Effectiveness of Vestibular Rehabilitation in Improving Health Status and Balance in Patients with Fibromyalgia Syndrome: A Single-Blind Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Sample Size Calculation
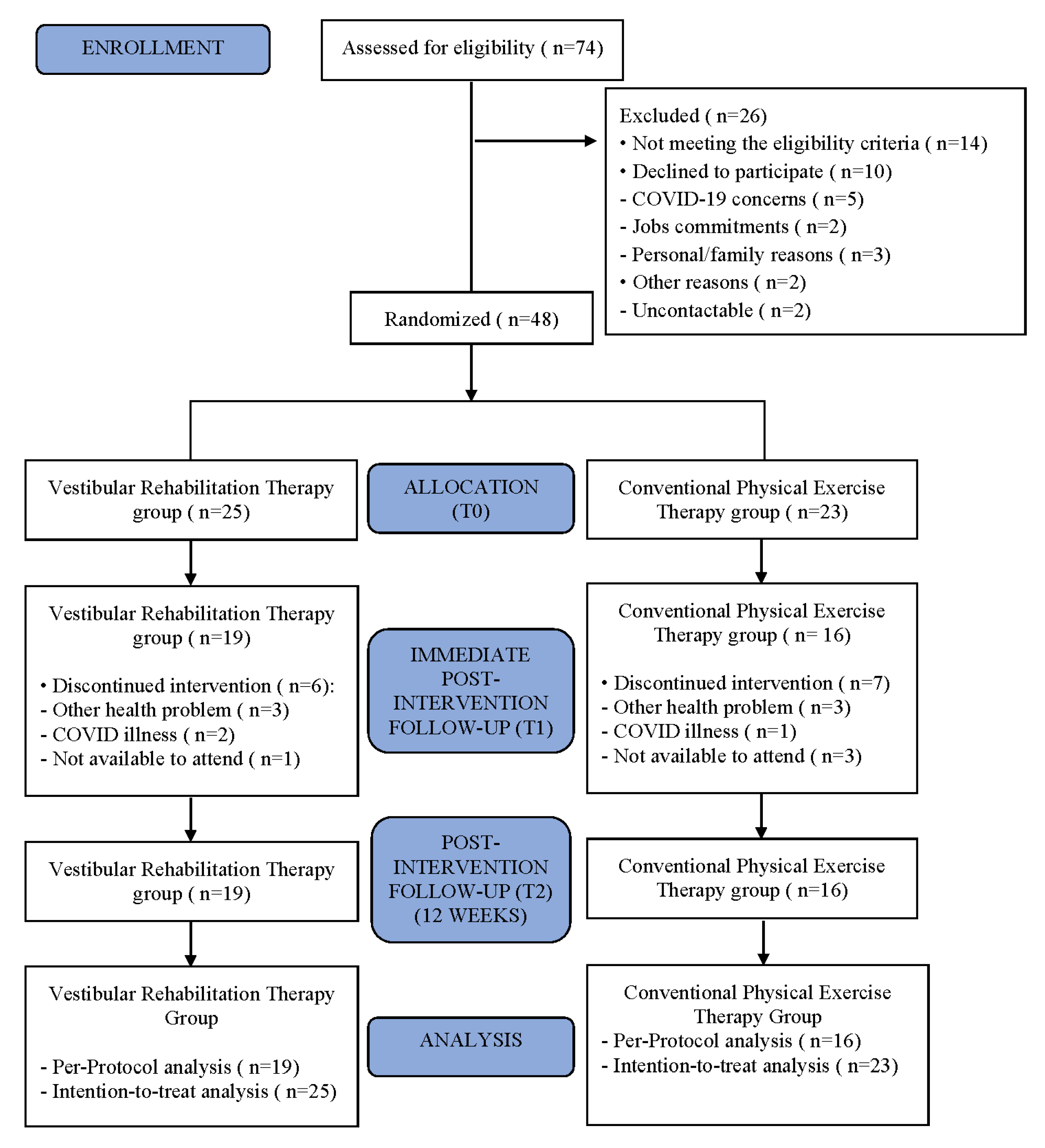
2.4. Randomization
2.5. Interventions
2.6. Intervention Protocols
2.7. Measurements
2.7.1. Self-Reported Outcome Measures
2.7.2. Objective Measures
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pujol, J.; Macià, D.; Garcia-Fontanals, A.; Blanco-Hinojo, L.; López-Solà, M.; Garcia-Blanco, S.; Poca-Dias, V.; Harrison, B.J.; Contreras-Rodríguez, O.; Monfort, J.; et al. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain 2014, 155, 1492–1503. [Google Scholar] [CrossRef]
- Klein, A.; Schankin, C.J. Visual snow syndrome, the spectrum of perceptual disorders, and migraine as a common risk factor: A narrative review. Headache 2021, 61, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Gyorfi, M.; Rupp, A.; Abd-Elsayed, A. Fibromyalgia Pathophysiology. Biomedicines 2022, 10, 3070. [Google Scholar] [CrossRef]
- Sempere-Rubio, N.; López-Pascual, J.; Aguilar-Rodríguez, M.; Cortés-Amador, S.; Espí-López, G.; Villarrasa-Sapiña, I.; Serra-Añó, P. Characterization of postural control impairment in women with fibromyalgia. PLoS ONE 2018, 13, e0196575. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet. Disord. 2007, 8, 27. [Google Scholar] [CrossRef]
- Villafaina, S.; Gusi, N.; Rodriguez-Generelo, S.; Martin-Gallego, J.d.D.; Fuentes-García, J.P.; Collado-Mateo, D. Influence of a Cell-Phone Conversation on Balance Performance in Women with Fibromyalgia: A Cross-Sectional Descriptive Study. BioMed Res. Int. 2019, 2019, 5132802. [Google Scholar] [CrossRef] [PubMed]
- Benito-Orejas, J.L.; Aylagas-Andrés, M.J.; Martín-Moratinos, C.; Gallardo-Chaparro, I.; Pérez-Hickman, L.; Aladro-Abad, V.; Plaza-García, N.; López-Franco, J.M.; Díez-Rabadán, B.; García-Franco, C.; et al. Terapia física en la hipofunción vestibular unilateral y bilateral. Rev. ORL 2019, 11, 51. [Google Scholar] [CrossRef]
- Jones, K.D.; Horak, F.B.; Winters-Stone, K.; Irvine, J.M.; Bennett, R.M. Fibromyalgia Is Associated with Impaired Balance and Falls. JCR 2009, 15, 16–21. [Google Scholar] [CrossRef]
- Jones, K.D.; King, L.A.; Mist, S.D.; Bennett, R.M.; Horak, F.B. Postural control deficits in people with fibromyalgia: A pilot study. Arthritis Res. Ther. 2011, 13, R127. [Google Scholar] [CrossRef]
- Núñez-Fuentes, D.; Obrero-Gaitán, E.; Zagalaz-Anula, N.; Ibáñez-Vera, A.J.; Achalandabaso-Ochoa, A.; López-Ruiz, M.d.C.; Rodríguez-Almagro, D.; Lomas-Vega, R. Alteration of Postural Balance in Patients with Fibromyalgia Syndrome—A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 127. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Rodríguez-Almagro, D.; Peinado-Rubia, A.B.; Zagalaz-Anula, N.; Molina, F.; Obrero-Gaitán, E.; Ibáñez-Vera, A.J.; Osuna-Pérez, M.C. Joint Assessment of Equilibrium and Neuromotor Function: A Validation Study in Patients with Fibromyalgia. Diagnostics 2020, 10, 1057. [Google Scholar] [CrossRef]
- Peinado-Rubia, A.; Osuna-Pérez, M.C.; Rodríguez-Almagro, D.; Zagalaz-Anula, N.; López-Ruiz, M.C.; Lomas-Vega, R. Impaired Balance in Patients with Fibromyalgia Syndrome: Predictors of the Impact of This Disorder and Balance Confidence. Int. J. Environ. Res. Public Health 2020, 17, 3160. [Google Scholar] [CrossRef]
