Gut Dysbiosis and Blood-Brain Barrier Alteration in Hepatic Encephalopathy: From Gut to Brain
Abstract
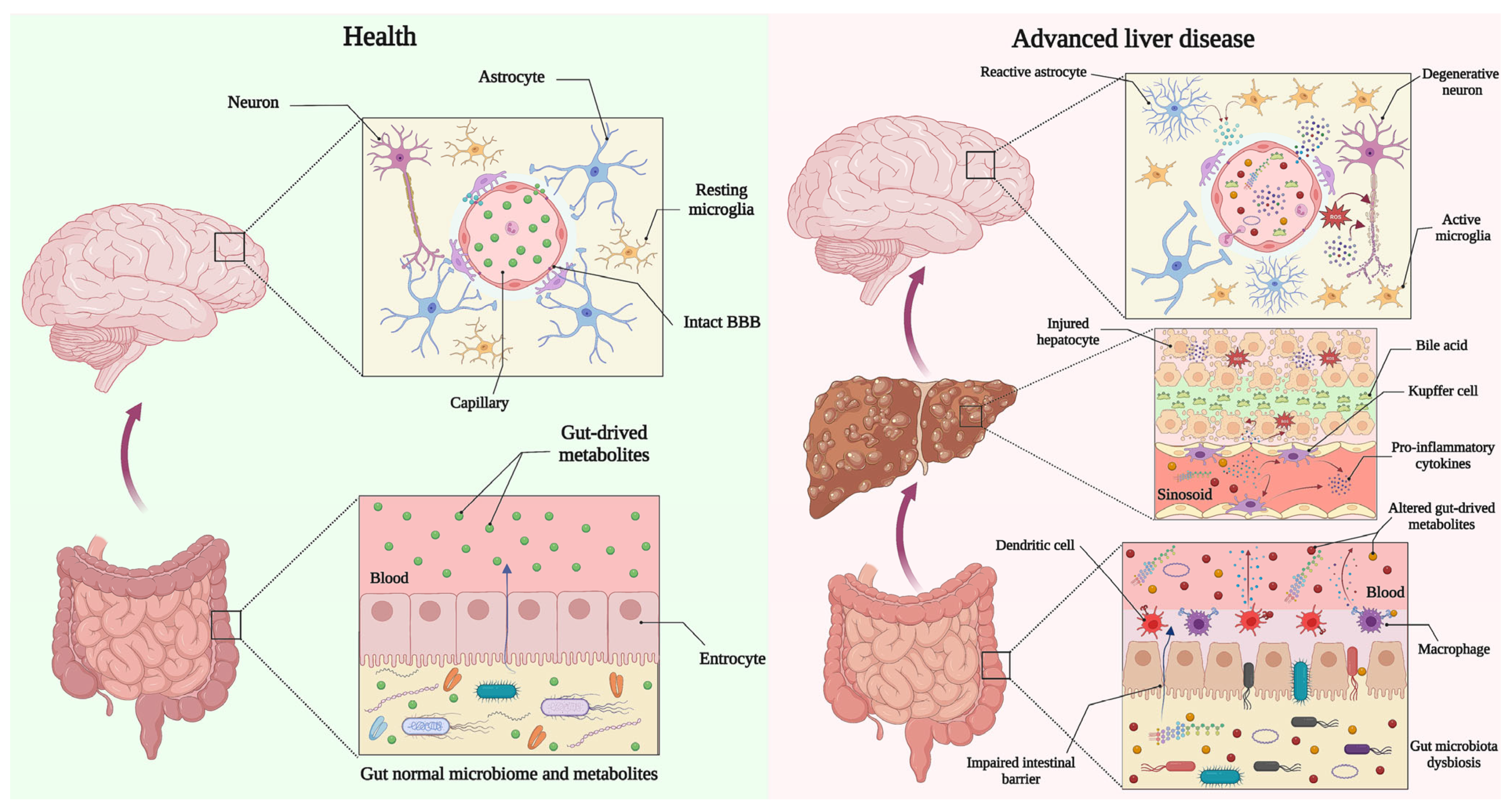
1. Introduction
2. Gut Dysbiosis in Hepatic Encephalopathy
3. Blood-Brain Barrier Structure and Transportation Systems
4. Altered Gut Microbial Metabolites and Molecules May Disrupt the Integrity of the BBB in HE
5. Therapeutic Targets
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Häussinger, D.; Dhiman, R.K.; Felipo, V.; Görg, B.; Jalan, R.; Kircheis, G.; Merli, M.; Montagnese, S.; Romero-Gomez, M.; Schnitzler, A.; et al. Hepatic encephalopathy. Nat. Rev. Dis. Prim. 2022, 8, 43. [Google Scholar] [CrossRef]
- Legaz, I.; Bolarin, J.M.; Campillo, J.A.; Moya, R.M.; Luna, A.; Osuna, E.; Minguela, A.; Sanchez-Bueno, F.; Alvarez, M.R.; Muro, M. Pretransplant ascites and encephalopathy and their influence on survival and liver graft rejection in alcoholic cirrhosis disease. Arch. Med. Sci. AMS 2021, 17, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Bajaj, J.S. Impact of Hepatic Encephalopathy in Cirrhosis on Quality-of-Life Issues. Drugs 2019, 79, 11–16. [Google Scholar] [CrossRef]
- Montagnese, S.; Rautou, P.-E.; Romero-Gómez, M.; Larsen, F.S.; Shawcross, D.L.; Thabut, D.; Vilstrup, H.; Weissenborn, K. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J. Hepatol. 2022, 77, 807–824. [Google Scholar] [CrossRef]
- Hartmann, I.J.; Groeneweg, M.; Quero, J.C.; Beijeman, S.J.; de Man, R.A.; Hop, W.C.; Schalm, S.W. The prognostic significance of subclinical hepatic encephalopathy. Am. J. Gastroenterol. 2000, 95, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Jesús Maldonado-Garza, H.; Vázquez-Elizondo, G.; Obed Gaytán-Torres, J.; Ricardo Flores-Rendón, Á.; Graciela Cárdenas-Sandoval, M.; Javier Bosques-Padilla, F. Prevalence of minimal hepatic encephalopathy in cirrhotic patients. Ann. Hepatol. 2011, 10, S40–S44. [Google Scholar] [CrossRef]
- Groeneweg, M.; Moerland, W.; Quero, J.C.; Hop, W.C.J.; Krabbe, P.F.; Schalm, S.W. Screening of subclinical hepatic encephalopathy. J. Hepatol. 2000, 32, 748–753. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, B.C.; Puri, V.; Sarin, S.K. Critical flicker frequency: Diagnostic tool for minimal hepatic encephalopathy. J. Hepatol. 2007, 47, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Legaz, I.; Navarro-Noguera, E.; Bolarín, J.M.; García-Alonso, A.M.; Luna Maldonado, A.; Mrowiec, A.; Campillo, J.A.; Gimeno, L.; Moya-Quiles, R.; Álvarez-López, M.d.R.; et al. Epidemiology, Evolution, and Long-Term Survival of Alcoholic Cirrhosis Patients Submitted to Liver Transplantation in Southeastern Spain. Alcohol Clin. Exp. Res. 2016, 40, 794–805. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Boza, F.; García-Valdecasas, M.S.; García, E.; Aguilar-Reina, J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am. J. Gastroenterol. 2001, 96, 2718–2723. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Jepsen, P.; Wong, F.; Ginès, P.; Córdoba, J.; Vilstrup, H. Satavaptan treatment for ascites in patients with cirrhosis: A meta-analysis of effect on hepatic encephalopathy development. Metab. Brain Dis. 2013, 28, 301–305. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P.; Del Piccolo, F.; Pettenò, E.; Mapelli, D.; Angeli, P.; Iemmolo, R.; Muraca, M.; Musto, C.; Gerunda, G.; Rizzo, C.; et al. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J. Hepatol. 2001, 35, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bass, N.M.; Mullen, K.D.; Sanyal, A.; Poordad, F.; Neff, G.; Leevy, C.B.; Sigal, S.; Sheikh, M.Y.; Beavers, K.; Frederick, T.; et al. Rifaximin Treatment in Hepatic Encephalopathy. N. Engl. J. Med. 2010, 362, 1071–1081. [Google Scholar] [CrossRef]
- Chen, Z.; Ruan, J.; Li, D.; Wang, M.; Han, Z.; Qiu, W.; Wu, G. The Role of Intestinal Bacteria and Gut-Brain Axis in Hepatic Encephalopathy. Front. Cell. Infect. Microbiol. 2021, 10, 595759. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Betrapally, N.S.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; White, M.B.; Unser, A.; Thacker, L.R.; Sanyal, A.J.; Kang, D.J.; et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 2015, 62, 1260–1271. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhai, H.; Geng, J.; Yu, R.; Ren, H.; Fan, H.; Shi, P. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am. J. Gastroenterol. 2013, 108, 1601–1611. [Google Scholar] [CrossRef]
- Sung, C.M.; Lin, Y.F.; Chen, K.F.; Ke, H.M.; Huang, H.Y.; Gong, Y.N.; Tsai, W.S.; You, J.F.; Lu, M.J.; Cheng, H.T.; et al. Predicting Clinical Outcomes of Cirrhosis Patients with Hepatic Encephalopathy From the Fecal Microbiome. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 301–318.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Bajaj, J.S.; Wang, J.B.; Shang, J.; Zhou, X.M.; Guo, X.L.; Zhu, X.; Meng, L.N.; Jiang, H.X.; Mi, Y.Q.; et al. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: A multicenter, randomized controlled trial. J. Dig. Dis. 2019, 20, 547–556. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, J.; Qian, G.; Fang, D.; Shi, D.; Guo, L.; Li, L. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J. Gastroenterol. Hepatol. 2015, 30, 1429–1437. [Google Scholar] [CrossRef]
- Yukawa-Muto, Y.; Kamiya, T.; Fujii, H.; Mori, H.; Toyoda, A.; Sato, I.; Konishi, Y.; Hirayama, A.; Hara, E.; Fukuda, S.; et al. Distinct responsiveness to rifaximin in patients with hepatic encephalopathy depends on functional gut microbial species. Hepatol. Commun. 2022, 6, 2090–2104. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Feng, H. Changes in intestinal microbiota of HBV-associated liver cirrhosis with/without hepatic encephalopathy. Medicine 2022, 101, e29935. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, G.; Feng, F.; Wang, M.; Long, F. Characterization of intestinal microbiota and serum metabolites in patients with mild hepatic encephalopathy. Open Life Sci. 2022, 17, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Sikaroodi, M.; Shamsaddini, A.; Henseler, Z.; Santiago-Rodriguez, T.; Acharya, C.; Fagan, A.; Hylemon, P.B.; Fuchs, M.; Gavis, E.; et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut 2021, 70, 1162–1173. [Google Scholar] [CrossRef]
- Kang, D.J.; Betrapally, N.S.; Ghosh, S.A.; Sartor, R.B.; Hylemon, P.B.; Gillevet, P.M.; Sanyal, A.J.; Heuman, D.M.; Carl, D.; Zhou, H.; et al. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice. Hepatology 2016, 64, 1232–1248. [Google Scholar] [CrossRef]
- Yang, B.; Sun, T.; Chen, Y.; Xiang, H.; Xiong, J.; Bao, S. The Role of Gut Microbiota in Mice with Bile Duct Ligation-Evoked Cholestatic Liver Disease-Related Cognitive Dysfunction. Front. Microbiol. 2022, 13, 909461. [Google Scholar] [CrossRef] [PubMed]
