Licochalcone A Exerts Anti-Cancer Activity by Inhibiting STAT3 in SKOV3 Human Ovarian Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Cell Viability Assay
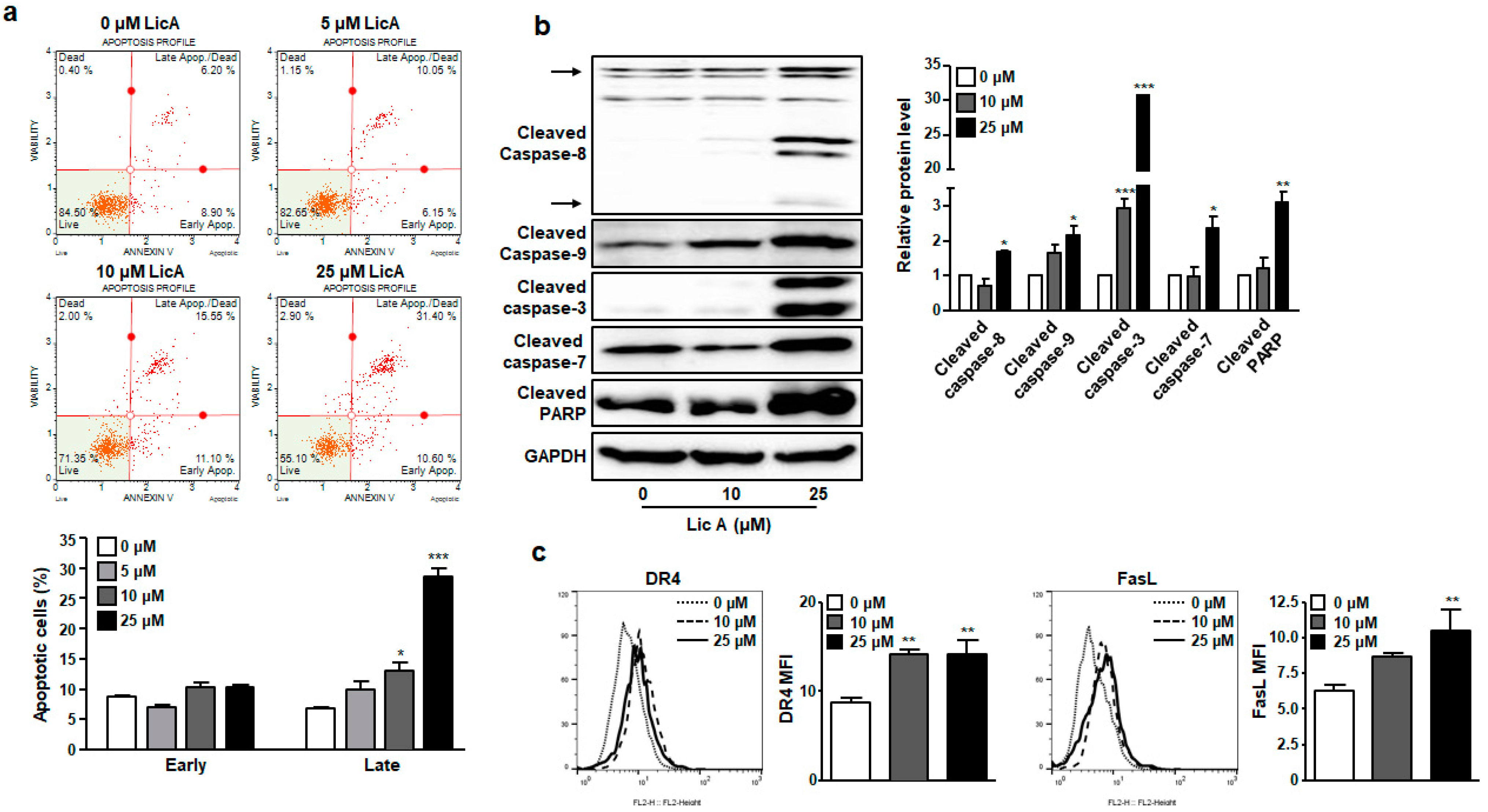
2.3. Measurement of Apoptotic Cells
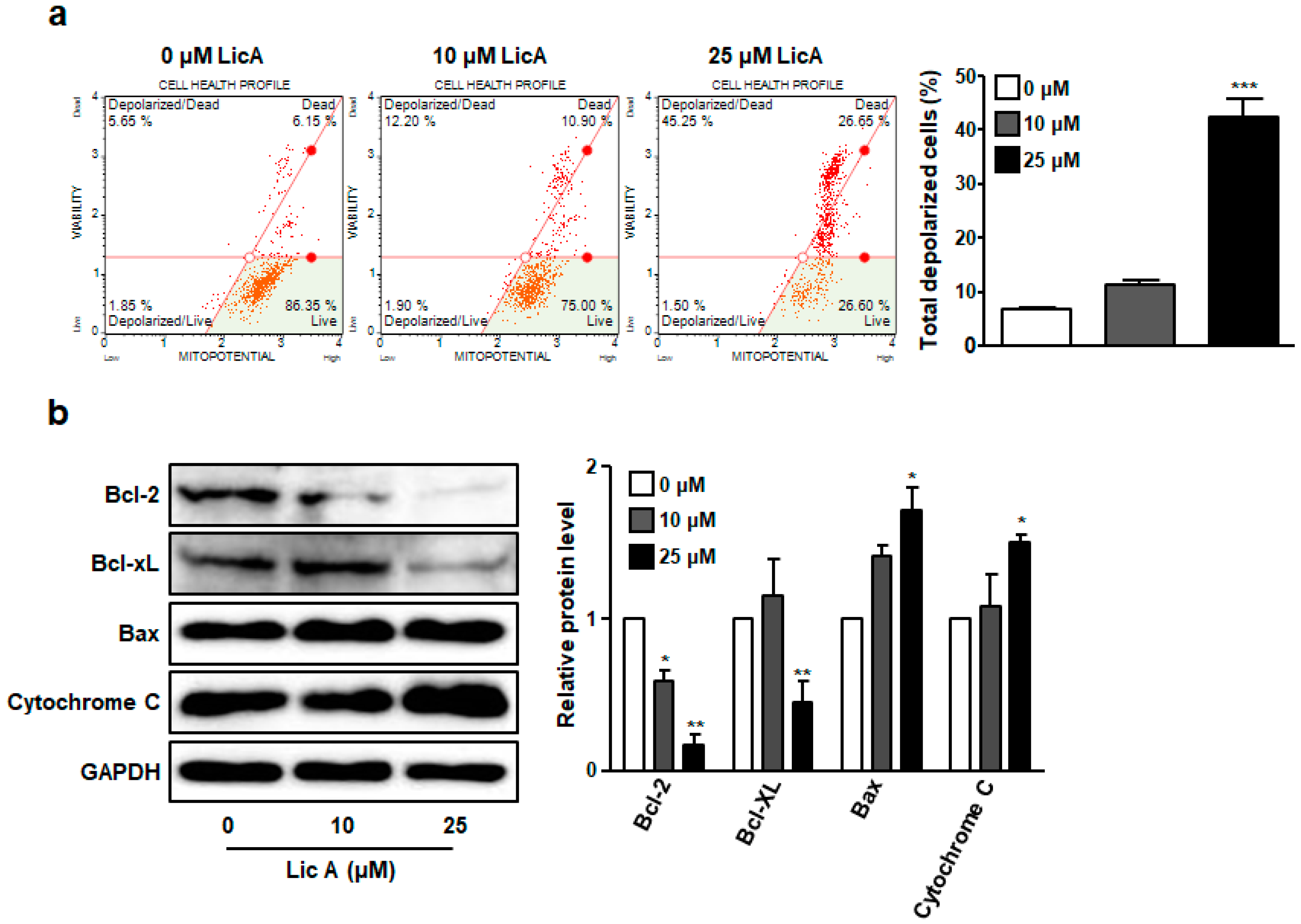
2.4. Determination of Mitochondrial Membrane Potential (MMP)
2.5. Measurement of Reactive Oxygen Species (ROS) Production
2.6. Western Blot Analysis
2.7. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. LicA Inhibits Proliferation of SKOV3 Cells by Inducing Apoptosis
3.2. LicA Reduces Mitochondrial Membrane Potential in SKOV3 Cells
3.3. LicA Suppresses Intracellular ROS Generation
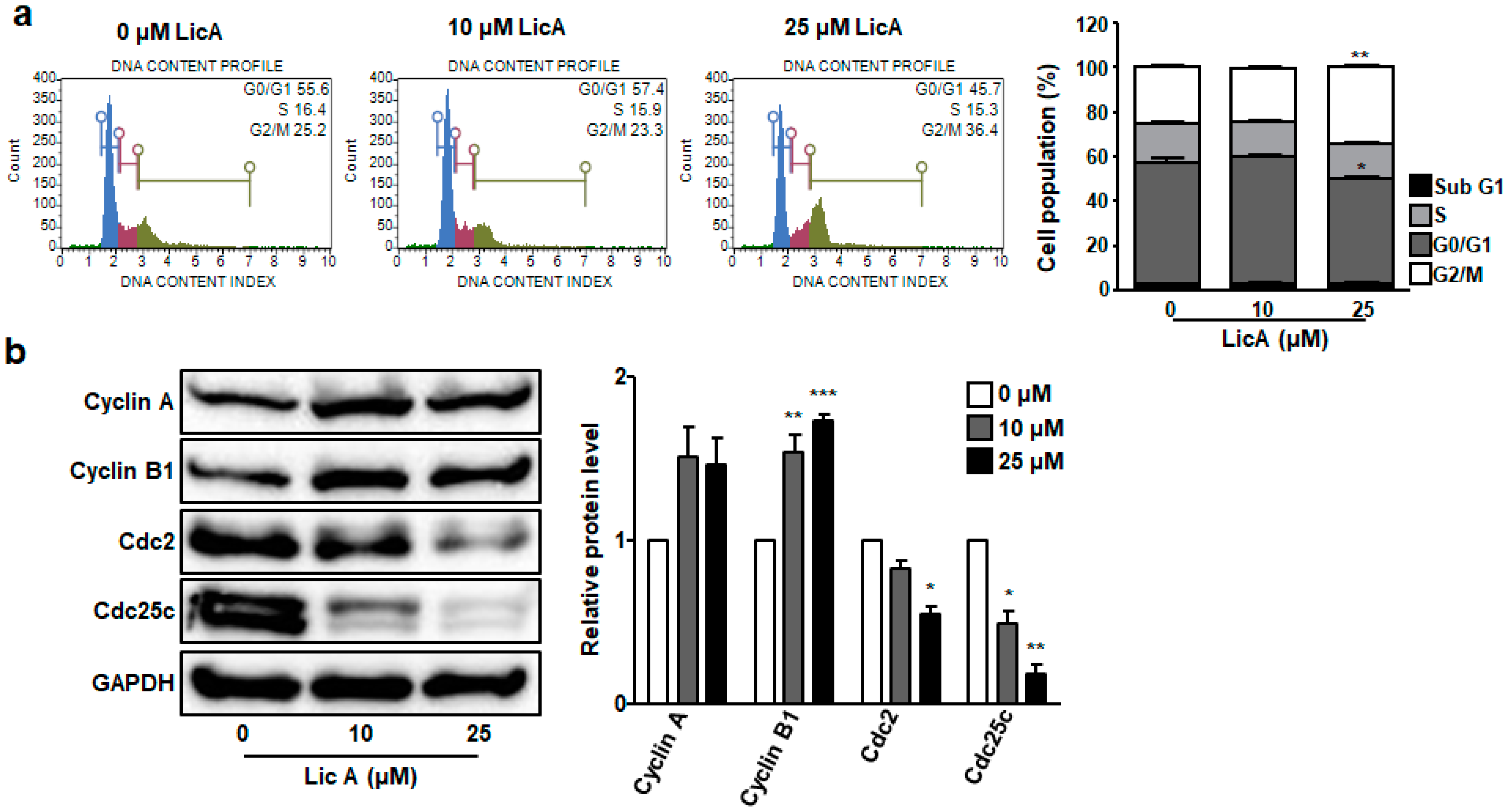
3.4. LicA Induces G2/M Phase Cell Cycle Arrest in SKOV3 Cells
3.5. LicA Blocks STAT3 Phosphorylation and Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2019. Cancer Res. Treat. 2022, 54, 330–344. [Google Scholar] [CrossRef]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Penny, S.M. Ovarian cancer: An overview. Radiol. Technol. 2020, 91, 561–575. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Kunnumakkara, A.B.; Harikumar, K.B.; Gupta, S.R.; Tharakan, S.T.; Koca, C.; Dey, S.; Sung, B. Signal transducer and activator of transcription-3, inflammation, and cancer: How intimate is the relationship? Ann. N. Y. Acad. Sci. 2009, 1171, 59–76. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Kasembeli, M.M.; Robinson, P.; Tweardy, D.J. Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: Rationale, progress, and caution. Pharmacol. Rev. 2020, 72, 486–526. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.L.; Naora, H.; Liu, J.; Cheng, W.; Montell, D.J. Activated signal transducer and activator of transcription (STAT) 3: Localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004, 64, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Page, C.; Reynolds, R.K.; Lin, J. Constitutive activation of STAT 3 oncogene product in human ovarian carcinoma cells. Gynecol. Oncol. 2000, 79, 67–73. [Google Scholar] [CrossRef]