- Shiozaki, T.; Ito, T.; Wada, Y.; Yamanaka, T.; Kitahara, T. Effects of Vestibular Rehabilitation on Physical Activity and Subjective Dizziness in Patients with Chronic Peripheral Vestibular Disorders: A Six-Month Randomized Trial. Front. Neurol. 2021, 12, 656157. [Google Scholar] [CrossRef] [PubMed]
- Buskila, D.; Neumann, L. Assessing Functional Disability and Health Status of Women with Fibromyalgia: Validation of a Hebrew Version of the Fibromyalgia Impact Questionnaire. J. Rheumatol. 1996, 23, 903–906. [Google Scholar]
- Toprak Celenay, S.; Mete, O.; Akan, S.; Un Yildirim, N.; Erten, S. Comparison of the effects of stabilization exercise plus kinesio taping and stabilization exercise alone on pain and well-being in fibromyalgia. Complement. Ther. Clin. Pract. 2020, 38, 101076. [Google Scholar] [CrossRef]
- Larsson, A.; Palstam, A.; Löfgren, M.; Ernberg, M.; Bjersing, J.; Bileviciute-Ljungar, I.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia—A randomized controlled trial. Arthritis Res. Ther. 2015, 17, 161. [Google Scholar] [CrossRef]
- Jahnke, R.; Larkey, L.; Rogers, C.; Etnier, J.; Lin, F. A Comprehensive Review of Health Benefits of Qigong and Tai Chi. Am. J. Health Promot. 2010, 24, e1–e25. [Google Scholar] [CrossRef]
- Rodríguez-Mansilla, J.; Mejías-Gil, A.; Garrido-Ardila, E.M.; Jiménez-Palomares, M.; Montanero-Fernández, J.; González-López-Arza, M.V. Effects of Non-Pharmacological Treatment on Pain, Flexibility, Balance and Quality of Life in Women with Fibromyalgia: A Randomised Clinical Trial. J. Clin. Med. 2021, 10, 3826. [Google Scholar] [CrossRef]
- Popkirov, S.; Staab, J.P.; Stone, J. Persistent postural-perceptual dizziness (PPPD): A common, characteristic and treatable cause of chronic dizziness. Pract. Neurol. 2017, 18, 5–13. [Google Scholar] [CrossRef]
- Herdman, D.; Norton, S.; Murdin, L.; Frost, K.; Pavlou, M.; Moss-Morris, R. The INVEST trial: A randomised feasibility trial of psychologically informed vestibular rehabilitation versus current gold standard physiotherapy for people with Persistent Postural Perceptual Dizziness. J. Neurol. 2022, 269, 4753–4763. [Google Scholar] [CrossRef]
- Bauer, M.; Benito-Orejas, J.I.; Ramírez-Salas, J.E. Rehabilitación vestibular en la dependencia visual y somatosensorial. Rev. ORL 2019, 11, 79. [Google Scholar] [CrossRef]
- Giray, M.; Kirazli, Y.; Karapolat, H.; Celebisoy, N.; Bilgen, C.; Kirazli, T. Short-Term Effects of Vestibular Rehabilitation in Patients with Chronic Unilateral Vestibular Dysfunction: A Randomized Controlled Study. Arch. Phys. Med. Rehabil. 2009, 90, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Benito-Orejas, J.I.; Alonso-Vielba, J.; Valda-Rodrigo, J.; Cifuentes-Navas, V.A. Resultados y seguimiento de la rehabilitación vestibular. Rev. ORL 2019, 11, 107. [Google Scholar] [CrossRef]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Cass, S.P.; Clendaniel, R.A.; Fife, T.D.; Furman, J.M.; Getchius, T.S.D.; Goebel, J.A.; Shepard, N.T.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction. J. Neurol. Phys. Ther. 2016, 40, 124–155. [Google Scholar] [CrossRef] [PubMed]
- Baydan, M.; Yigit, O.; Aksoy, S. Does vestibular rehabilitation improve postural control of subjects with chronic subjective dizziness? PLoS ONE 2020, 15, e0238436. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Lewis, M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Teare, M.D.; Dimairo, M.; Shephard, N.; Hayman, A.; Whitehead, A.; Walters, S.J. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: A simulation study. Trials 2014, 15, 264. [Google Scholar] [CrossRef]