- Đurašević, S.; Pejić, S.; Grigorov, I.; Nikolić, G.; Mitić-Ćulafić, D.; Dragićević, M.; Đorđević, J.; Todorović Vukotić, N.; Đorđević, N.; Todorović, A.; et al. Effects of C60 Fullerene on Thioacetamide-Induced Rat Liver Toxicity and Gut Microbiome Changes. Antioxidants 2021, 10, 911. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Wang, J.; Jiang, R.; Zhao, T. Gut microbiota combined with metabolomics reveal the mechanism of curcumol on liver fibrosis in mice. Biomed. Pharmacother. 2022, 152, 113204. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, D.; Zhu, B.; Wang, S.; Xu, Y.; Zhang, C.; Yang, H.; Wang, S.; Liu, P.; Qin, L.; et al. Rubus chingii Hu. unripe fruits extract ameliorates carbon tetrachloride-induced liver fibrosis and improves the associated gut microbiota imbalance. Chin. Med. 2022, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Patterson, A.M.; Cotter, P.D.; Beraza, N. Cholestasis induced by bile duct ligation promotes changes in the intestinal microbiome in mice. Sci. Rep. 2019, 9, 12324. [Google Scholar] [CrossRef]
- De Minicis, S.; Rychlicki, C.; Agostinelli, L.; Saccomanno, S.; Candelaresi, C.; Trozzi, L.; Mingarelli, E.; Facinelli, B.; Magi, G.; Palmieri, C.; et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology 2014, 59, 1738–1749. [Google Scholar] [CrossRef]
- Li, Y.; Lv, L.; Ye, J.; Fang, D.; Shi, D.; Wu, W.; Wang, Q.; Wu, J.; Yang, L.; Bian, X.; et al. Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in d-galactosamine-treated rats. Appl. Microbiol. Biotechnol. 2019, 103, 375–393. [Google Scholar] [CrossRef]
- Terrón-Camero, L.C.; Gordillo-González, F.; Salas-Espejo, E.; Andrés-León, E. Comparison of Metagenomics and Metatranscriptomics Tools: A Guide to Making the Right Choice. Genes 2022, 13, 2280. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Dong, Y.-Q.; Yin, J.; Qiu, Y.-Q. Diagnostic accuracy of metagenomic next-generation sequencing in diagnosing infectious diseases: A meta-analysis. Sci. Rep. 2022, 12, 21032. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, M. Long-read metagenomics paves the way toward a complete microbial tree of life. Nat. Methods 2023, 20, 30–31. [Google Scholar] [CrossRef]
- Madison, J.; LaBumbard, B.; Woodhams, D. Shotgun Metagenomic and 16S rRNA Gene Sequencing of Museum-Derived Rana pipiens Gut Microbiota Generate Differing Community Diversity Metrics Based on Commonly Used Analysis Pipelines. Authorea Prepr. 2023. [Google Scholar]
- Zuo, W.; Wang, B.; Bai, X.; Luan, Y.; Fan, Y.; Michail, S.; Sun, F. 16S rRNA and metagenomic shotgun sequencing data revealed consistent patterns of gut microbiome signature in pediatric ulcerative colitis. Sci. Rep. 2022, 12, 6421. [Google Scholar] [CrossRef]
- Morgan, X.C.; Huttenhower, C. Chapter 12: Human microbiome analysis. PLoS Comput. Biol. 2012, 8, e1002808. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, G.; Campanaro, S.; Angione, C.; Treu, L. Metatranscriptomics-guided genome-scale metabolic modeling of microbial communities. Cell Rep. Methods 2023, 3, 100383. [Google Scholar] [CrossRef] [PubMed]
- Heravi, F.S.; Zakrzewski, M.; Vickery, K.; Malone, M.; Hu, H. Metatranscriptomic Analysis Reveals Active Bacterial Communities in Diabetic Foot Infections. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Aguiar-Pulido, V.; Huang, W.; Suarez-Ulloa, V.; Cickovski, T.; Mathee, K.; Narasimhan, G. Metagenomics, Metatranscriptomics, and Metabolomics Approaches for Microbiome Analysis. Evol. Bioinform. Online 2016, 12, 5–16. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood–brain barrier. Handb. Clin. Neurol. 2016, 133, 39–59. [Google Scholar]
- Tietz, S.; Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Thompson, H.P.; Goodman, G.W.; Li, J.; Urayama, A. Mucopolysaccharidoses and the blood–brain barrier. Fluids Barriers CNS 2022, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular transport and regulation of transcytosis across the blood–brain barrier. Cell. Mol. Life Sci. 2019, 76, 1081–1092. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613. [Google Scholar] [CrossRef]
- Ayloo, S.; Gu, C. Transcytosis at the blood–brain barrier. Curr. Opin. Neurobiol. 2019, 57, 32–38. [Google Scholar] [CrossRef]
- Deng, H.; Dutta, P.; Liu, J. Stochastic simulations of nanoparticle internalization through transferrin receptor dependent clathrin-mediated endocytosis. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 2104–2111. [Google Scholar] [CrossRef]
- Sorets, A.G.; Rosch, J.C.; Duvall, C.L.; Lippmann, E.S. Caveolae-mediated transport at the injured blood-brain barrier as an underexplored pathway for central nervous system drug delivery. Curr. Opin. Chem. Eng. 2020, 30, 86–95. [Google Scholar] [CrossRef]
- Zhou, M.; Shi, S.X.; Liu, N.; Jiang, Y.; Karim, M.S.; Vodovoz, S.J.; Wang, X.; Zhang, B.; Dumont, A.S. Caveolae-mediated endothelial transcytosis across the blood-brain barrier in acute ischemic stroke. J. Clin. Med. 2021, 10, 3795. [Google Scholar] [CrossRef]
- De Boer, A.; van der Sandt, I.; Gaillard, P. The role of drug transporters at the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–brain barrier endogenous transporters as therapeutic targets: A new model for small molecule CNS drug discovery. Expert Opin. Ther. Targets 2015, 19, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Eng, M.E.; Imperio, G.E.; Bloise, E.; Matthews, S.G. ATP-binding cassette (ABC) drug transporters in the developing blood-brain barrier: Role in fetal brain protection. Cell. Mol. Life Sci. 2022, 79, 415. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Terasaki, T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm. Res. 2007, 24, 1745–1758. [Google Scholar] [CrossRef]
- Veys, K.; Fan, Z.; Ghobrial, M.; Bouché, A.; García-Caballero, M.; Vriens, K.; Conchinha, N.V.; Seuwen, A.; Schlegel, F.; Gorski, T. Role of the GLUT1 glucose transporter in postnatal CNS angiogenesis and blood-brain barrier integrity. Circ. Res. 2020, 127, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Kang, Y.-S. In vivo and in vitro evidence for brain uptake of 4-phenylbutyrate by the monocarboxylate transporter 1 (MCT1). Pharm. Res. 2016, 33, 1711–1722. [Google Scholar] [CrossRef]
- Schäfer, A.M.; Meyer zu Schwabedissen, H.E.; Grube, M. Expression and Function of Organic Anion Transporting Polypeptides in the Human Brain: Physiological and Pharmacological Implications. Pharmaceutics 2021, 13, 834. [Google Scholar] [CrossRef]