- Duan, Z.; Foster, R.; Bell, D.A.; Mahoney, J.; Wolak, K.; Vaidya, A.; Hampel, C.; Lee, H.; Seiden, M.V. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin. Cancer Res. 2006, 12, 5055–5063. [Google Scholar] [CrossRef]
- Liang, R.; Chen, X.; Chen, L.; Wan, F.; Chen, K.; Sun, Y.; Zhu, X. STAT3 signaling in ovarian cancer: A potential therapeutic target. J. Cancer 2020, 11, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; George, J.; Kulbe, H.; Friedlander, M.; Rischin, D.; Lemech, C.; Power, J.; Coward, J.; Cowin, P.A.; House, C.M.; et al. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin. Cancer Res. 2011, 17, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Martincuks, A.; Li, P.C.; Zhao, Q.; Zhang, C.; Li, Y.J.; Yu, H.; Rodriguez-Rodriguez, L. CD44 in ovarian cancer progression and therapy resistance-a critical role for STAT3. Front. Oncol. 2020, 10, 589601. [Google Scholar] [CrossRef] [PubMed]
- Li, M.T.; Xie, L.; Jiang, H.M.; Huang, Q.; Tong, R.S.; Li, X.; Xie, X.; Liu, H.M. Role of Licochalcone A in potential pharmacological therapy: A review. Front. Pharmacol. 2022, 13, 878776. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shin, E.K.; Park, J.H.; Kim, Y.H.; Park, J.H. Antitumor and antimetastatic effects of Licochalcone A in mouse models. J. Mol. Med. 2010, 88, 829–838. [Google Scholar] [CrossRef]
- Kim, Y.H.; Shin, E.K.; Kim, D.H.; Lee, H.H.; Park, J.H.; Kim, J.K. Antiangiogenic effect of Licochalcone A. Biochem. Pharmacol. 2010, 80, 1152–1159. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jung, E.B.; Myung, S.C.; Kim, W.; Lee, C.S. Licochalcone A enhances geldanamycin-induced apoptosis through reactive oxygen species-mediated caspase activation. Pharmacology 2013, 92, 49–59. [Google Scholar] [CrossRef]