- Ricci, N.A.; Aratani, M.C.; Caovilla, H.H.; Ganança, F.F. Effects of Vestibular Rehabilitation on Balance Control in Older People with Chronic Dizziness. Am. J. Phys. Med. Rehabil. 2016, 95, 256–269. [Google Scholar] [CrossRef]
- Aratani, M.C.; Ricci, N.A.; Caovilla, H.H.; Ganança, F.F. Benefits of vestibular rehabilitation on patient-reported outcomes in older adults with vestibular disorders: A randomized clinical trial. Braz. J. Phys. Ther. 2020, 24, 550–559. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Herdman, D.; Norton, S.; Pavlou, M.; Murdin, L.; Moss-Morris, R. Protocol for a randomised controlled feasibility study of psychologically informed vestibular rehabilitation for people with persistent dizziness: INVEST trial. Pilot Feasibility Stud. 2021, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; González, T. The Fibromyalgia Impact Questionnaire: A validated Spanish Version to Assess the Health Status in Women with Fibromyalgia. Clin. Exp. Rheumatol. 2004, 22, 554–560. [Google Scholar] [PubMed]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Vilagut, G.; María Valderas, J.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: Componentes físico y mental. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Roldan-Jimenez, C.; Neblett, R.; Gatchel, R.J. Cross-cultural adaptation and validity of the Spanish central sensitization inventory. SpringerPlus 2016, 5, 1837. [Google Scholar] [CrossRef]
- Téllez, N.; Río, J.; Tintoré, M.; Nos, C.; Galán, I.; Montalban, X. Does the Modified Fatigue Impact Scale Offer a More Comprehensive Assessment of Fatigue in MS? Mult. Scler. J. 2005, 11, 198–202. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de la versión española de la escala de la catastrofización ante el dolor (Pain Catastrophizing Scale) en la fibromialgia. Med. Clin. 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Gómez-Pérez, L.; López-Martínez, A.E.; Ruiz-Párraga, G.T. Psychometric Properties of the Spanish Version of the Tampa Scale for Kinesiophobia (TSK). J. Pain 2011, 12, 425–435. [Google Scholar] [CrossRef]
- Pérez, N.; Garmendia, I.; Martín, E.; García-Tapia, R. Adaptación cultural de dos cuestionarios de medida de la salud en pacientes con vértigo [Cultural adaptation of 2 questionnaires for health measurement in patients with vertigo]. Acta Otorrinolaringol. Esp. 2000, 51, 572–580. [Google Scholar]
- Montilla-Ibáñez, A.; Martínez-Amat, A.; Lomas-Vega, R.; Cruz-Díaz, D.; Torre-Cruz, M.J.D.l.; Casuso-Pérez, R.; Hita-Contreras, F. The Activities-Specific Balance Confidence Scale: Reliability and Validity in Spanish Patients with Vestibular Disorders. Disabil. Rehabil. 2016, 39, 697–703. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Hita-Contreras, F.; Mendoza, N.; Martínez-Amat, A. Cross-cultural adaptation and validation of the Falls Efficacy Scale International in Spanish postmenopausal women. Menopause 2012, 19, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.A.; Antero, D.C.; Kulczycki, M.M.; Skare, T.L. Prevalence of falls in fibromyalgia patients. Acta Ortop. Bras. 2014, 22, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, A.; Mast, F. Assessing Otolith Function by the Subjective Visual Vertical. Ann. N.Y. Acad. Sci. 1999, 871, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Oltman, P.K. A Portable Rod-And-Frame Apparatus. Percept. Mot. Ski. 1968, 26, 503–506. [Google Scholar] [CrossRef]
