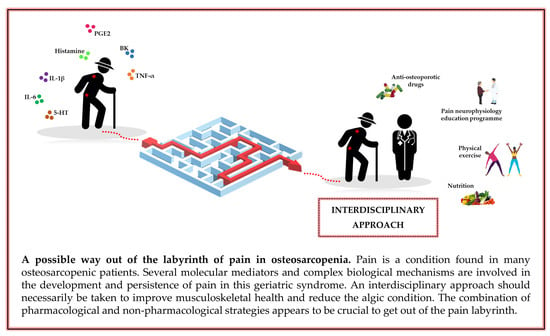
Osteosarcopenia and Pain: Do We Have a Way Out?
Abstract
:1. Introduction
2. Literature Search Strategy
3. Chronic Pain in OSP
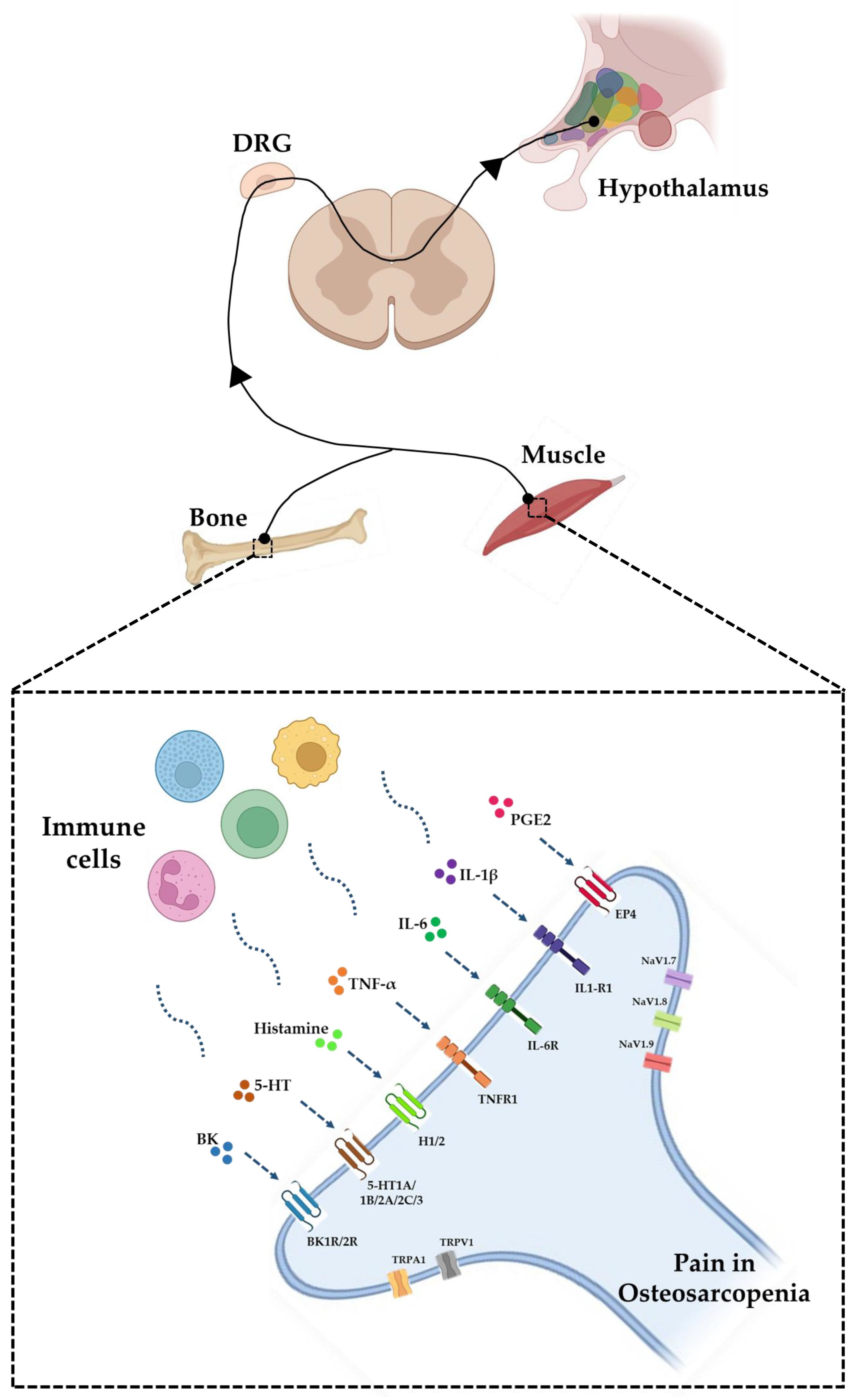
3.1. The Ion Channels of Pain
3.2. The Crosstalk between the Immune System and the Nervous System
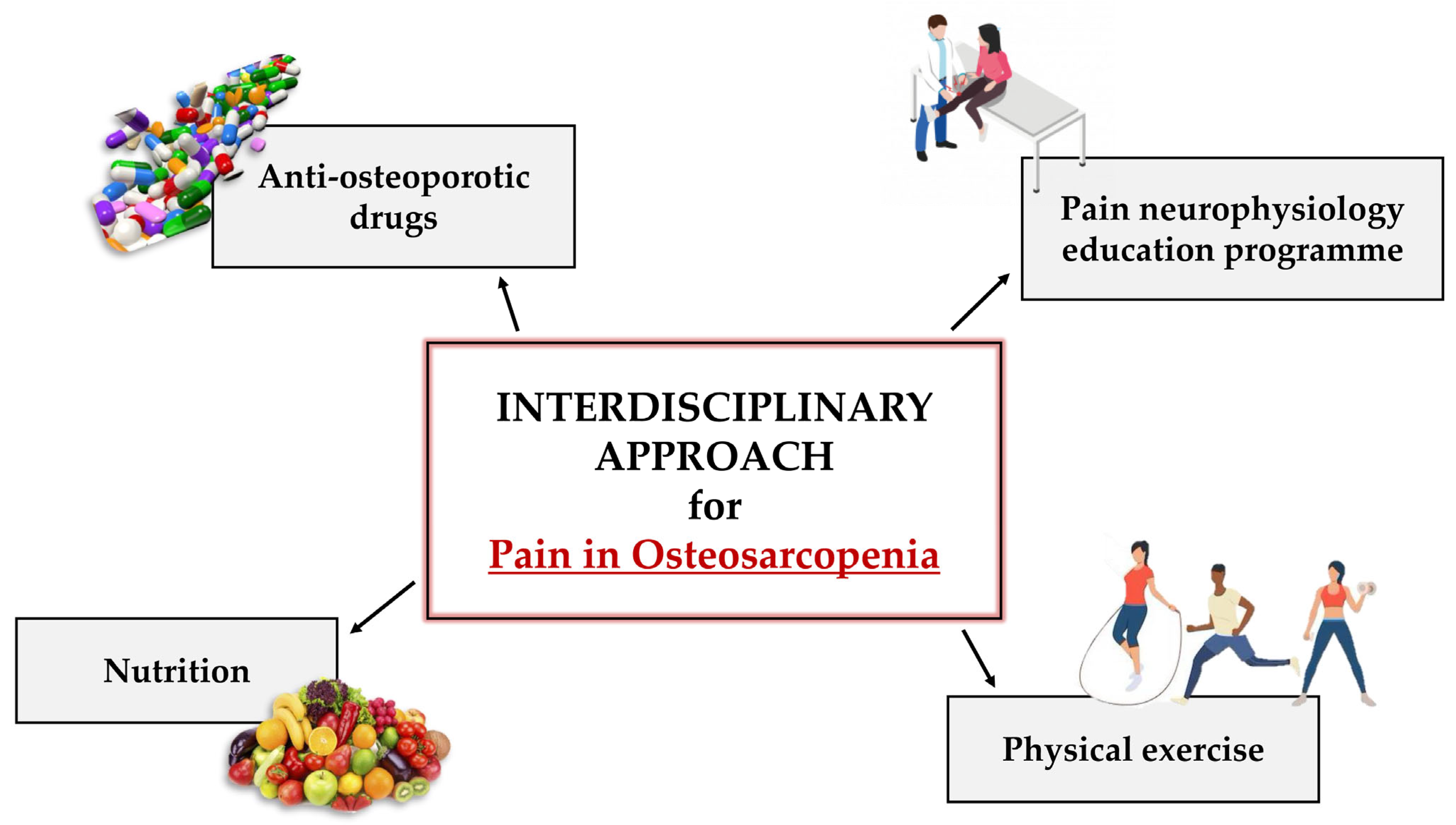
4. Management of Pain in OSP Patients
4.1. Pharmacological Therapy: The Power of Anti-Osteoporotic Drugs
4.1.1. Bisphosphonates
4.1.2. Denosumab
4.1.3. Teriparatide
4.2. Non-Pharmacological Therapy: From Mind to Body
4.2.1. PNE
4.2.2. Physical Exercise
4.2.3. Nutrition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckingham, M.; Bajard, L.; Chang, T.; Daubas, P.; Hadchouel, J.; Meilhac, S.; Montarras, D.; Rocancourt, D.; Relaix, F. The formation of skeletal muscle: From somite to limb. J. Anat. 2003, 202, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castillo, N.; Johnson, M.L. Bone-Muscle Mutual Interactions. Curr. Osteoporos. Rep. 2020, 18, 408–421. [Google Scholar] [CrossRef]
- DiGirolamo, D.J.; Kiel, D.P.; Esser, K.A. Bone and skeletal muscle: Neighbors with close ties. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone-Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef]
- He, C.; He, W.; Hou, J.; Chen, K.; Huang, M.; Yang, M.; Luo, X.; Li, C. Bone and Muscle Crosstalk in Aging. Front. Cell Dev. Biol. 2020, 8, 585644. [Google Scholar] [CrossRef]
- Tominari, T.; Ichimaru, R.; Taniguchi, K.; Yumoto, A.; Shirakawa, M.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Itoh, Y.; Shiba, D.; et al. Hypergravity and microgravity exhibited reversal effects on the bone and muscle mass in mice. Sci. Rep. 2019, 9, 6614. [Google Scholar] [CrossRef]
- Aryana, I.G.P.S.; Rini, S.S.; Soejono, C.H. Importance of Sclerostin as Bone-Muscle Mediator Crosstalk. Ann. Geriatr. Med. Res. 2022, 26, 72–82. [Google Scholar] [CrossRef]
- Cariati, I.; Scimeca, M.; Bonanni, R.; Triolo, R.; Naldi, V.; Toro, G.; Marini, M.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; et al. Role of Myostatin in Muscle Degeneration by Random Positioning Machine Exposure: An in vitro Study for the Treatment of Sarcopenia. Front. Physiol. 2022, 13, 782000. [Google Scholar] [CrossRef]
- Tarantino, U.; Cariati, I.; Marini, M.; D’Arcangelo, G.; Tancredi, V.; Primavera, M.; Iundusi, R.; Gasbarra, E.; Scimeca, M. Effects of Simulated Microgravity on Muscle Stem Cells Activity. Cell. Physiol. Biochem. 2020, 54, 736–747. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Scimeca, M.; Rinaldi, A.M.; Marini, M.; Tarantino, U.; Tancredi, V. Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line. Life 2022, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, H.; Mani, D.; Singh, D.; Gupta, A. The underlying pathophysiology and therapeutic approaches for osteoporosis. Med. Res. Rev. 2018, 38, 2024–2057. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.J.S.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Litwic, A.; Cooper, C.; Dennison, E. Determinants of Muscle and Bone Aging. J. Cell. Physiol. 2015, 230, 2618–2625. [Google Scholar] [CrossRef]