- Lee, C.S.; Kwak, S.W.; Kim, Y.J.; Lee, S.A.; Park, E.S.; Myung, S.C.; Kim, W.; Lee, M.S.; Lee, J.J. Guanylate cyclase activator YC-1 potentiates apoptotic effect of Licochalcone A on human epithelial ovarian carcinoma cells via activation of death receptor and mitochondrial pathways. Eur. J. Pharmacol. 2012, 683, 54–62. [Google Scholar] [CrossRef]
- Bortolotto, L.F.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of trans-chalcone and Licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 2017, 85, 425–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Chen, L.; Zhou, L.; Bian, S.; Lv, Y. Licochalcone A restrains microphthalmia-associated transcription factor expression and growth by activating autophagy in melanoma cells via miR-142-3p/Rheb/mTOR pathway. Phytother. Res. 2020, 34, 349–358. [Google Scholar] [CrossRef]
- Lu, W.J.; Wu, G.J.; Chen, R.J.; Chang, C.C.; Lien, L.M.; Chiu, C.C.; Tseng, M.F.; Huang, L.T.; Lin, K.H. Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest. Food Funct. 2018, 9, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mahfouz, R.Z.; Sharma, R.K.; Sarkar, O.; Mangrola, D.; Mathur, P.P. Potential biological role of poly (ADP-ribose) polymerase (PARP) in male gametes. Reprod. Biol. Endocrinol. 2009, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Loveless, J.; Shay, C.; Teng, Y. Targeting ROS-mediated crosstalk between autophagy and apoptosis in cancer. Adv. Exp. Med. Biol. 2020, 1260, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Merrick, W.C. eIF4F: A retrospective. J. Biol. Chem. 2015, 290, 24091–24099. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed]
- Rais, J.; Jafri, A.; Siddiqui, S.; Tripathi, M.; Arshad, M. Phytochemicals in the treatment of ovarian cancer. Front. Biosci. 2017, 9, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef]