- Negrillo-Cárdenas, J.; Rueda-Ruiz, A.J.; Ogayar-Anguita, C.J.; Lomas-Vega, R.; Segura-Sánchez, R.J. A System for the Measurement of the Subjective Visual Vertical Using a Virtual Reality Device. J. Med. Syst. 2018, 42, 124. [Google Scholar] [CrossRef]
- Bagust, J.; Docherty, S.; Haynes, W.; Telford, R.; Isableu, B. Changes in Rod and Frame Test Scores Recorded in Schoolchildren during Development—A Longitudinal Study. PLoS ONE 2013, 8, e65321. [Google Scholar] [CrossRef]
- Romero-Franco, N.; Martínez-López, E.J.; Lomas-Vega, R.; Hita-Contreras, F.; Osuna-Pérez, M.C.; Martínez-Amat, A. Short-term Effects of Proprioceptive Training with Unstable Platform on Athletes’ Stabilometry. J. Strength Cond. Res. 2013, 27, 2189–2197. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Dunlap, P.M.; Holmberg, J.M.; Whitney, S.L. Vestibular rehabilitation. Curr. Opin. Neurol. 2019, 32, 137–144. [Google Scholar] [CrossRef]
- Mucci, V.; Demori, I.; Rapallo, F.; Molinari, E.; Losacco, S.; Marinelli, L.; Browne, C.J.; Burlando, B. Vestibular Disability/Handicap in Fibromyalgia: A Questionnaire Study. J. Clin. Med. 2022, 11, 4017. [Google Scholar] [CrossRef]
- Russek, L.N.; Fulk, G.D. Pilot study assessing balance in women with fibromyalgia syndrome. Physiother. Theory Pract. 2009, 25, 555–565. [Google Scholar] [CrossRef]
- Zeigelboim, B.S.; Moreira, D.N. Achados vestibulares em pacientes portadores de fibromialgia. Arq. Int. Otorrinolaringol. 2011, 15, 283–289. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Oh, S.-Y.; Kim, H.-J.; Koo, J.-W.; Kim, J.S. Vestibular dysfunction in migraine: Effects of associated vertigo and motion sickness. J. Neurol. 2009, 257, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Sarıhan, K.; Uzkeser, H.; Erdal, A. Evaluation of balance, fall risk, and related factors in patients with fibromyalgia syndrome. Turk. J. Phys. Med. Rehabil. 2021, 67, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Gallego-Diaz, J.M.; Adsuar, J.C.; Domínguez-Muñoz, F.J.; Olivares, P.R.; Gusi, N. Fear of Falling in Women with Fibromyalgia and Its Relation with Number of Falls and Balance Performance. BioMed Res. Int. 2015, 2015, 589014. [Google Scholar] [CrossRef]
- Rossi-Izquierdo, M.; Gayoso-Diz, P.; Santos-Pérez, S.; Del-Río-Valeiras, M.; Faraldo-García, A.; Vaamonde-Sánchez-Andrade, I.; Lirola-Delgado, A.; Soto-Varela, A. Vestibular rehabilitation in elderly patients with postural instability: Reducing the number of falls—A randomized clinical trial. Aging Clin. Exp. Res. 2018, 30, 1353–1361. [Google Scholar] [CrossRef]
- Roberts, R.E.; Da Silva Melo, M.; Siddiqui, A.A.; Arshad, Q.; Patel, M. Vestibular and oculomotor influences on visual dependency. J. Neurophysiol. 2016, 116, 1480–1487. [Google Scholar] [CrossRef]
- Lord, S.R.; Webster, I.W. Visual Field Dependence in Elderly Fallers and Non-Fallers. Int. J. Aging Hum. Dev. 1990, 31, 267–277. [Google Scholar] [CrossRef]
- Hafstrom, A.; Modig, F.; Karlberg, M.; Fransson, P.-A. Increased visual dependence and otolith dysfunction with alcohol intoxication. Neuroreport 2007, 18, 391–394. [Google Scholar] [CrossRef]