- Fougère, B.; Boulanger, E.; Nourhashémi, F.; Guyonnet, S.; Cesari, M. Chronic Inflammation: Accelerator of Biological Aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2017, 72, 1218–1225. [Google Scholar] [CrossRef]
- Polito, A.; Barnaba, L.; Ciarapica, D.; Azzini, E. Osteosarcopenia: A Narrative Review on Clinical Studies. Int. J. Mol. Sci. 2022, 23, 5591. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Greggi, C.; Visconti, V.V.; Cariati, I.; Tallarico, M.; Fauceglia, M.; Iundusi, R.; Albanese, M.; Chiaramonte, C.; Gasbarra, E. T-Score and Handgrip Strength Association for the Diagnosis of Osteosarcopenia: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2597. [Google Scholar] [CrossRef]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Muir, S.W.; Montero-Odasso, M.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of Osteosarcopenia in Older Individuals With a History of Falling. J. Am. Med. Dir. Assoc. 2015, 16, 290–295. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia. Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Chapter 53—Calcitonin: History, Physiology, Pathophysiology and Therapeutic Applications; Orwoll, E.S., Bilezikian, J.P., Vanderschueren, D.B.T.-O., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 653–666. ISBN 978-0-12-374602-3. [Google Scholar]
- Hou, W.; Chen, S.; Zhu, C.; Gu, Y.; Zhu, L.; Zhou, Z. Associations between smoke exposure and osteoporosis or osteopenia in a US NHANES population of elderly individuals. Front. Endocrinol. 2023, 14, 1074574. [Google Scholar] [CrossRef] [PubMed]
- Scheuren, A.C.; D’Hulst, G.; Kuhn, G.A.; Masschelein, E.; Wehrle, E.; De Bock, K.; Müller, R. Hallmarks of frailty and osteosarcopenia in prematurely aged PolgA((D257A/D257A)) mice. J. Cachexia. Sarcopenia Muscle 2020, 11, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Maeda, K.; Satake, S.; Matsui, Y.; Arai, H. Osteosarcopenia, the co-existence of osteoporosis and sarcopenia, is associated with social frailty in older adults. Aging Clin. Exp. Res. 2022, 34, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Citko, A.; Górski, S.; Marcinowicz, L.; Górska, A. Sedentary Lifestyle and Nonspecific Low Back Pain in Medical Personnel in North-East Poland. BioMed Res. Int. 2018, 2018, 1965807. [Google Scholar] [CrossRef]
- Mendonça, C.R.; Noll, M.; de Carvalho Santos, A.S.E.A.; Rodrigues, A.P.D.S.; Silveira, E.A. High prevalence of musculoskeletal pain in individuals with severe obesity: Sites, intensity, and associated factors. Korean J. Pain 2020, 33, 245–257. [Google Scholar] [CrossRef]
- Abate, M.; Vanni, D.; Pantalone, A.; Salini, V. Cigarette smoking and musculoskeletal disorders. Muscles Ligaments Tendons J. 2013, 3, 63–69. [Google Scholar] [CrossRef]
- Patrick, N.; Emanski, E.; Knaub, M.A. Acute and chronic low back pain. Med. Clin. North Am. 2014, 98, 777–789. [Google Scholar] [CrossRef]
- Lin, T.; Dai, M.; Xu, P.; Sun, L.; Shu, X.; Xia, X.; Zhao, Y.; Song, Q.; Guo, D.; Deng, C.; et al. Prevalence of Sarcopenia in Pain Patients and Correlation Between the Two Conditions: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2022, 23, 902.e1–902.e20. [Google Scholar] [CrossRef]