- Häfliger, P.; Charles, R.P. The L-Type Amino Acid Transporter LAT1-An Emerging Target in Cancer. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- O’Donnell, M.E. Blood-brain barrier Na transporters in ischemic stroke. Adv. Pharmacol. 2014, 71, 113–146. [Google Scholar]
- Gerhardt, H.; Wolburg, H.; Redies, C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2000, 218, 472–479. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ramsauer, M.; Krause, D.; Dermietzel, R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002, 16, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W.; Scofield, M.D. Astrocyte regulation of synaptic signaling in psychiatric disorders. Neuropsychopharmacology 2023, 48, 21–36. [Google Scholar] [CrossRef]
- Gordon, G.R.; Howarth, C.; MacVicar, B.A. Bidirectional control of arteriole diameter by astrocytes. Exp. Physiol. 2011, 96, 393–399. [Google Scholar] [CrossRef]
- Bihlmaier, R.; Deffner, F.; Mattheus, U.; Neckel, P.H.; Hirt, B.; Mack, A.F. Aquaporin-1 and Aquaporin-4 Expression in Ependyma, Choroid Plexus and Surrounding Transition Zones in the Human Brain. Biomolecules 2023, 13, 212. [Google Scholar] [CrossRef]
- Jahncke, J.N.; Wright, K.M. The many roles of dystroglycan in nervous system development and function: Dystroglycan and neural circuit development. Dev. Dyn. 2023, 252, 61–80. [Google Scholar] [CrossRef]
- Nicchia, G.P.; Cogotzi, L.; Rossi, A.; Basco, D.; Brancaccio, A.; Svelto, M.; Frigeri, A. Expression of multiple AQP4 pools in the plasma membrane and their association with the dystrophin complex. J. Neurochem. 2008, 105, 2156–2165. [Google Scholar] [CrossRef]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.-O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef] [PubMed]
- 83. Peng, X.; Luo, Z.; He, S.; Zhang, L.; Li, Y. Blood-Brain Barrier Disruption by Lipopolysaccharide and Sepsis-Associated Encephalopathy. Front. Cell Infect Microbiol. 2021, 11, 768108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Xu, P.; Sun, B.; Zhong, Z.; Liu, C.; Ling, Z.; Chen, Y.; Shu, N.; Zhao, K. Acute liver failure impairs function and expression of breast cancer-resistant protein (BCRP) at rat blood–brain barrier partly via ammonia-ROS-ERK 1/2 activation. J. Neurochem. 2016, 138, 282–294. [Google Scholar] [CrossRef]
- Ott, P.; Larsen, F.S. Blood-brain barrier permeability to ammonia in liver failure: A critical reappraisal. Neurochem. Int. 2004, 44, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Sun, C.-M.; Liu, P. Alterations of blood-brain barrier and associated factors in acute liver failure. Gastroenterol. Res. Pract. 2013, 2013, 841707. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Zarifkar, A.; Namvar, G.; Shahbazi, A.; Williams, R. Astrocyte swelling in hepatic encephalopathy: Molecular perspective of cytotoxic edema. Metab. Brain Dis. 2020, 35, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Dohi, K.; Banks, W.A. Neuroinflammation: A common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation 2012, 19, 121–130. [Google Scholar] [CrossRef]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Kato, M.; Hughes, R.D.; Keays, R.T.; Williams, R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 1992, 15, 1060–1066. [Google Scholar] [CrossRef]
- Lv, S.; Song, H.L.; Zhou, Y.; Li, L.X.; Cui, W.; Wang, W.; Liu, P. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010, 30, 1198–1210. [Google Scholar] [CrossRef]
- Livingstone, A.S.; Potvin, M.; Goresky, C.A.; Finlayson, M.H.; Hinchey, E.J. Changes in the Blood-Brain Barrier in Hepatic Coma after Hepatectomy in the Rat. Gastroenterology 1977, 73, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.E.; Ede, R.J.; Davis, M.; Williams, R. Experimental studies of blood brain barrier permeability in acute hepatic failure. Hepatology 1984, 4, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Mossakowski, M.J.; Borowicz, J. Early electron-microscopic changes in hepatogenic encephalopathy induced by thioacetamide intoxication in rats. Neuropatol. Pol. 1985, 23, 375–387. [Google Scholar] [PubMed]
- Traber, P.G.; Dal Canto, M.; Ganger, D.R.; Blei, A.T. Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: Ultrastructure and integrity of the blood-brain barrier. Hepatology 1987, 7, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.H.; Yamamoto, S.; Steers, J.; Sevlever, D.; Lin, W.; Shimojima, N.; Castanedes-Casey, M.; Genco, P.; Golde, T.; Richelson, E.; et al. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J. Hepatol. 2006, 44, 1105–1114. [Google Scholar] [CrossRef]
- Chen, F.; Ohashi, N.; Li, W.; Eckman, C.; Nguyen, J.H. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 2009, 50, 1914–1923. [Google Scholar] [CrossRef]
- Kristiansen, R.G.; Lindal, S.; Myreng, K.; Revhaug, A.; Ytrebø, L.M.; Rose, C.F. Neuropathological changes in the brain of pigs with acute liver failure. Scand. J. Gastroenterol. 2010, 45, 935–943. [Google Scholar] [CrossRef]
- Wang, W.; Lv, S.; Zhou, Y.; Fu, J.; Li, C.; Liu, P. Tumor necrosis factor-α affects blood-brain barrier permeability in acetaminophen-induced acute liver failure. Eur. J. Gastroenterol. Hepatol. 2011, 23, 552–558. [Google Scholar] [CrossRef]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef]
- Chastre, A.; Bélanger, M.; Nguyen, B.N.; Butterworth, R.F. Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int. 2014, 34, 353–361. [Google Scholar] [CrossRef]
- Faleiros, B.E.; Miranda, A.S.; Campos, A.C.; Gomides, L.F.; Kangussu, L.M.; Guatimosim, C.; Camargos, E.R.S.; Menezes, G.B.; Rachid, M.A.; Teixeira, A.L. Up-regulation of brain cytokines and chemokines mediates neurotoxicity in early acute liver failure by a mechanism independent of microglial activation. Brain Res. 2014, 1578, 49–59. [Google Scholar] [CrossRef]
- McMillin, M.A.; Frampton, G.A.; Seiwell, A.P.; Patel, N.S.; Jacobs, A.N.; DeMorrow, S. TGFβ1 exacerbates blood-brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab. Investig. 2015, 95, 903–913. [Google Scholar] [CrossRef]
- Thabut, D.; Mouri, S.; El Mourabit, H.; Morichon, R.; Wandum, D.; Lasnier, E.; Housset, C.; Weiss, N. Sodium benzoate and rifaximin are able to restore blood-brain barrier integrity in the cirrhotic rats. Intensive Care Med. Exp. 2015, 3, A691. [Google Scholar] [CrossRef]
- Grant, S.; McMillin, M.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jaeger, V.; Kain, J.; DeMorrow, S. Direct Comparison of the Thioacetamide and Azoxymethane Models of Type A Hepatic Encephalopathy in Mice. Gene Expr. 2018, 18, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-M.; Oh, K.K.; Gupta, H.; Ganesan, R.; Sharma, S.P.; Jeong, J.-J.; Yoon, S.J.; Jeong, M.K.; Min, B.H.; Hyun, J.Y.; et al. The Link between Gut Microbiota and Hepatic Encephalopathy. Int. J. Mol. Sci. 2022, 23, 8999. [Google Scholar] [CrossRef] [PubMed]