- Bonan, I.V.; Leman, M.C.; Legargasson, J.F.; Guichard, J.P.; Yelnik, A.P. Evolution of Subjective Visual Vertical Perturbation After Stroke. Neurorehabilit. Neural Repair 2006, 20, 484–491. [Google Scholar] [CrossRef]
- Einarsson, E.-J.; Patel, M.; Petersen, H.; Wiebe, T.; Fransson, P.-A.; Magnusson, M.; Moëll, C. Elevated visual dependency in young adults after chemotherapy in childhood. PLoS ONE 2018, 13, e0193075. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C. Relationship of visual dependence to age, balance, attention, and vertigo. J. Phys. Ther. Sci. 2017, 29, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.S.; Tedla, J.S.; Dixit, S.; Raizah, A.; Al-Otaibi, M.L.; Gular, K.; Ahmad, I.; Sirajudeen, M.S. Cervical Joint Position Sense and Its Correlations with Postural Stability in Subjects with Fibromyalgia Syndrome. Life 2022, 12, 1817. [Google Scholar] [CrossRef]
- Gucmen, B.; Kocyigit, B.F.; Nacitarhan, V.; Berk, E.; Koca, T.T.; Akyol, A. The relationship between cervical proprioception and balance in patients with fibromyalgia syndrome. Rheumatol. Int. 2022, 42, 311–318. [Google Scholar] [CrossRef]
- Albuquerque, M.L.L.; Monteiro, D.; Marinho, D.A.; Vilarino, G.T.; Andrade, A.; Neiva, H.P. Effects of different protocols of physical exercise on fibromyalgia syndrome treatment: Systematic review and meta-analysis of randomized controlled trials. Rheumatol. Int. 2022, 42, 1893–1908. [Google Scholar] [CrossRef]
- Kundakci, B.; Kaur, J.; Goh, S.l.; Hall, M.; Doherty, M.; Zhang, W.; Abhishek, A. Efficacy of nonpharmacological interventions for individual features of fibromyalgia. Pain 2021, 163, 1432–1445. [Google Scholar] [CrossRef]
- Del-Moral-García, M.; Obrero-Gaitán, E.; Rodríguez-Almagro, D.; Rodríguez-Huguet, M.; Osuna-Pérez, M.C.; Lomas-Vega, R. Effectiveness of Active Therapy-Based Training to Improve the Balance in Patients with Fibromyalgia: A Systematic Review with Meta-Analysis. J. Clin. Med. 2020, 9, 3771. [Google Scholar] [CrossRef]
- Alghwiri, A.; Alghadir, A.; Whitney, S.L. The vestibular activities and participation measure and vestibular disorders. J. Vestib. Res. 2013, 23, 305–312. [Google Scholar] [CrossRef]
- Ekwall, A.; Lindberg, Å.; Magnusson, M. Dizzy—Why Not Take a Walk? Low Level Physical Activity Improves Quality of Life among Elderly with Dizziness. Gerontology 2009, 55, 652–659. [Google Scholar] [CrossRef]
- Saracoglu, I.; Akin, E.; Aydin Dincer, G.B. Efficacy of adding pain neuroscience education to a multimodal treatment in fibromyalgia: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2022, 25, 394–404. [Google Scholar] [CrossRef]
- Axer, H.; Finn, S.; Wassermann, A.; Guntinas-Lichius, O.; Klingner, C.M.; Witte, O.W. Multimodal treatment of persistent postural–perceptual dizziness. Brain Behav. 2020, 10, e01864. [Google Scholar] [CrossRef] [PubMed]
- Serrat, M.; Sanabria-Mazo, J.P.; Almirall, M.; Musté, M.; Feliu-Soler, A.; Méndez-Ulrich, J.L.; Sanz, A.; Luciano, J.V. Effectiveness of a Multicomponent Treatment Based on Pain Neuroscience Education, Therapeutic Exercise, Cognitive Behavioral Therapy, and Mindfulness in Patients with Fibromyalgia (FIBROWALK Study): A Randomized Controlled Trial. Phys. Ther. 2021, 101, pzab200. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Fernández-de-las-Peñas, C.; Navarro-Santana, M.J.; Plaza-Manzano, G. Efficacy of Dry Needling and Acupuncture in Patients with Fibromyalgia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 9904. [Google Scholar] [CrossRef]