- Aragonès, E.; Rambla, C.; López-Cortacans, G.; Tomé-Pires, C.; Sánchez-Rodríguez, E.; Caballero, A.; Miró, J. Effectiveness of a collaborative care intervention for managing major depression and chronic musculoskeletal pain in primary care: A cluster-randomised controlled trial. J. Affect. Disord. 2019, 252, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Patchay, S.; Soundy, A.; Schofield, P. The avoidance of activities due to fear of falling contributes to sedentary behavior among community-dwelling older adults with chronic musculoskeletal pain: A multisite observational study. Pain Med. 2014, 15, 1861–1871. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Perrot, S.; Cohen, M.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. The IASP classification of chronic pain for ICD-11: Chronic secondary musculoskeletal pain. Pain 2019, 160, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Alsoof, D.; Anderson, G.; McDonald, C.L.; Basques, B.; Kuris, E.; Daniels, A.H. Diagnosis and Management of Vertebral Compression Fracture. Am. J. Med. 2022, 135, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, S.; Hashida, R.; Matsuse, H.; Higashi, E.; Bekki, M.; Iwanaga, S.; Hara, K.; Higuchi, T.; Hirakawa, Y.; Kubota, A.; et al. The impact of sarcopenia on low back pain and quality of life in patients with osteoporosis. BMC Musculoskelet Disord. 2022, 23, 142. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Wood, J.N.; Boorman, J.P.; Okuse, K.; Baker, M.D. Voltage-gated sodium channels and pain pathways. J. Neurobiol. 2004, 61, 55–71. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef]
- Yoshino, K.; Suzuki, M.; Kawarai, Y.; Sakuma, Y.; Inoue, G.; Orita, S.; Yamauchi, K.; Aoki, Y.; Ishikawa, T.; Miyagi, M.; et al. Increase of TRPV1-immunoreactivity in dorsal root ganglia neurons innervating the femur in a rat model of osteoporosis. Yonsei Med. J. 2014, 55, 1600–1605. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Bloom, A.P.; Mantyh, W.G.; Koewler, N.J.; Freeman, K.T.; Delong, D.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Capsaicin-sensitive sensory nerve fibers contribute to the generation and maintenance of skeletal fracture pain. Neuroscience 2009, 162, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Chapman, H.; Jalava, N.; Korjamo, T.; Saarnilehto, M.; Lindstedt, K.; Pertovaara, A. TRPA1: A transducer and amplifier of pain and inflammation. Basic Clin. Pharmacol. Toxicol. 2014, 114, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ibe, K.; Iba, K.; Hanaka, M.; Kiyomoto, K.; Hayakawa, H.; Teramoto, A.; Emori, M.; Yamashita, T. Hypersensitivity to cold stimulation associated with regional osteoporotic changes in tail-suspended mice. J. Bone Miner. Metab. 2020, 38, 469–480. [Google Scholar] [CrossRef]
- Huang, K.-C.; Chiang, Y.-F.; Huang, T.-C.; Chen, H.-Y.; Lin, P.-H.; Ali, M.; Hsia, S.-M. Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo. J. Cachexia. Sarcopenia Muscle 2023, 14, 182–197. [Google Scholar] [CrossRef]
- Wang, S.; Brigoli, B.; Lim, J.; Karley, A.; Chung, M.-K. Roles of TRPV1 and TRPA1 in Spontaneous Pain from Inflamed Masseter Muscle. Neuroscience 2018, 384, 290–299. [Google Scholar] [CrossRef]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The Na(V)1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef]
