cellular | Review | N/A | DCs, T-cells | [96] | |
| both | In vitro, murine | N/A | ACPA ICs, nicotine, GERD EMT, | [97,105,107] | |
| molecular | Cross-sectional | 55 */36 ** | ACPAs, NETosis, cytokines | [98] | |
| molecular | In vitro/cross-sectional | 4 */6 ** | IL-17A | [99] | |
| molecular | Cross-sectional | 8 ** | TGF-β1 | [100] | |
| cellular | Cohort retrospective | 31 * | Smoking, DCs | [101] | |
| molecular | Cohort retrospective/murine/in vitro | 4 **/4 ** | Smoking, NETosis | [102] | |
| cellular | Cross-sectional/In vitro/murine | 24 **/8 ** | Smoking, DCs | [104] | |
| Bronchiectasis | N/A | reviews | N/A | Pathogenesis theories | [86,108,110,111] |
| N/A | Retrospective | 40 * + 7 ** | Infections, biologics | [109] | |
| molecular | Cohort prospective | 165 **/34 ** | IL-17a, IL-1β, IL-8 | [112] | |
| molecular | Cross-sectional | 40 **/20 ** | TNF-α, IIL-8, IL-10 | [113] | |
| molecular | Cohort prospective | 12 * + 42 ±±/15 ** | ACPAs | [114] | |
| molecular | Cross-sectional | 50 */50 * + 79 ** | ACPAs | [115] | |
| RN | both | Reviews | N/A | Pathogenesis theories | [116,117] |
| cellular | Observational | 11 * | Histology | [120] | |
| cellular | Case report | 2 * | Histology, B-cells | [122] | |
| cellular | Cross-sectional | 13 * | Vasculitis | [123] | |
| molecular | Cross-sectional | 10 * | IL-1, IL-12, IL-18, IL-15, IL-10 | [124] | |
| Pleural effusion | both | Case series/literature review | 2 * + 28 * | Cytology, biochem. analysis | [125] |
| both | Case report/literature review | 1 * | Cytology, RF, ICs, complement | [131] | |
| both | Literature review | N/A | Cytology, RF, ICs, complement | [129] | |
| Pericardial effusion | molecular | Case reports | 1 *, 1 *, 1 * | IL-6, IL-1, ACPA | [132,133,135] |
| molecular | Cross-sectional | 51 ** | TNF-α, IL-6, TGF-β1, IFN-γ | [134] | |
| molecular | Cross-sectional | 20 */67 * | Albumins, RF | [127] | |
| Meningitis | both | Case report/literature review | 48 * | Cytology, RF, biochem. analysis | [126] |
| molecular | Case report/literature review | 24 * | IL-6 ***, inflammation site | [136] | |
| molecular | Case report | 1 * | RF, ICs, inflammation site | [138] | |
| Ocular | cellular | Cross-sectional | 52 */24 ** | LCs | [143] |
| cellular | Cross-sectional | 50 */35 ** | LCs, nerve fibers | [144] | |
| both | Cohort prospective | 24 **** | LCs, IL-1a,-6, -8, TNF-a | [145] | |
| molecular | Cross-sectional | 142 **/28 ** | IL-17 | [146] | |
| molecular | Review articles | N/A | IL-1, TNF-α, MMP | [149,150,152] | |
| both | Murine/case report | 1 * | Cytology, IL-6, TNF-α | [151] | |
| Anemia | both | Cohort prospective/in vitro | 45 */24 ** | TNF-α, apoptosis | [154] |
| Cross-sectional | 105 */127 * | TNFα, IL-1β, IL-6 | [155] | ||
| N/A | Review | N/A | Etiology, Iron-deficiency | [153] | |
| Molecular | Case-control | 34 */17 * | Hepcidin, iron | [156] | |
| Felty syndrome | both | Reviews | N/A | BM histology, T-cells, humoral | [157,158,159,165] |
| molecular | Cross-sectional | 14 */20 * + 17 ** | STAT-3 | [162] | |
| molecular | Cross-sectional | 15 */16 * | G-CSF antibodies | [164] | |