- Saboo, K.; Petrakov, N.V.; Shamsaddini, A.; Fagan, A.; Gavis, E.A.; Sikaroodi, M.; McGeorge, S.; Gillevet, P.M.; Iyer, R.K.; Bajaj, J.S. Stool microbiota are superior to saliva in distinguishing cirrhosis and hepatic encephalopathy using machine learning. J. Hepatol. 2022, 76, 600–607. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, R.; Mu, Y.; Song, Y.; Hao, N.; Wei, Y.; Wang, Q.; Mackay, C.R. Propionate Ameliorates Alcohol-Induced Liver Injury in Mice via the Gut-Liver Axis: Focus on the Improvement of Intestinal Permeability. J. Agric. Food Chem. 2022, 70, 6084–6096. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, Y.; Zhang, Y.; Xia, C.; Lai, Q.; Dong, Z.; Kuang, W.; Yang, C.; Su, D.; Li, H.; et al. Potential effects of antibiotic-induced gut microbiome alteration on blood-brain barrier permeability compromise in rhesus monkeys. Ann. N. Y. Acad. Sci. 2020, 1470, 14–24. [Google Scholar] [CrossRef]
- Bloom, P.P.; Luévano, J.M.; Miller, K.J.; Chung, R.T. Deep stool microbiome analysis in cirrhosis reveals an association between short-chain fatty acids and hepatic encephalopathy. Ann. Hepatol. 2021, 25, 100333. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, L.F.; Dai, T.Y.; Qi, X.; Wang, Y.; Zheng, T.Z.; Gao, X.Y.; Zhang, Y.J.; Ai, Y.; Ma, L.; et al. Short-Chain Fatty Acids Produced by Ruminococcaceae Mediate α-Linolenic Acid Promote Intestinal Stem Cells Proliferation. Mol. Nutr. Food Res. 2022, 66, e2100408. [Google Scholar] [CrossRef]
- Jin, M.; Kalainy, S.; Baskota, N.; Chiang, D.; Deehan, E.C.; McDougall, C.; Tandon, P.; Martínez, I.; Cervera, C.; Walter, J.; et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019, 39, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Moodley, P.; Dhanda, A. The effect of increasing intestinal short-chain fatty acid concentration on gut permeability and liver injury in the context of liver disease: A systematic review. J Gastroenterol. Hepatol. 2022, 37, 1498–1506. [Google Scholar] [CrossRef]
- Tsiaoussis, G.I.; Assimakopoulos, S.F.; Tsamandas, A.C.; Triantos, C.K.; Thomopoulos, K.C. Intestinal barrier dysfunction in cirrhosis: Current concepts in pathophysiology and clinical implications. World J. Hepatol. 2015, 7, 2058–2068. [Google Scholar] [CrossRef]
- Fukui, H. Leaky Gut and Gut-Liver Axis in Liver Cirrhosis: Clinical Studies Update. Gut Liver 2021, 15, 666–676. [Google Scholar] [CrossRef]
- Philipp, B. Bacterial degradation of bile salts. Appl. Microbiol. Biotechnol. 2011, 89, 903–915. [Google Scholar] [CrossRef]
- Guo, X.; Okpara, E.S.; Hu, W.; Yan, C.; Wang, Y.; Liang, Q.; Chiang, J.Y.; Han, S. Interactive Relationships between Intestinal Flora and Bile Acids. Int. J. Mol. Sci. 2022, 23, 8343. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Mencarelli, A.; Chini, M.G.; Distrutti, E.; Renga, B.; Bifulco, G.; Baldelli, F.; Donini, A.; Fiorucci, S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE 2011, 6, e25637. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.; Renooij, W.; Murzilli, S.; Klomp, L.W.; Siersema, P.D.; Schipper, M.E.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef]
- Acharya, C.; Bajaj, J.S. Chronic Liver Diseases and the Microbiome-Translating Our Knowledge of Gut Microbiota to Management of Chronic Liver Disease. Gastroenterology 2021, 160, 556–572. [Google Scholar] [CrossRef]
- Úbeda, M.; Lario, M.; Muñoz, L.; Borrero, M.J.; Rodríguez-Serrano, M.; Sánchez-Díaz, A.M.; Del Campo, R.; Lledó, L.; Pastor, Ó.; García-Bermejo, L.; et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J. Hepatol. 2016, 64, 1049–1057. [Google Scholar] [CrossRef]
- Neale, G.; Lewis, B.; Weaver, V.; Panveliwalla, D. Serum bile acids in liver disease. Gut 1971, 12, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Suh, D.J. Profiles of serum bile acids in liver diseases. Korean J. Intern. Med. 1986, 1, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Balazs, I.; Horvath, A.; Leber, B.; Feldbacher, N.; Sattler, W.; Rainer, F.; Fauler, G.; Vermeren, S.; Stadlbauer, V. Serum bile acids in liver cirrhosis promote neutrophil dysfunction. Clin. Transl. Med. 2022, 12, e735. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Gómez-Hurtado, I.; Such, J.; Francés, R. Microbiome and bacterial translocation in cirrhosis. Gastroenterol. Y Hepatol. 2016, 39, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Rayes, N.; Seehofer, D.; Müller, A.R.; Hansen, S.; Bengmark, S.; Neuhaus, P. Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery—Results of a prospective trial. Z. Gastroenterol. 2002, 40, 869–876. [Google Scholar] [CrossRef]
- Such, J.; Francés, R.; Muñoz, C.; Zapater, P.; Casellas, J.A.; Cifuentes, A.; Rodríguez-Valera, F.; Pascual, S.; Sola-Vera, J.; Carnicer, F.; et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology 2002, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, C.; Jensen, J.S.; Hobolth, L.; Dam-Larsen, S.; Madsen, B.S.; Andersen, O.; Møller, S.; Bendtsen, F. Association of markers of bacterial translocation with immune activation in decompensated cirrhosis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1360–1366. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Hu, Y.; Zhou, L.; Tan, Q. Changes in gut toll-like receptor-4 and nod-like receptor family pyrin domain containing-3 innate pathways in liver cirrhosis rats with bacterial translocation. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.; José Borrero, M.; Ubeda, M.; Lario, M.; Díaz, D.; Francés, R.; Monserrat, J.; Pastor, O.; Aguado-Fraile, E.; Such, J.; et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology 2012, 56, 1861–1869. [Google Scholar] [CrossRef]
- Bellot, P.; Francés, R.; Such, J. Pathological bacterial translocation in cirrhosis: Pathophysiology, diagnosis and clinical implications. Liver Int. 2013, 33, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Männistö, V.; Färkkilä, M.; Pussinen, P.; Jula, A.; Männistö, S.; Lundqvist, A.; Valsta, L.; Salomaa, V.; Perola, M.; Åberg, F. Serum lipopolysaccharides predict advanced liver disease in the general population. JHEP Rep. Innov. Hepatol. 2019, 1, 345–352. [Google Scholar] [CrossRef]
- Meng, J.; Wang, Q.; Liu, K.; Yang, S.; Fan, X.; Liu, B.; He, C.; Wu, X. Systemic and Splanchnic Lipopolysaccharide and Endothelin-1 Plasma Levels in Liver Cirrhosis before and after Transjugular Intrahepatic Portosystemic Shunt. Gastroenterol. Res. Pract. 2016, 2016, 8341030. [Google Scholar] [CrossRef]