- Tokle, G.; Mørkved, S.; Bråthen, G.; Goplen, F.K.; Salvesen, Ø.; Arnesen, H.; Holmeslet, B.; Nordahl, S.H.G.; Wilhelmsen, K.T. Efficacy of Vestibular Rehabilitation Following Acute Vestibular Neuritis. Otol. Neurotol. 2020, 41, 78–85. [Google Scholar] [CrossRef] [PubMed]
| CPE Group (n = 23) | VR Group (n = 25) | p-Value | ||||
|---|---|---|---|---|---|---|
| F | % | F | % | X2 tests | ||
| Gender | Female | 22.0 | 95.7 | 24.0 | 96.0 | 0.734 |
| Male | 1.0 | 4.3 | 1.0 | 4.0 | ||
| Civil Status | Single | 0.0 | 0.0 | 2.0 | 8.0 | 0.107 |
| Married | 20.0 | 87.0 | 14.0 | 56.0 | ||
| Divorced | 2.0 | 8.7 | 7.0 | 28.0 | ||
| Widow | 1.0 | 4.3 | 2.0 | 8.0 | ||
| Education | No studies | 2.0 | 8.7 | 0.0 | 0.0 | 0.225 |
| Primary | 10.0 | 43.5 | 7.0 | 28.0 | ||
| Secondary | 7.0 | 30.4 | 13.0 | 52.0 | ||
| University | 4.0 | 17.4 | 5.0 | 20.0 | ||
| Occupation | Active | 5.0 | 21.7 | 9.0 | 36.0 | 0.551 |
| Sick leave | 3.0 | 13.0 | 5.0 | 20.0 | ||
| Unemployed | 4.0 | 17.4 | 3.0 | 12.0 | ||
| Housewife | 1.0 | 4.3 | 2.0 | 8.0 | ||
| Retired | 10.0 | 43.5 | 6.0 | 24.0 | ||
| Dizziness last 3 months | Never | 0.0 | 0.0 | 1.0 | 4.0 | 0.430 |
| Ever | 9.0 | 39.1 | 14.0 | 56.0 | ||
| Frequently | 9.0 | 39.1 | 7.0 | 28.0 | ||
| Always | 5.0 | 21.7 | 3.0 | 12.0 | ||
| Mean | SD | Mean | SD | t Test | ||
| Age | 55.48 | 6.85 | 51.24 | 6.06 | 0.028 * | |
| Weight | 78.22 | 18.62 | 76.32 | 15.94 | 0.706 | |
| Height | 159.17 | 6.53 | 162.84 | 5.92 | 0.047 | |
| Body Mass Index | 30.88 | 7.09 | 28.86 | 6.28 | 0.299 | |
| CPE Group (n = 23) | VR Group (n = 25) | t Test | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| Falls Last Three Months | 1.13 | 1.18 | 1.40 | 1.47 | 0.490 |
| FIQ TOTAL | 66.02 | 15.78 | 67.50 | 12.89 | 0.723 |
| NPRS | 6.17 | 2.23 | 5.84 | 1.72 | 0.563 |
| PCS of SF-12 | 39.09 | 4.46 | 42.74 | 4.20 | 0.005 ** |
| MCS of SF-12 | 24.48 | 8.99 | 24.42 | 7.36 | 0.981 |
| FSS | 52.61 | 8.43 | 52.60 | 7.48 | 0.997 |
| CSI TOTAL | 64.39 | 14.72 | 63.72 | 7.86 | 0.843 |
| TSK | 29.22 | 7.43 | 26.52 | 6.36 | 0.182 |
| PCS | 28.74 | 15.16 | 24.92 | 10.95 | 0.319 |
| DHI TOTAL | 62.04 | 26.48 | 52.92 | 18.00 | 0.166 |
| ABC-16 | 52.53 | 36.69 | 51.78 | 17.41 | 0.927 |
| FES-I | 39.48 | 12.27 | 34.64 | 9.20 | 0.127 |
| JAEN Scale | 40.00 | 11.92 | 34.60 | 10.09 | 0.096 |
| SVV | 4.20 | 6.39 | 2.55 | 1.41 | 0.214 |
| RFT | 11.34 | 9.12 | 11.84 | 11.45 | 0.869 |
| Sway Area EO | 513.46 | 651.96 | 309.88 | 511.23 | 0.233 |
| Velocity EO | 21.29 | 5.02 | 18.59 | 5.07 | 0.070 |
| RMSX EO | 0.39 | 0.10 | 0.36 | 0.10 | 0.312 |
| RMSY EO | 0.46 | 0.12 | 0.38 | 0.12 | 0.018 * |
| Mean CoP X axis EO | −2.51 | 6.71 | −0.64 | 6.39 | 0.328 |
| Mean CoP Y axis EO | −20.30 | 10.97 | −15.87 | 10.76 | 0.165 |
| Sway Area EC | 692.49 | 971.60 | 607.83 | 743.55 | 0.737 |
| Velocity EC | 24.66 | 6.04 | 21.52 | 5.50 | 0.069 |
| RMSX EC | 0.48 | 0.18 | 0.42 | 0.14 | 0.200 |
| RMSY EC | 0.57 | 0.18 | 0.49 | 0.15 | 0.081 |
| Mean CoP X axis EC | −2.58 | 7.85 | −0.15 | 4.93 | 0.205 |
| Mean CoP Y axis EC | −17.42 | 13.98 | −15.74 | 12.18 | 0.661 |
| CPE Group (n = 16) | VR Group (n = 19) | Differences | Effect Size | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SE | p-Value | ETA2 | |