- Wang, Z.F.; Liu, J.; Yang, Y.A.; Zhu, H.L. A review: The anti-inflammatory, anticancer and antibacterial properties of four kinds of licorice flavonoids isolated from licorice. Curr. Med. Chem. 2020, 27, 1997–2011. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Y.; Li, M.; Zhang, Z.; Wang, J.; Ri, M.; Jin, C.; Xu, G.; Piao, L.; Jin, H.; et al. Licochalcone A inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the NF-κB and Ras/Raf/MEK pathways. J. Ethnopharmacol. 2021, 273, 113989. [Google Scholar] [CrossRef]
- Yuan, L.W.; Jiang, X.M.; Xu, Y.L.; Huang, M.Y.; Chen, Y.C.; Yu, W.B.; Su, M.X.; Ye, Z.H.; Chen, X.; Wang, Y.; et al. Licochalcone A inhibits interferon-gamma-induced programmed death-ligand 1 in lung cancer cells. Phytomedicine 2021, 80, 153394. [Google Scholar] [CrossRef]
- Hong, S.H.; Cha, H.J.; Hwang-Bo, H.; Kim, M.Y.; Kim, S.Y.; Ji, S.Y.; Cheong, J.; Park, C.; Lee, H.; Kim, G.Y.; et al. Anti-proliferative and pro-apoptotic effects of Licochalcone A through ROS-mediated cell cycle arrest and apoptosis in human bladder cancer cells. Int. J. Mol. Sci. 2019, 20, 3820. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Seo, J.H.; Oh, H.; Yoon, G.; Chae, J.I.; Shim, J.H. Licochalcone A suppresses specificity protein 1 as a novel target in human breast cancer cells. J. Cell. Biochem. 2017, 118, 4652–4663. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Kariya, R.; Boonnate, P.; Kawaguchi, A.; Okada, S. Induction of apoptosis by shikonin through ROS-mediated intrinsic and extrinsic apoptotic pathways in primary effusion lymphoma. Transl. Oncol. 2021, 14, 101006. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef]
- Trivedi, R.; Maurya, R.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, T.; Zhang, W.; Zhou, L.; Yu, B.; Wang, W.; Yang, Z.; Liu, Z.; Zou, P.; Liang, G. Licochalcone A inhibits the proliferation of human lung cancer cell lines A549 and H460 by inducing G2/M cell cycle arrest and ER stress. Int. J. Mol. Sci. 2017, 18, 1761. [Google Scholar] [CrossRef]