- Li, X.; Jiang, S.; Tapping, R.I. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010, 49, 1–9. [Google Scholar] [CrossRef]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Vidya, M.K.; Kumar, V.G.; Sejian, V.; Bagath, M.; Krishnan, G.; Bhatta, R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2018, 37, 20–36. [Google Scholar] [CrossRef]
- Ozato, K.; Tsujimura, H.; Tamura, T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. BioTechniques 2002, 33, S66–S75. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Abrial, C.; Salvator, H.; Brollo, M.; Naline, E.; Devillier, P. The Role of Toll-Like Receptors in the Production of Cytokines by Human Lung Macrophages. J. Innate Immun. 2020, 12, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L. Lipopolysaccharides in liver injury: Molecular mechanisms of Kupffer cell activation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G256–G265. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine Attenuates LPS-Induced Liver Injury and Improves Survival of Mice through Suppressing Oxidative Stress, Inflammation, and Apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.-y.; Sun, Y.; Wang, H.; Shan, H.; Wang, S. Baicalin protects LPS-induced blood–brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int. Immunopharmacol. 2021, 96, 107725. [Google Scholar] [CrossRef]
- Sewal, R.K.; Modi, M.; Saikia, U.N.; Chakrabarti, A.; Medhi, B. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res. 2017, 135, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Jangula, A.; Murphy, E.J. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci. Lett. 2013, 551, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.E.; Wardle, E.N.; Canalese, J.; Ede, R.J.; Williams, R. Potential toxins of acute liver failure and their effects on blood-brain barrier permeability. Experientia 1983, 39, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.A.; Ganey, P.E.; Ju, C.; Kamendulis, L.M.; Rusyn, I.; Klaunig, J.E. Role of the Kupffer Cell in Mediating Hepatic Toxicity and Carcinogenesis. Toxicol. Sci. 2007, 96, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Thurman, R.G.; Bradford, B.U.; Iimuro, Y.; Knecht, K.T.; Connor, H.D.; Adachi, Y.; Wall, C.; Arteel, G.E.; Raleigh, J.A.; Forman, D.T.; et al. Role of Kupffer Cells, Endotoxin and Free Radicals in Hepatotoxicity Due to Prolonged Alcohol Consumption: Studies in Female and Male Rats. J. Nutr. 1997, 127, 903S–906S. [Google Scholar] [CrossRef]
- McCuskey, P.A.; McCuskey, R.S. Electron microscopic study of the effects of endotoxin on the cells of the hepatic sinusoid in normal and BCG sensitized mice. Histol. Histopathol. 1991, 6, 353–362. [Google Scholar] [PubMed]
- McMillin, M.; Galindo, C.; Pae, H.Y.; Frampton, G.; Di Patre, P.L.; Quinn, M.; Whittington, E.; DeMorrow, S. Gli1 activation and protection against hepatic encephalopathy is suppressed by circulating transforming growth factor β1 in mice. J. Hepatol. 2014, 61, 1260–1266. [Google Scholar] [CrossRef]
- Dhanda, S.; Sandhir, R. Blood-Brain Barrier Permeability Is Exacerbated in Experimental Model of Hepatic Encephalopathy via MMP-9 Activation and Downregulation of Tight Junction Proteins. Mol. Neurobiol. 2018, 55, 3642–3659. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, B.; Ali, M.; Grant, S.; Frampton, G.; Ploof, M.; Andry, S.; DeMorrow, S.; McMillin, M. Thrombospondin-1 Exacerbates Acute Liver Failure and Hepatic Encephalopathy Pathology in Mice by Activating Transforming Growth Factor β1. Am. J. Pathol. 2020, 190, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Azhari, H.; Swain, M.G. Role of Peripheral Inflammation in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2018, 8, 281–285. [Google Scholar] [CrossRef]
- Balzano, T.; Leone, P.; Ivaylova, G.; Castro, M.C.; Reyes, L.; Ramón, C.; Malaguarnera, M.; Llansola, M.; Felipo, V. Rifaximin Prevents T-Lymphocytes and Macrophages Infiltration in Cerebellum and Restores Motor Incoordination in Rats with Mild Liver Damage. Biomedicines 2021, 9, 1002. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef]
- Barajon, I.; Serrao, G.; Arnaboldi, F.; Opizzi, E.; Ripamonti, G.; Balsari, A.; Rumio, C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J. Histochem. Cytochem. 2009, 57, 1013–1023. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Barba, A.; Eyre, E.; Romero, C.; Neunlist, M.; Fernández, E. TLR2 and TLR9 modulate enteric nervous system inflammatory responses to lipopolysaccharide. J. Neuroinflamm. 2016, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.H.; Zhu, Y.; Li, Q.L.; Zhao, C.; Zhou, P.H. Enteric Nervous System: The Bridge Between the Gut Microbiota and Neurological Disorders. Front. Aging Neurosci. 2022, 14, 810483. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Seier Poulsen, S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as Cosensors for Short-Chain Fatty Acids in Enteroendocrine Cells vs FFAR3 in Enteric Neurons and FFAR2 in Enteric Leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef] [PubMed]
- Soret, R.; Chevalier, J.; de Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef]
- Linan-Rico, A.; Ochoa-Cortes, F.; Beyder, A.; Soghomonyan, S.; Zuleta-Alarcon, A.; Coppola, V.; Christofi, F.L. Mechanosensory Signaling in Enterochromaffin Cells and 5-HT Release: Potential Implications for Gut Inflammation. Front. Neurosci. 2016, 10, 564. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Muscularis macrophages: Trained guardians of enteric neurons. Cell Res. 2022, 32, 229–230. [Google Scholar] [CrossRef]
- Nijhuis, L.E.; Olivier, B.J.; de Jonge, W.J. Neurogenic regulation of dendritic cells in the intestine. Biochem. Pharmacol. 2010, 80, 2002–2008. [Google Scholar] [CrossRef]
- Progatzky, F.; Pachnis, V. The role of enteric glia in intestinal immunity. Curr. Opin. Immunol. 2022, 77, 102183. [Google Scholar] [CrossRef]
- Xu, X.; Chen, R.; Zhan, G.; Wang, D.; Tan, X.; Xu, H. Enterochromaffin Cells: Sentinels to Gut Microbiota in Hyperalgesia? Front. Cell. Infect. Microbiol. 2021, 11, 760076. [Google Scholar] [CrossRef]
- Arora, T.; Vanslette, A.M.; Hjorth, S.A.; Bäckhed, F. Microbial regulation of enteroendocrine cells. Med 2021, 2, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chalazonitis, A.; Huang, Y.Y.; Mann, J.J.; Margolis, K.G.; Yang, Q.M.; Kim, D.O.; Côté, F.; Mallet, J.; Gershon, M.D. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 2011, 31, 8998–9009. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Grasset, E.; Mannerås Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [PubMed]