| FIQ | 67.64 | 14.39 | 62.15 | 13.52 | 6.94 | 4.18 | 0.108 | 0.090 |
| NPRS | 5.81 | 1.38 | 5.63 | 1.95 | 0.27 | 0.42 | 0.513 | 0.013 |
| PCS of SF-12 | 41.10 | 6.75 | 43.25 | 5.80 | −0.50 | 2.20 | 0.824 | 0.002 |
| MCS of SF-12 | 27.38 | 10.59 | 25.46 | 8.35 | 0.94 | 2.86 | 0.745 | 0.003 |
| FSS | 45.50 | 12.05 | 47.32 | 8.13 | −1.62 | 2.70 | 0.553 | 0.011 |
| CSI | 58.88 | 12.09 | 59.32 | 7.29 | 1.98 | 2.52 | 0.439 | 0.019 |
| TSK | 28.00 | 7.56 | 26.58 | 4.83 | 1.35 | 1.54 | 0.386 | 0.024 |
| PCS | 24.38 | 12.75 | 24.16 | 13.04 | 0.05 | 2.84 | 0.986 | 0.000 |
| Falls | 1.13 | 0.27 | 0.52 | 0.25 | 0.60 | 0.38 | 0.118 | 0.075 |
| DHI-Total | 52.88 | 28.71 | 47.26 | 19.37 | 2.78 | 4.89 | 0.573 | 0.010 |
| DHI Emotional | 16.38 | 11.18 | 12.42 | 7.82 | 1.05 | 1.69 | 0.540 | 0.012 |
| DHI Functional | 19.38 | 10.78 | 17.37 | 7.03 | 1.63 | 1.98 | 0.416 | 0.021 |
| DHI Physical | 17.13 | 8.03 | 17.47 | 6.10 | 0.20 | 1.97 | 0.918 | 0.000 |
| ABC-16 | 55.59 | 20.26 | 58.45 | 18.60 | −6.94 | 5.69 | 0.232 | 0.044 |
| FES-I | 34.13 | 9.98 | 31.05 | 7.32 | 0.99 | 2.06 | 0.633 | 0.007 |
| JAEN-Total Score | 34.75 | 12.95 | 22.84 | 10.31 | 7.74 | 3.45 | 0.032 * | 0.136 |
| JAEN-HM | 6.88 | 4.54 | 3.42 | 3.24 | 3.17 | 0.97 | 0.002 ** | 0.252 |
| JAEN-SR | 21.00 | 6.50 | 15.84 | 6.03 | 2.51 | 2.12 | 0.246 | 0.042 |
| JAEN-GEO | 4.13 | 2.87 | 1.16 | 1.30 | 2.90 | 0.66 | 0.000 *** | 0.379 |
| JAEN-SWEC | 2.75 | 1.91 | 2.42 | 1.30 | 0.18 | 0.56 | 0.748 | 0.003 |
| SVV | 2.55 | 1.52 | 2.40 | 1.26 | 0.07 | 0.52 | 0.892 | 0.001 |
| RFT | 12.48 | 13.75 | 8.52 | 8.82 | 3.25 | 2.71 | 0.241 | 0.051 |
| Sway Area EO | 261.67 | 294.08 | 194.01 | 281.22 | −48.51 | 89.48 | 0.592 | 0.010 |
| Velocity EO | 17.58 | 4.70 | 16.54 | 4.37 | 0.09 | 1.43 | 0.951 | 0.000 |
| RMSX EO | 0.36 | 0.08 | 0.32 | 0.07 | 0.03 | 0.03 | 0.189 | 0.059 |
| RMSY EO | 0.35 | 0.12 | 0.33 | 0.12 | −0.02 | 0.04 | 0.550 | 0.012 |
| Mean CoP X axis EO | −3.73 | 7.37 | −0.62 | 6.32 | −3.20 | 2.22 | 0.161 | 0.067 |
| Mean CoP Y axis EO | −19.71 | 8.20 | −14.93 | 6.79 | −4.09 | 2.82 | 0.158 | 0.068 |
| Sway Area EC | 815.84 | 1170.98 | 508.33 | 1078.73 | 92.49 | 375.95 | 0.807 | 0.002 |
| Velocity EC | 19.90 | 5.68 | 19.43 | 6.70 | −1.03 | 1.92 | 0.593 | 0.010 |
| RMSX EC | 0.40 | 0.13 | 0.38 | 0.13 | −0.01 | 0.04 | 0.866 | 0.001 |
| RMSY EC | 0.46 | 0.12 | 0.45 | 0.21 | −0.02 | 0.06 | 0.721 | 0.004 |
| Mean CoP X axis EC | −6.87 | 14.79 | −1.00 | 5.28 | −5.21 | 3.16 | 0.110 | 0.086 |
| Mean CoP Y axis EC | −20.86 | 8.45 | −17.69 | 9.96 | −2.91 | 3.26 | 0.379 | 0.027 |
| CPE Group (n = 16) | VR Group (n = 19) | Differences | Effect Size | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SE | p-Value | ETA2 | |
| FIQ | 62.35 | 18.80 | 64.58 | 9.78 | 0.80 | 4.29 | 0.854 | 0.001 |
| NPRS | 6.38 | 1.96 | 6.61 | 1.14 | −0.11 | 0.43 | 0.794 | 0.002 |
| PCS of SF-12 | 38.04 | 4.66 | 43.36 | 5.52 | −4.36 | 1.88 | 0.027* | 0.148 |
| MCS of SF-12 | 30.32 | 7.79 | 24.88 | 9.58 | 5.01 | 3.03 | 0.108 | 0.081 |
| FSS | 49.06 | 12.85 | 46.83 | 9.79 | 2.50 | 3.54 | 0.485 | 0.016 |
| CSI | 56.63 | 12.62 | 60.44 | 6.40 | −0.49 | 2.34 | 0.836 | 0.001 |
| TSK | 27.13 | 6.17 | 25.67 | 6.36 | 0.38 | 1.42 | 0.791 | 0.002 |
| PCS | 23.63 | 12.21 | 20.44 | 9.61 | 2.95 | 2.04 | 0.158 | 0.063 |
| Falls | 1.13 | 1.82 | 0.39 | 0.61 | 0.98 | 0.44 | 0.033 * | 0.139 |
| DHITotal | 54.50 | 20.66 | 47.56 | 15.92 | 5.18 | 4.24 | 0.231 | 0.046 |