- Wu, P.; Yu, T.; Wu, J.; Chen, J. Licochalcone A induces ROS-mediated apoptosis through TrxR1 inactivation in colorectal cancer cells. Biomed Res. Int. 2020, 2020, 5875074. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Keane, L.; Antignano, I.; Riechers, S.P.; Zollinger, R.; Dumas, A.A.; Offermann, N.; Bernis, M.E.; Russ, J.; Graelmann, F.; McCormick, P.N.; et al. mTOR-dependent translation amplifies microglia priming in aging mice. J. Clin. Investig. 2021, 131, e132727. [Google Scholar] [CrossRef]
- Satheesha, S.; Cookson, V.J.; Coleman, L.J.; Ingram, N.; Madhok, B.; Hanby, A.M.; Suleman, C.A.; Sabine, V.S.; Macaskill, E.J.; Bartlett, J.M.; et al. Response to mTOR inhibition: Activity of eIF4E predicts sensitivity in cell lines and acquired changes in eIF4E regulation in breast cancer. Mol. Cancer 2011, 10, 19. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.M.; Tago, K.; Nishizawa, C.; Takahashi, K.; Mashino, T.; Iwata, S.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A is a potent inhibitor of TEL-Jak2-mediated transformation through the specific inhibition of Stat3 activation. Biochem Pharmacol. 2008, 76, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Cui, X.; Liu, X.; Yu, W.; Zhang, W.; Huo, X.; Lu, C. Licochalcone A inhibits IgE-mediated allergic reaction through PLC/ERK/STAT3 pathway. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221135462. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.; Lee, D.E.; Kim, S.M.; Kim, E.; Kim, J.-K. Licochalcone A Exerts Anti-Cancer Activity by Inhibiting STAT3 in SKOV3 Human Ovarian Cancer Cells. Biomedicines 2023, 11, 1264. https://doi.org/10.3390/biomedicines11051264
Seo J, Lee DE, Kim SM, Kim E, Kim J-K. Licochalcone A Exerts Anti-Cancer Activity by Inhibiting STAT3 in SKOV3 Human Ovarian Cancer Cells. Biomedicines. 2023; 11(5):1264. https://doi.org/10.3390/biomedicines11051264
Chicago/Turabian StyleSeo, Jeonghyeon, Da Eun Lee, Seong Mi Kim, Eunjung Kim, and Jin-Kyung Kim. 2023. "Licochalcone A Exerts Anti-Cancer Activity by Inhibiting STAT3 in SKOV3 Human Ovarian Cancer Cells" Biomedicines 11, no. 5: 1264. https://doi.org/10.3390/biomedicines11051264