- Bauché, D.; Marie, J.C. Transforming growth factor β: A master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; von Wright, A.; Vilpponen-Salmela, T.; Ben-Amor, K.; Akkermans, A.D.; de Vos, W. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl. Environ. Microbiol. 2002, 68, 3401–3407. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside—from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Lkhagva, E.; Jung, S.; Kim, H.-J.; Chung, H.-J.; Hong, S.-T. Fecal Microbiome Does Not Represent Whole Gut Microbiome. Cell. Microbiol. 2023, 2023, 6868417. [Google Scholar] [CrossRef]
- Patidar, K.R.; Bajaj, J.S. Antibiotics for the treatment of hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 307–312. [Google Scholar] [CrossRef]
- Tapper, E.B.; Essien, U.R.; Zhao, Z.; Ufere, N.N.; Parikh, N.D. Racial and ethnic disparities in rifaximin use and subspecialty referrals for patients with hepatic encephalopathy in the United States. J. Hepatol. 2022, 77, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic acid prebiotic plus Lacticaseibacillus rhamnosus GG probiotic as a novel synbiotic promoted their separate neuroprotection against Parkinson’s disease. Food Res. Int. 2022, 155, 111067. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, N.; Hao, X.; Zhao, J.; Zhao, Y.; Li, Y.; Li, Z. Manual acupuncture benignly regulates blood-brain barrier disruption and reduces lipopolysaccharide loading and systemic inflammation, possibly by adjusting the gut microbiota. Front. Aging Neurosci. 2022, 14, 1018371. [Google Scholar] [CrossRef]
- Birmann, P.T.; Casaril, A.M.; Pesarico, A.P.; Caballero, P.S.; Smaniotto, T.Â.; Rodrigues, R.R.; Moreira, Â.N.; Conceição, F.R.; Sousa, F.S.S.; Collares, T.; et al. Komagataella pastoris KM71H modulates neuroimmune and oxidative stress parameters in animal models of depression: A proposal for a new probiotic with antidepressant-like effect. Pharmacol. Res. 2021, 171, 105740. [Google Scholar] [CrossRef]
- Ranuh, R.G.; Athiyyah, A.F.; Darma, A.; Riawan, W.; Gunawan, P.I.; Surono, I.S.; Sudarmo, S.M. Probiotic Lactobacillus plantarum IS-10506, Expression of Glial Fibrillary Acidic Protein and Platelet Endothelial Cell Adhesion Molecule-1 by Astrocytes and Endothelial Integrity: The Importance of Intestinal Microbiota as Blood Brain-Barrier Stabilizer. Int. J. Probiotics Prebiotics 2022, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.; Singh, D.P.; Singh, S.; Pinnaka, A.K.; Boparai, R.K.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Jiang, S.; Xu, M.; Ma, T.; Sun, Z.; Zhang, J. Lactobacillus fermentum HNU312 alleviated oxidative damage and behavioural abnormalities during brain development in early life induced by chronic lead exposure. Ecotoxicol. Environ. Saf. 2023, 251, 114543. [Google Scholar] [CrossRef]
- Du, J.; Yang, X.; Yu, D.; Xue, L. Probiotics Improve Flora Alternation and Memory Deficits in Aged Mice. FASEB J. 2019, 33, 516–517. [Google Scholar] [CrossRef]
- Saha, P.; Skidmore, P.T.; Holland, L.A.; Mondal, A.; Bose, D.; Seth, R.K.; Sullivan, K.; Janulewicz, P.A.; Horner, R.; Klimas, N. Andrographolide attenuates gut-brain-axis associated pathology in gulf war illness by modulating bacteriome-virome associated inflammation and microglia-neuron proinflammatory crosstalk. Brain Sci. 2021, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Chan, L.; Chen, K.Y.; Lee, H.H.; Huang, L.K.; Yang, Y.S.H.; Liu, Y.R.; Hu, C.J. Rifaximin Modifies Gut Microbiota and Attenuates Inflammation in Parkinson’s Disease: Preclinical and Clinical Studies. Cells 2022, 11, 3468. [Google Scholar] [CrossRef] [PubMed]
- Kanamaru, H.; Kawakita, F.; Nishikawa, H.; Nakano, F.; Asada, R.; Suzuki, H. Clarithromycin Ameliorates Early Brain Injury after Subarachnoid Hemorrhage via Suppressing Periostin-Related Pathways in Mice. Neurotherapeutics 2021, 18, 1880–1890. [Google Scholar] [CrossRef]
- Luo, A.; Li, S.; Wang, X.; Xie, Z.; Li, S.; Hua, D. Cefazolin Improves Anesthesia and Surgery-Induced Cognitive Impairments by Modulating Blood-Brain Barrier Function, Gut Bacteria and Short Chain Fatty Acids. Front. Aging Neurosci. 2021, 13, 748637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Li, K.; Wei, S.; Hu, L.; Yin, X.; Mai, Y.; Jiang, C.; Peng, X.; Cao, X.; Huang, Z.; Zhou, H. Protection of Fecal Microbiota Transplantation in a Mouse Model of Multiple Sclerosis. Mediat. Inflamm 2020, 2020, 2058272. [Google Scholar] [CrossRef]
- Jing, Y.; Bai, F.; Wang, L.; Yang, D.; Yan, Y.; Wang, Q.; Zhu, Y.; Yu, Y.; Chen, Z. Fecal Microbiota Transplantation Exerts Neuroprotective Effects in a Mouse Spinal Cord Injury Model by Modulating the Microenvironment at the Lesion Site. Microbiol. Spectr. 2022, 10, e0017722. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Hu, H.; Wang, F.; Li, L.; Zhu, W.; Shen, Y.; Xiu, J.; Xu, Q. Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav. Immun. 2021, 92, 102–114. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, L.; Wang, F.; Wang, Z.; Tong, X.; Yan, H. Orally Administered Brain Protein Combined with Probiotics Increases Treg Differentiation to Reduce Secondary Inflammatory Damage Following Craniocerebral Trauma. Front. Immunol. 2022, 13, 928343. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Surawicz, C.M. Fecal microbiota transplantation. Gastroenterol. Clin. 2017, 46, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, B.P.; Rank, K.M.; Khoruts, A. Fecal microbiota transplantation: Current status in treatment of GI and liver disease. Clin. Gastroenterol. Hepatol. 2019, 17, 353–361. [Google Scholar] [CrossRef] [PubMed]

| Study | Groups and Gender (Women/Men) | Specimen/Samples | Microbiota Alternations | Location | References | |
|---|---|---|---|---|---|---|
| Decreased | Increased | |||||
| Clinical studies | ||||||
| Bajaj et al., 2014 | Cirrhosis with HE vs healthy control (193/64) | Multi-tagged pyrosequencing on fecal samples | Clostridiales XIV; Ruminococcaceae; Lachnospiraceae | Enterococcaeae Staphylococcaceae; Enterobacteriaceae | United States | [21] |
| Bajaj et al., 2015 | Cirrhosis with HE and without HE vs healthy control (18/84) | Fecal specimen analysis using multi-tagged pyrosequencing techniques | Lachnospiraceae; Ruminococcaceae; Clostridiales XIV | Enterobacteriaceae; Enterococcaceae | United States | [22] |
| Bajaj et al., 2012 | Cirrhosis with HE/cirrhosis without HE (10/50) | Sigmoid mucosal specimen using 16S ribosomal RNA (rRNA) sequencing | Roseburia | Enterococcus; Veillonella; Megasphaera; Burkholderia | United States | [23] |
| Zhang et al., 2013 | Cirrhosis with MHE/cirrhosis without MHE (40/37) | Fecal specimen analysis using 16S rRNA-based pyrosequencing | - | Streptococcus salivarius (as a gut urease-containing bacteria) | China | [24] |