- Amir, R.; Argoff, C.E.; Bennett, G.J.; Cummins, T.R.; Durieux, M.E.; Gerner, P.; Gold, M.S.; Porreca, F.; Strichartz, G.R. The role of sodium channels in chronic inflammatory and neuropathic pain. J. Pain 2006, 7, S1–S29. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, R.; McGrew, S.Y.; Baxter, J.C.; Kiveric, E.; Elmslie, K.S. Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J. Neurophysiol. 2012, 108, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Verri, W.A.J.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Ma, W.; Li, L.; Xing, S. PGE2/EP4 receptor and TRPV1 channel are involved in repeated restraint stress-induced prolongation of sensitization pain evoked by subsequent PGE2 challenge. Brain Res. 2019, 1721, 146335. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.T.V.; Palla, A.R.; Blake, M.R.; Yucel, N.D.; Wang, Y.X.; Magnusson, K.E.G.; Holbrook, C.A.; Kraft, P.E.; Delp, S.L.; Blau, H.M. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl. Acad. Sci. USA 2017, 114, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.R.; Ravichandran, M.; Wang, Y.X.; Alexandrova, L.; Yang, A.V.; Kraft, P.; Holbrook, C.A.; Schürch, C.M.; Ho, A.T.V.; Blau, H.M. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science 2021, 371, eabc8059. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Brown, L.A.; Lim, E.; Castor-Macias, J.A.; Larouche, J.; Macpherson, P.C.D.; Davis, C.; Aguilar, C.A.; Maddipati, K.R.; Brooks, S. V Metabolipidomic profiling reveals an age-related deficiency of skeletal muscle pro-resolving mediators that contributes to maladaptive tissue remodeling. Aging Cell 2021, 20, e13393. [Google Scholar] [CrossRef]
- Liu, X.-H.; Kirschenbaum, A.; Yao, S.; Levine, A.C. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann. N. Y. Acad. Sci. 2006, 1068, 225–233. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Ding, Y.; Xie, W.; Li, H.; Li, S.; Li, Y.; Cai, M. Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis. Cells 2022, 11, 2760. [Google Scholar] [CrossRef]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef]
- Canlı, K.; Billens, A.; Van Oosterwijck, J.; Meeus, M.; De Meulemeester, K. Systemic Cytokine Level Differences in Patients with Chronic Musculoskeletal Spinal Pain Compared to Healthy Controls and Its Association with Pain Severity: A Systematic Review. Pain Med. 2022, 23, 1947–1964. [Google Scholar] [CrossRef]
- Mailhot, B.; Christin, M.; Tessandier, N.; Sotoudeh, C.; Bretheau, F.; Turmel, R.; Pellerin, È.; Wang, F.; Bories, C.; Joly-Beauparlant, C.; et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J. Exp. Med. 2020, 217, e20191430. [Google Scholar] [CrossRef]
- Svensson, C.I. Interleukin-6: A local pain trigger? Arthritis Res. Ther. 2010, 12, 145. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A. Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.-L.; Hu, H.-Y.; Rong, Y.-D.; Wang, J.; Wang, J.-X.; Zhou, X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.-D.; Bian, A.-L.; Hu, H.-Y.; Ma, Y.; Zhou, X.-Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Kato, S.; Nagao, N.; Miyamura, G.; Naito, Y.; Sudo, A. Interleukin-6 Inhibitor Suppresses Hyperalgesia Without Improvement in Osteoporosis in a Mouse Pain Model of Osteoporosis. Calcif. Tissue Int. 2019, 104, 658–666. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Miyamura, G.; Nagao, N.; Kato, S.; Naito, Y.; Sudo, A. Functional Block of Interleukin-6 Reduces a Bone Pain Marker but Not Bone Loss in Hindlimb-Unloaded Mice. Int. J. Mol. Sci. 2020, 21, 3521. [Google Scholar] [CrossRef]