| DHI_Emotional | 16.63 | 9.68 | 11.67 | 6.37 | 2.57 | 1.60 | 0.119 | 0.077 |
| DHI_Functional | 19.63 | 7.84 | 18.33 | 6.80 | 1.19 | 1.85 | 0.527 | 0.013 |
| DHI_Physical | 18.25 | 5.16 | 17.56 | 5.55 | 1.15 | 1.54 | 0.458 | 0.018 |
| ABC-16 | 57.30 | 21.84 | 54.13 | 17.97 | −1.06 | 5.96 | 0.860 | 0.001 |
| FES-I | 32.88 | 10.35 | 31.83 | 8.74 | −0.02 | 2.35 | 0.994 | 0.000 |
| JAÉN-Total Score | 34.44 | 12.18 | 25.28 | 7.92 | 6.16 | 3.33 | 0.074 | 0.099 |
| JAEN-HM | 6.00 | 4.82 | 4.67 | 3.01 | 1.09 | 1.29 | 0.405 | 0.022 |
| JAEN-SR | 21.25 | 5.88 | 16.11 | 5.09 | 2.26 | 1.73 | 0.202 | 0.052 |
| JAEN-GEO | 3.88 | 2.36 | 1.94 | 1.11 | 1.90 | 0.57 | 0.002 ** | 0.265 |
| JAEN-SWEC | 3.31 | 1.74 | 2.56 | 0.86 | 0.69 | 0.48 | 0.163 | 0.062 |
| SVV | 3.48 | 3.04 | 2.70 | 1.17 | 0.19 | 0.70 | 0.783 | 0.003 |
| RFT | 10.10 | 7.52 | 6.26 | 4.40 | 3.61 | 1.51 | 0.024 * | 0.164 |
| Sway Area EO | 216.50 | 401.86 | 112.18 | 98.12 | −37.78 | 62.75 | 0.552 | 0.012 |
| Velocity EO | 14.90 | 3.87 | 16.42 | 6.18 | −2.65 | 1.72 | 0.134 | 0.076 |
| RMSX EO | 0.29 | 0.06 | 0.32 | 0.10 | −0.04 | 0.03 | 0.220 | 0.051 |
| RMSY EO | 0.31 | 0.11 | 0.34 | 0.16 | −0.07 | 0.04 | 0.092 | 0.095 |
| Mean CoP X axis EO | 0.23 | 7.30 | −1.06 | 7.74 | 1.19 | 2.45 | 0.630 | 0.008 |
| Mean CoP Y axis EO | −19.56 | 9.82 | −14.18 | 7.15 | −3.84 | 3.18 | 0.237 | 0.048 |
| Sway Area EC | 254.44 | 444.79 | 265.04 | 341.83 | −100.49 | 101.60 | 0.331 | 0.033 |
| Velocity EC | 17.94 | 4.80 | 18.52 | 8.61 | −1.86 | 2.45 | 0.454 | 0.019 |
| RMSX EC | 0.34 | 0.07 | 0.37 | 0.15 | −0.05 | 0.04 | 0.195 | 0.057 |
| RMSY EC | 0.41 | 0.14 | 0.40 | 0.21 | −0.03 | 0.06 | 0.637 | 0.008 |
| Mean CoP X axis EC | 0.98 | 6.56 | 0.29 | 6.66 | 1.08 | 2.23 | 0.633 | 0.008 |
| Mean CoP Y axis EC | −21.78 | 8.91 | −13.55 | 6.83 | −7.88 | 2.80 | 0.009 ** | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peinado-Rubia, A.B.; Osuna-Pérez, M.C.; Cortés-Pérez, I.; Rojas-Navarrete, A.; Ibancos-Losada, M.d.R.; Lomas-Vega, R. Effectiveness of Vestibular Rehabilitation in Improving Health Status and Balance in Patients with Fibromyalgia Syndrome: A Single-Blind Randomized Controlled Trial. Biomedicines 2023, 11, 1297. https://doi.org/10.3390/biomedicines11051297
Peinado-Rubia AB, Osuna-Pérez MC, Cortés-Pérez I, Rojas-Navarrete A, Ibancos-Losada MdR, Lomas-Vega R. Effectiveness of Vestibular Rehabilitation in Improving Health Status and Balance in Patients with Fibromyalgia Syndrome: A Single-Blind Randomized Controlled Trial. Biomedicines. 2023; 11(5):1297. https://doi.org/10.3390/biomedicines11051297
Chicago/Turabian StylePeinado-Rubia, Ana Belén, María Catalina Osuna-Pérez, Irene Cortés-Pérez, Alicia Rojas-Navarrete, María del Rocío Ibancos-Losada, and Rafael Lomas-Vega. 2023. "Effectiveness of Vestibular Rehabilitation in Improving Health Status and Balance in Patients with Fibromyalgia Syndrome: A Single-Blind Randomized Controlled Trial" Biomedicines 11, no. 5: 1297. https://doi.org/10.3390/biomedicines11051297
APA StylePeinado-Rubia, A. B., Osuna-Pérez, M. C., Cortés-Pérez, I., Rojas-Navarrete, A., Ibancos-Losada, M. d. R., & Lomas-Vega, R. (2023). Effectiveness of Vestibular Rehabilitation in Improving Health Status and Balance in Patients with Fibromyalgia Syndrome: A Single-Blind Randomized Controlled Trial. Biomedicines, 11(5), 1297. https://doi.org/10.3390/biomedicines11051297