| Sung et al., 2019 | Acute episode of OHE/compensated cirrhosis (36/129) | Profiled fecal microbiome alternations from cohort | Bacteroidetes phylum | Firmicute; Proteobacteria; Actinobacteria | Taiwan | [25] |
| Wang et al., 2019 | Cirrhosis with MHE/ healthy controls (0/91) | 16S rRNA sequencing on stool | - | Pasteurellaceae Haemophilus; Alcaligenaceae Parasutterella | China | [26] |
| Bajaj et al., 2012 | Cirrhosis with HE/cirrhosis without HE (4/29) | Fecal specimen analysis using 16S rRNA sequencing | - | Veillonellaceae | United States | [27] |
| Bajaj et al., 2012 | Cirrhosis with HE/healthy controls (4/29) | Fecal specimen analysis using 16S rRNA sequencing) | Clostridiales_Incertae Sedis XIV; Ruminococcaceae; Lachnospiraceae | Leuconostocaceae; Enterobacteriaceae | United States | [27] |
| Chen et al., 2012 | Acute-on-chronic liver failure with HE/healthy controls (42/161) | Fecal microbiota analysis (16S rRNA sequencing) | Lachnospiraceae | - | China | [28] |
| Yukawa-Muto et al., 2022 | Cirrhosis with HE/cirrhosis without HE and healthy controls (34/45) | Fecal specimen analysis using16S rRNA and metagenomic sequencing | - | Streptococcus salivarius | Japan | [29] |
| Hua et al., 2022 | Cirrhosis with HE/cirrhosis without HE and healthy controls (13/37) | 16S rRNA analysis on fecal samples | Lachnospiraceae; Turicibacterales; Turicibacter; Turicibacteraceae | Pasteurellales; Pasteurellaceae; Haemophilus; Selenomonas | China | [30] |
| Lin et al., 2022 | Cirrhosis with MHE/ healthy controls (-) | 16S rRNA high-throughput sequencing on fecal specimen | Lachnospiraceae; Roseburia; Coprpcpccus | Veillonella | China | [31] |
| Bajaj et al., 2021 | Cirrhosis with HE/ healthy controls (0/150) | Stool metagenomics sequencing | Faecalibacterium phage; Myoviridae | - | United States | [32] |
| In Vivo studies | ||||||
| Kang, D. J. et al., 2016 | Carbon tetrachloride (CCL4)-induced HE/control C57BL/6 mice | Fecal samples from large intestine and cecum | Lachnospiraceae; Ruminococcaceae; Clostridiales XIV; Bifidobacteriaceae; | Staphylococcaceae; Enterobacteriaceae; Lactobacillaceae | - | [33] |
| Yang et al., 2022 | Bile duct ligation (BDL)-induced HE/control C57BL/6 mice | Fecal samples collected from sterile cage bottom | Bacteroidetes; Bacteroidia; MB-A2-108; Erysipelotrichia; Bacteroidales; Erysipelotrichales; Muribaculaceae; Tannerellaceae; Erysipelotrichaceae; Parabacteroides; GCA-900066225 (a genus of the Lachnospiraceae family) | Firmicutes; Bacilli; Clostridiales; Clostridia; Lactobacillales; Lachnospiraceae; Alistipes; Lactobacillus murinus; Lactobacillaceae; Lachnospiraceae; Rikenellaceae; Lactobacillus | - | [34] |
| Đurašević et al., 2021 | Thioacetamide (TAA)-induced liver injury/control rats | Fecal sample | Muribaculaceae; Desulfovibrionaceae; Lachnospiraceae | Christensenella; Rikenellaceae; Bacteroidaceae; Lactobacillaceae | - | [35] |
| Yang et al., 2022 | CCL4-induced liver fibrosis/control C57BL/6 mice | Fecal sample | Staphylococcus | Bacteroides; Acinetobacter | - | [36] |
| Wu et al., 2022 | CCL4-induced liver fibrosis/control C57BL/6 mice | Pyrosequencing analysis on fecal samples | Bifidobacterium; Turicibacter; (In addition to these 2 genera, 22 bacterial genera had lower abundance) | Lactobacillus; In addition to this genus, 6 bacterial genera had higher abundance | - | [37] |
| Cabrera-Rubio et al., 2019 | BDL-induced liver injury/control C57BL/6 mice | Pyrosequencing on fecal sample | Faecalibacterium | prausnitzii; Akkermansia; Prevotella; Bacteroides; unclassified Ruminococcaceae | - | [38] |
| De Minicis et al., 2014 | BDL-induced liver injury/control C57BL/6 mice | Fecal samples from cecum | Erysipelotrichaceae | Lachnospiraceae; Ruminococcaceae | - | [39] |
| Li et al., 2019 | D-galactosamine (GalN)-induced ALF/control Sprague–Dawley rats | Fecal sample | Christensenellaceae; Fastidiosipila; Romboutsia | Betaproteobacteria; Burkholderiales | - | [40] |
| Study | Case/Model | Method | Findings | Reference |
|---|---|---|---|---|
| Human studies | ||||
| Kato et al., 1992 | Postmortem on 9 patients with ALF | Electron microscopic study on cerebral cortex capillaries | Swollen and vacuolated endothelial cells, intact tight junctions, enlargement and vacuolated basement membrane, vacuolated pericytes and swollen perivascular astrocyte end-feet | [90] |
| Sa et al., 2010 | Postmortem analysis of brain samples from 14 patients with ALF | Electron microscopy | shrunken and vacuolated endothelial cells disrupted tight junctions and mitochondria, as well as swollen perivascular astrocyte end-feet | [91] |
| Animal studies | ||||
| Livingstone et al., 1977 | devascularization and total hepatectomy-induced HE/control Wistar rats | Trypan blue, 14C-inulin, 14C-sucrose, 14C-glucose, 14C-phenylalanine, electron microscopy | Increased brain uptake index of 14C-substrates; raised in the cerebral concentration of trypan blue; Swollen and vacuolated perivascular astrocyte end-feet and their mitochondria | [92] |
| Zaki et al., 1984 | Devascularized and GalN-induced ALF/control albino rats | 14C-inulin, 14C-sucrose, 14C-glucose | Increased brain uptake index of 14C substrates | [93] |
| Mossakowski et al., 1985 | TAA-induced HE rats | Electron microscopy on the cerebral cortex | Degenerative mitochondria and organelles in astrocytes, | [94] |
| Traber et al., 1987 | GalN-induced ALF/control New Zealand White rabbits | Horseradish peroxidase injection and electron microscopy | swollen and vacuolated astrocytic foot processes, intact endothelial cells | [95] |
| Nguyen et al., 2006 | AOM-induced ALF/control C57BL/6 mice | Evans blue dye extravasation, electron microscopy | Increased level of Evans blue dye in cerebral tissues; swollen perivascular astrocyte end-feet | [96] |
| Chen et al., 2009 | AOM-induced ALF/control mice | Western blot analysis of tight junction proteins | Decreased cerebral proteins of occludin, claudin-5 and ZO-1 | [97] |
| Kristiansen et al., 2010 | Hepatic devascularization and portocaval anastomosis-induced ALF/control Norwegian Landrace pigs | Electron microscopy examination on frontal lobe, cerebellum, and brain stem | Perivascular edema, abnormal processes of astrocytes and pericytes, swollen neuron | [98] |
| Sa et al., 2010 | GalN+LPS induced ALF/control BALB/c mice | Electron microscopy, immunohistochemistry, Evans blue dye extravasation | Shrunken and vacuolated endothelial cells, disrupted tight junctions, swollen perivascular astrocyte end-feet; lower protein levels of occludin; Increased cerebral level of Evans blue | [91] |