- Yue, J.-X.; Wang, R.-R.; Yu, J.; Tang, Y.-Y.; Hou, W.-W.; Lou, G.-D.; Zhang, S.-H.; Chen, Z. Histamine upregulates Nav1.8 expression in primary afferent neurons via H2 receptors: Involvement in neuropathic pain. CNS Neurosci. Ther. 2014, 20, 883–892. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Mistry, S.; Paule, C.C.; Varga, A.; Photiou, A.; Jenes, A.; Avelino, A.; Buluwela, L.; Nagy, I. Prolonged exposure to bradykinin and prostaglandin E2 increases TRPV1 mRNA but does not alter TRPV1 and TRPV1b protein expression in cultured rat primary sensory neurons. Neurosci. Lett. 2014, 564, 89–93. [Google Scholar] [CrossRef]
- Uchitomi, R.; Hatazawa, Y.; Senoo, N.; Yoshioka, K.; Fujita, M.; Shimizu, T.; Miura, S.; Ono, Y.; Kamei, Y. Metabolomic Analysis of Skeletal Muscle in Aged Mice. Sci. Rep. 2019, 9, 10425. [Google Scholar] [CrossRef]
- Fitzpatrick, L.A.; Buzas, E.; Gagne, T.J.; Nagy, A.; Horvath, C.; Ferencz, V.; Mester, A.; Kari, B.; Ruan, M.; Falus, A.; et al. Targeted deletion of histidine decarboxylase gene in mice increases bone formation and protects against ovariectomy-induced bone loss. Proc. Natl. Acad. Sci. USA 2003, 100, 6027–6032. [Google Scholar] [CrossRef]
- Lesclous, P.; Schramm, F.; Gallina, S.; Baroukh, B.; Guez, D.; Saffar, J.L. Histamine mediates osteoclastic resorption only during the acute phase of bone loss in ovariectomized rats. Exp. Physiol. 2006, 91, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; Tarantino, U. Chronic Pain in Musculoskeletal Diseases: Do You Know Your Enemy? J. Clin. Med. 2022, 11, 2609. [Google Scholar] [CrossRef] [PubMed]
- Gerdle, B.; Ghafouri, B.; Ernberg, M.; Larsson, B. Chronic musculoskeletal pain: Review of mechanisms and biochemical biomarkers as assessed by the microdialysis technique. J. Pain Res. 2014, 7, 313–326. [Google Scholar] [CrossRef]
- Pace, M.C.; Passavanti, M.B.; De Nardis, L.; Bosco, F.; Sansone, P.; Pota, V.; Barbarisi, M.; Palagiano, A.; Iannotti, F.A.; Panza, E.; et al. Nociceptor plasticity: A closer look. J. Cell Physiol. 2018, 233, 2824–2838. [Google Scholar] [CrossRef]
- Babatunde, O.O.; Jordan, J.L.; Van der Windt, D.A.; Hill, J.C.; Foster, N.E.; Protheroe, J. Effective treatment options for musculoskeletal pain in primary care: A systematic overview of current evidence. PLoS ONE 2017, 12, e0178621. [Google Scholar] [CrossRef]
- Vellucci, R.; Terenzi, R.; Kanis, J.A.; Kress, H.G.; Mediati, R.D.; Reginster, J.-Y.; Rizzoli, R.; Brandi, M.L. Understanding osteoporotic pain and its pharmacological treatment. Osteoporos. Int. 2018, 29, 1477–1491. [Google Scholar] [CrossRef]
- Das, S.; Crockett, J.C. Osteoporosis—A current view of pharmacological prevention and treatment. Drug Des. Devel. Ther. 2013, 7, 435–448. [Google Scholar] [CrossRef]