| cellular | Cross-sectional | 20 */24 * + 34 ** | Neutrophils adherence | [166] | |
| molecular | Cross-sectional/murine | 8 */6 * + 4 ** | FS sera, mice neutrophils | [167] | |
| molecular | Cross-sectional | 23 */37 * + 10 ** | NETosis | [168] | |
| cellular | Case series | 3 * | Spleen histopathology | [169] | |
| N/A | Literature review | 118 * | Splenectomy | [170] | |
| Osteoporosis | N/A | Review | N/A | Risk factors, inflammation | [171,172] |
| molecular | Cross-sectional | 62 */54 * | TNFα, Il-4, IL-6, IL-10, IL-17 | [173] | |
| both | Cohort prospective/in vitro | 107 */113 ** | T-cells, TNF-α, IL-6, IL-15 | [174] | |
| molecular | Cross-sectional | 15 ±±/15 ** | ACPAs | [175] | |
| molecular | Cohort | 238 * | ACPAs, RF | [176] | |
| N/A | Meta-analysis/Systematic review | 46,711 */857 ** | Glucocorticoids | [177] | |
| molecular | Cohort prospective | 2567 * | Vitamin D | [178] | |
| N/A | Observational retrospective | 551 * | Gender, menopause | [179] | |
| N/A | Cohort prospective | 535 * | Fall risk | [180] | |
| Vasculitis | molecular | Review | N/A | RF, ACPAs | [183] |
| cellular | Cross-sectional | 12 */14 * | Histopathology | [182] | |
| molecular | Meta-analysis | 129 */1439 * | HLA-DRB1 shared epitope | [184] | |
| molecular | Cohort prospective | 11 */11 * | RF ICs, C3, complement | [186] | |
| molecular | Cross-sectional | 16 */28 * + 9 ** | RF ICs, complement | [187] | |
| Renal | N/A | reviews | N/A | Histopathology, treatment | [190,194] |
| N/A | Cohort retrospective | 128,062 * | Renal impairment prevalence | [191] | |
| N/A | Cross-sectional | 400 * | Risk factors | [192] | |
| molecular | Cross-sectional | 41 */134 * | HLA antigens | [193] | |
| molecular | Cohort retrospective | 37 */37 ** | Immunofluorescence, RF, ICs | [195] | |
| N/A | Cohort retrospective | 100 * | medication | [197] | |
| N/A | Cohort retrospective | 158 * | medication | [198] | |
| N/A | Cohort retrospective | 53 * | medication | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrović, J.; Hrkač, S.; Tečer, J.; Golob, M.; Ljilja Posavec, A.; Kolar Mitrović, H.; Grgurević, L. Pathogenesis of Extraarticular Manifestations in Rheumatoid Arthritis—A Comprehensive Review. Biomedicines 2023, 11, 1262. https://doi.org/10.3390/biomedicines11051262
Mitrović J, Hrkač S, Tečer J, Golob M, Ljilja Posavec A, Kolar Mitrović H, Grgurević L. Pathogenesis of Extraarticular Manifestations in Rheumatoid Arthritis—A Comprehensive Review. Biomedicines. 2023; 11(5):1262. https://doi.org/10.3390/biomedicines11051262
Chicago/Turabian StyleMitrović, Joško, Stela Hrkač, Josip Tečer, Majda Golob, Anja Ljilja Posavec, Helena Kolar Mitrović, and Lovorka Grgurević. 2023. "Pathogenesis of Extraarticular Manifestations in Rheumatoid Arthritis—A Comprehensive Review" Biomedicines 11, no. 5: 1262. https://doi.org/10.3390/biomedicines11051262
APA StyleMitrović, J., Hrkač, S., Tečer, J., Golob, M., Ljilja Posavec, A., Kolar Mitrović, H., & Grgurević, L. (2023). Pathogenesis of Extraarticular Manifestations in Rheumatoid Arthritis—A Comprehensive Review. Biomedicines, 11(5), 1262. https://doi.org/10.3390/biomedicines11051262