| Wang et al., 2011 | Acetaminophen-induced ALF/control BALB/c mice | Evans blue dye extravasation, electron microscopy, western blot analysis | Increased level of Evans blue dye in brain tissues; shrunken and vacuolated endothelial cells, incomplete tight junctions, swollen perivascular astrocyte end-feet; decreased the protein expression of occludin in cerebral tissues | [99] |
| Quinn et al., 2014 | BDL-induced liver injury/control Sprague Dawley rats | Immunofluorescence staining of brain microvasculature, Evans blue dye extravasation | losing the microvessel integrity, increased level of Evans blue dye in brain tissues; | [100] |
| Chastre et al., 2014 | Azoxymethane (AOM)+LPS-induced ALF and coma/control C57BL/6 | IgG extravasation | Increased protein expression of IgG in brain tissues | [101] |
| Faleiros et al., 2015 | TAA-induced ALF/control C57BL/6 mice | Electron microscopy | Abnormal structure of brain capillary activated endothelial cells and disrupted tight junctions, enlargement of perivascular astrocyte end-feet | [102] |
| McMillin et al., 2015 | AOM-induced ALF/control C57BL/6 mice | Evans blue dye extravasation | Increased cerebral level of Evans blue | [103] |
| Thabut et al., 2015 | BDL+NH3-induced cirrhosis/control rats | Fluorochrome extravasation | Increased cerebral fluorescence intensity | [104] |
| Grant et al., 2018 | TAA and AOM-induced HE/control C57BL/6 mice | Evans blue dye extravasation | Increased level of Evans blue dye in brain tissues | [105] |
| Disease (Animal Model) | Interventions | Findings | Reference |
|---|---|---|---|
| Probiotic and prebiotic | |||
| Chronic Parkinson’s disease mouse model | Probiotic (Lacticaseibacillus rhamnosus GG) + prebiotic (polymannuronic acid) | Improved integrity of the BBB, prevented dopaminergic neuronal loss and increased glial cell-derived neurotrophic factor and BDNF in striatum, and inhibited striatal apoptosis | [184] |
| APP/PS1 mouse model of Alzheimer’s disease | Probiotic supplement (several beneficial species) | Decreased fluorescence intensity of Evans blue in hippocampus, increased expression of tight junction proteins ZO-1 and occludin in brain, and reduced concentration of LPS and pro-inflammatory cytokines in brain | [185] |
| Mouse model of depressive-like behavior (repeated restraint stress and lipopolysaccharide) | New probiotic agent (Komagataella pastoris KM71H) | Decreased extravasation of Evans blue dye in prefrontal cortex, and prevented neuroinflammation and cerebral oxidative stress | [186] |
| LPS-induced model of systemic inflammation in rat | Probiotic (Lactobacillus plantarum IS 10506) | Upregulated glial fibrillary acidic protein (GFAP) and platelet endothelial cell adhesion molecule-1 (PECAM1)in brain | [187] |
| Stress (chronic unpredictable mild stress and sleep deprivation in mice) | Probiotic (Lactobacillus plantarum MTCC 9510) | Decreased Evans blue concentration in brain, increased hippocampal BDNF, prevented neuroinflammation and oxidative stress | [188] |
| Lead toxicity in mice | Probiotic (Lactobacillus fermentum HNU312) | Increased the integrity of the BBB, decreased neuroinflammation, and improved anxiety-like and depression-like behaviors | [189] |
| Senescence-accelerated mouse prone 8 mouse model of aging | Probiotics (species of Lactobacillus and Bifidobacterium) | Improved disruption of the BBB, decreased astrocyte reactivity and microglial activation, reduced plasma and cerebral LPS concentrations, decreased mRNA expression of toll-like receptor 4 and nuclear factor-κB, and reduced neuroinflammation in the brain | [190] |
| Acute mice models of Gulf War illness | Prebiotic (andrographolide) | Restored claudin-5 protein level, increased BDNF, and decreased microglial activation in brain | [191] |
| Antibiotic therapy | |||
| Transgenic MitoPark mouse model of Parkinson’s disease | Rifaximin | Decreased circulatory levels of claudin-5 and occludin (protected the BBB), suppressed systemic inflammation, reduced astrocyte reactivity, and decreased microglial activation | [192] |
| Mouse model of subarachnoid hemorrhage | Clarithromycin | Reduced extravasation of immunoglobulin G, and increased ZO-1 protein expression, | [193] |
| Mouse model of postoperative cognitive dysfunction | Cefazolin | Increased expression of ZO-1 and occludin, and decreased extravasation of Evans blue in brain tissue | [194] |
| Fecal microbiota transplantation | |||
| Chronic rotenone-induced Parkinson’s disease mouse model | FMT (from control mice) | Restored tight junction proteins occludin, claudin-5, and ZO-1, decreased astrocyte reactivity, reduced microglial activation, decreased concentration of lipopolysaccharide, and suppressed neuroinflammation (TLR4/MyD88/NF-κB signaling pathway) in substantia nigra | [195] |
| Experimental autoimmune encephalomyelitis mouse model of multiple sclerosis | FMT (from control mice) | Decreased extravasation of Evans blue, increased protein expression of occludin-5 in the spinal cord, and reduced astrocyte reactivity, as well as decreased microglial activation in brain tissue | [196] |
| Mouse model of spinal cord injury | FMT (from control mice) | Reduced the level of Evans blue, restored expression of ZO-1 and occludin, decreased astrocyte reactivity, and reduced microglial activation in the spinal cord | [197] |
| Antibiotics-induced microbiome depletion and BBB permeabilization | FMT (pathogen-free mice) | Increased expression of ZO-1 and ZO-2 proteins in cerebral microvessels | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbazi, A.; Sepehrinezhad, A.; Vahdani, E.; Jamali, R.; Ghasempour, M.; Massoudian, S.; Sahab Negah, S.; Larsen, F.S. Gut Dysbiosis and Blood-Brain Barrier Alteration in Hepatic Encephalopathy: From Gut to Brain. Biomedicines 2023, 11, 1272. https://doi.org/10.3390/biomedicines11051272
Shahbazi A, Sepehrinezhad A, Vahdani E, Jamali R, Ghasempour M, Massoudian S, Sahab Negah S, Larsen FS. Gut Dysbiosis and Blood-Brain Barrier Alteration in Hepatic Encephalopathy: From Gut to Brain. Biomedicines. 2023; 11(5):1272. https://doi.org/10.3390/biomedicines11051272
Chicago/Turabian StyleShahbazi, Ali, Ali Sepehrinezhad, Edris Vahdani, Raika Jamali, Monireh Ghasempour, Shirin Massoudian, Sajad Sahab Negah, and Fin Stolze Larsen. 2023. "Gut Dysbiosis and Blood-Brain Barrier Alteration in Hepatic Encephalopathy: From Gut to Brain" Biomedicines 11, no. 5: 1272. https://doi.org/10.3390/biomedicines11051272
APA StyleShahbazi, A., Sepehrinezhad, A., Vahdani, E., Jamali, R., Ghasempour, M., Massoudian, S., Sahab Negah, S., & Larsen, F. S. (2023). Gut Dysbiosis and Blood-Brain Barrier Alteration in Hepatic Encephalopathy: From Gut to Brain. Biomedicines, 11(5), 1272. https://doi.org/10.3390/biomedicines11051272