- Black, D.M.; Thompson, D.E.; Bauer, D.C.; Ensrud, K.; Musliner, T.; Hochberg, M.C.; Nevitt, M.C.; Suryawanshi, S.; Cummings, S.R. Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. FIT Research Group. J. Clin. Endocrinol. Metab. 2000, 85, 4118–4124. [Google Scholar] [CrossRef]
- Ohtori, S.; Akazawa, T.; Murata, Y.; Kinoshita, T.; Yamashita, M.; Nakagawa, K.; Inoue, G.; Nakamura, J.; Orita, S.; Ochiai, N.; et al. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2010, 17, 209–213. [Google Scholar] [CrossRef]
- Iwamoto, J.; Makita, K.; Sato, Y.; Takeda, T.; Matsumoto, H. Alendronate is more effective than elcatonin in improving pain and quality of life in postmenopausal women with osteoporosis. Osteoporos. Int. 2011, 22, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Shiao, M.-S.; Mao, T.-Y. Retrospective Study of the Effects of Zoledronic Acid on Muscle Mass in Osteoporosis Patients. Drug Des. Devel. Ther. 2021, 15, 3711–3715. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R. Denosumab for the treatment of osteoporosis. Osteoporos. Sarcopenia 2017, 3, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Tetsunaga, T.; Tetsunaga, T.; Nishida, K.; Tanaka, M.; Sugimoto, Y.; Takigawa, T.; Takei, Y.; Ozaki, T. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2017, 22, 230–236. [Google Scholar] [CrossRef]
- Petranova, T.; Sheytanov, I.; Monov, S.; Nestorova, R.; Rashkov, R. Denosumab improves bone mineral density and microarchitecture and reduces bone pain in women with osteoporosis with and without glucocorticoid treatment. Biotechnol. Biotechnol. Equip. 2014, 28, 1127–1137. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef]
- Aryana, I.G.P.S.; Rini, S.S.; Setiati, S. Denosumab’s Therapeutic Effect for Future Osteosarcopenia Therapy: A Systematic Review and Meta-Analysis. Ann. Geriatr. Med. Res. 2023, 27, 32–41. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, W.; Zhao, S.; Mo, X.; Yuan, W.; Cheung, W.H.; Fu, D.; Chen, B. Effect of Teriparatide on pain relief, and quality of life in postmenopausal females with osteoporotic vertebral compression fractures, a retrospective cohort study. Ann. Palliat. Med. 2021, 10, 4000–4007. [Google Scholar] [CrossRef]
- Ifuku, E.; Yoshimura, T.; Uzawa, T.; Hokonohara, T. Safety and efficacy in actual clinical practice of once-weekly subcutaneous teriparatide for osteoporosis patients with a high fracture risk. Osteoporos. Sarcopenia 2019, 5, 44–50. [Google Scholar] [CrossRef]
- Kato, S.; Wakabayashi, H.; Nakagawa, T.; Miyamura, G.; Naito, Y.; Iino, T.; Sudo, A. Teriparatide improves pain-related behavior and prevents bone loss in ovariectomized mice. J. Orthop. Surg. 2020, 28, 2309499019893194. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Miyakoshi, N.; Kasukawa, Y.; Nozaka, K.; Tsuchie, H.; Nagahata, I.; Yuasa, Y.; Abe, K.; Saito, H.; Shoji, R.; et al. Teriparatide and exercise improve bone, skeletal muscle, and fat parameters in ovariectomized and tail-suspended rats. J. Bone Miner. Metab. 2021, 39, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, T.; Ando, T.; Hata, T.; Takayama, Y.; Ohba, T.; Ichikawa, J.; Takiyama, Y.; Tatsuno, R.; Koyama, K.; Haro, H. Exogenous parathyroid hormone attenuates ovariectomy-induced skeletal muscle weakness in vivo. Bone 2021, 151, 116029. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.; Diener, I.; Butler, D.S.; Puentedura, E.J. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch. Phys. Med. Rehabil. 2011, 92, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.L.; Ryan, C.G.; Martin, D.J. Pain neurophysiology education for the management of individuals with chronic low back pain: Systematic review and meta-analysis. Man. Ther. 2011, 16, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Saraçoğlu, İ.; Kaya, İ.; Cingöz, İ.D.; Emre Aydın, H. Preoperative pain neurophysiology education for lumbar radiculopathy: A randomized-controlled trial. Turkish J. Phys. Med. Rehabil. 2021, 67, 328–335. [Google Scholar] [CrossRef]
- Wijma, A.J.; van Wilgen, C.P.; Meeus, M.; Nijs, J. Clinical biopsychosocial physiotherapy assessment of patients with chronic pain: The first step in pain neuroscience education. Physiother. Theory Pract. 2016, 32, 368–384. [Google Scholar] [CrossRef]
- Bodes Pardo, G.; Lluch Girbés, E.; Roussel, N.A.; Gallego Izquierdo, T.; Jiménez Penick, V.; Pecos Martín, D. Pain Neurophysiology Education and Therapeutic Exercise for Patients With Chronic Low Back Pain: A Single-Blind Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 338–347. [Google Scholar] [CrossRef]
- Miller, J.; MacDermid, J.C.; Walton, D.M.; Richardson, J. Chronic Pain Self-Management Support With Pain Science Education and Exercise (COMMENCE) for People With Chronic Pain and Multiple Comorbidities: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2020, 101, 750–761. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Papadimitriou, K.; Voulgaridou, G.; Georgaki, E.; Tsotidou, E.; Zantidou, O.; Papandreou, D. Exercise and Nutrition Impact on Osteoporosis and Sarcopenia-The Incidence of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 4499. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Jakob, F.; Engelke, K.; von Stengel, S. Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study. Nutrients 2020, 12, 2341. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. BioMed Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Onorato, F.; Mastrogregori, A.; Rossi, D.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Role of Physical Activity in Bone-Muscle Crosstalk: Biological Aspects and Clinical Implications. J. Funct. Morphol. Kinesiol. 2021, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Liu, L.; Guo, J.; Chen, X.; Tong, X.; Xu, J.; Zou, J. The Role of Irisin in Exercise-Mediated Bone Health. Front. cell Dev. Biol. 2021, 9, 668759. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Romagnoli, C.; D’Arcangelo, G.; Annino, G.; Tancredi, V. Whole Body Vibration: A Valid Alternative Strategy to Exercise? J. Funct. Morphol. Kinesiol. 2022, 7, 99. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Pallone, G.; Romagnoli, C.; Rinaldi, A.M.; Annino, G.; D’Arcangelo, G.; Tancredi, V. Whole Body Vibration Improves Brain and Musculoskeletal Health by Modulating the Expression of Tissue-Specific Markers: FNDC5 as a Key Regulator of Vibration Adaptations. Int. J. Mol. Sci. 2022, 23, 10388. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Annino, G.; Scimeca, M.; Bonanno, E.; D’Arcangelo, G.; Tancredi, V. Dose-Response Effect of Vibratory Stimulus on Synaptic and Muscle Plasticity in a Middle-Aged Murine Model. Front. Physiol. 2021, 12, 678449. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef]
- Izquierdo-Alventosa, R.; Inglés, M.; Cortés-Amador, S.; Gimeno-Mallench, L.; Chirivella-Garrido, J.; Kropotov, J.; Serra-Añó, P. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3634. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Beavers, D.P.; Queen, K.; Mihalko, S.L.; Miller, G.D.; Losina, E.; Katz, J.N.; Loeser, R.F.; DeVita, P.; Hunter, D.J.; et al. Effect of Diet and Exercise on Knee Pain in Patients With Osteoarthritis and Overweight or Obesity: A Randomized Clinical Trial. JAMA 2022, 328, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Do Nutritional Factors Interact with Chronic Musculoskeletal Pain? A Systematic Review. J. Clin. Med. 2020, 9, 702. [Google Scholar] [CrossRef]
- Perna, S.; Alalwan, T.A.; Al-Thawadi, S.; Negro, M.; Parimbelli, M.; Cerullo, G.; Gasparri, C.; Guerriero, F.; Infantino, V.; Diana, M.; et al. Evidence-Based Role of Nutrients and Antioxidants for Chronic Pain Management in Musculoskeletal Frailty and Sarcopenia in Aging. Geriatrics 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J. Bioinformatic approaches to interrogating vitamin D receptor signaling. Mol. Cell Endocrinol. 2017, 453, 3–13. [Google Scholar] [CrossRef]
- Koundourakis, N.E.; Avgoustinaki, P.D.; Malliaraki, N.; Margioris, A.N. Muscular effects of vitamin D in young athletes and non-athletes and in the elderly. Hormones 2016, 15, 471–488. [Google Scholar] [CrossRef]
- Wu, Z.; Malihi, Z.; Stewart, A.W.; Lawes, C.M.; Scragg, R. Effect of Vitamin D Supplementation on Pain: A Systematic Review and Meta-analysis. Pain Physician 2016, 19, 415–427. [Google Scholar]
- Lombardo, M.; Feraco, A.; Ottaviani, M.; Rizzo, G.; Camajani, E.; Caprio, M.; Armani, A. The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain. Nutrients 2022, 14, 3010. [Google Scholar] [CrossRef]
- Helde-Frankling, M.; Björkhem-Bergman, L. Vitamin D in Pain Management. Int. J. Mol. Sci. 2017, 18, 2170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, R.; Gino Grillo, S.; Cariati, I.; Tranquillo, L.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Osteosarcopenia and Pain: Do We Have a Way Out? Biomedicines 2023, 11, 1285. https://doi.org/10.3390/biomedicines11051285
Bonanni R, Gino Grillo S, Cariati I, Tranquillo L, Iundusi R, Gasbarra E, Tancredi V, Tarantino U. Osteosarcopenia and Pain: Do We Have a Way Out? Biomedicines. 2023; 11(5):1285. https://doi.org/10.3390/biomedicines11051285
Chicago/Turabian StyleBonanni, Roberto, Sonia Gino Grillo, Ida Cariati, Lucia Tranquillo, Riccardo Iundusi, Elena Gasbarra, Virginia Tancredi, and Umberto Tarantino. 2023. "Osteosarcopenia and Pain: Do We Have a Way Out?" Biomedicines 11, no. 5: 1285. https://doi.org/10.3390/biomedicines11051285
APA StyleBonanni, R., Gino Grillo, S., Cariati, I., Tranquillo, L., Iundusi, R., Gasbarra, E., Tancredi, V., & Tarantino, U. (2023). Osteosarcopenia and Pain: Do We Have a Way Out? Biomedicines, 11(5), 1285. https://doi.org/10.3390/biomedicines11051285