Spheroid Culture System, a Promising Method for Chondrogenic Differentiation of Dental Mesenchymal Stem Cells
Abstract
1. Introduction
- Secondly, their exosomes could be used for cartilage repair [5].
- They are expandable and have relative genomic stability for a long period of time [18].
- They can differentiate into multiple lineages including odontoblasts, osteoblasts, chondrocytes, myocytes, neurocytes, adipocytes, corneal epithelial cells, and melanocytes [19].
- The mean doubling time frequency of dental pulp MSCs is comparable to that of bone marrow MSCs [13].
- The frequency of colony-forming cells from dental pulp is high compared to those from bone marrow [13].
- They have demonstrated immunomodulatory properties due to secreting cytokines [18].
- They have been found in various dental tissues (dental pulp, apical papilla, periodontal ligament, gingiva, dental follicle, tooth germ, and alveolar bone) [18].
2. Materials and Methods
2.1. Isolation and Culture
2.2. Characterization
- To confirm the stemness of the hDPSC, their ability to form colonies was assessed with the Colony-Forming Assay.
- Then, to ensure that the hDPSC were still functional, their capacity for in vitro differentiation into adipocyte and osteoblast lineages was evaluated.
2.2.1. Phenotypic Analysis by Flow Cytometry
2.2.2. Functional Characterization
Colony-Forming Assay
Osteogenic Differentiation
Adipogenic Differentiation
2.3. Chondrogenic Differentiation
2.3.1. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.3.2. Alcian Blue Staining
2.4. Statistical Analysis
3. Results
3.1. Cell Lines Isolation and Characterization
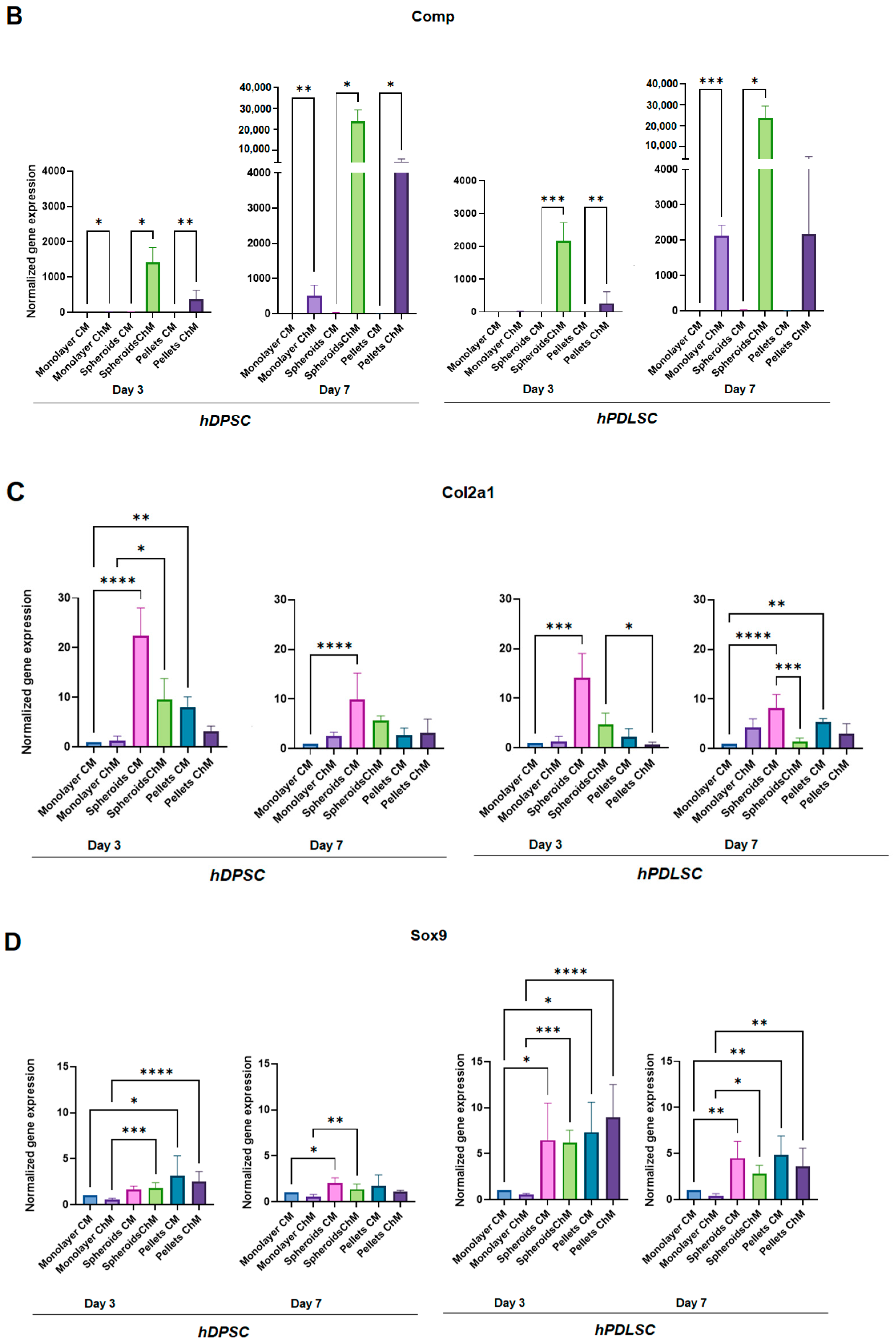
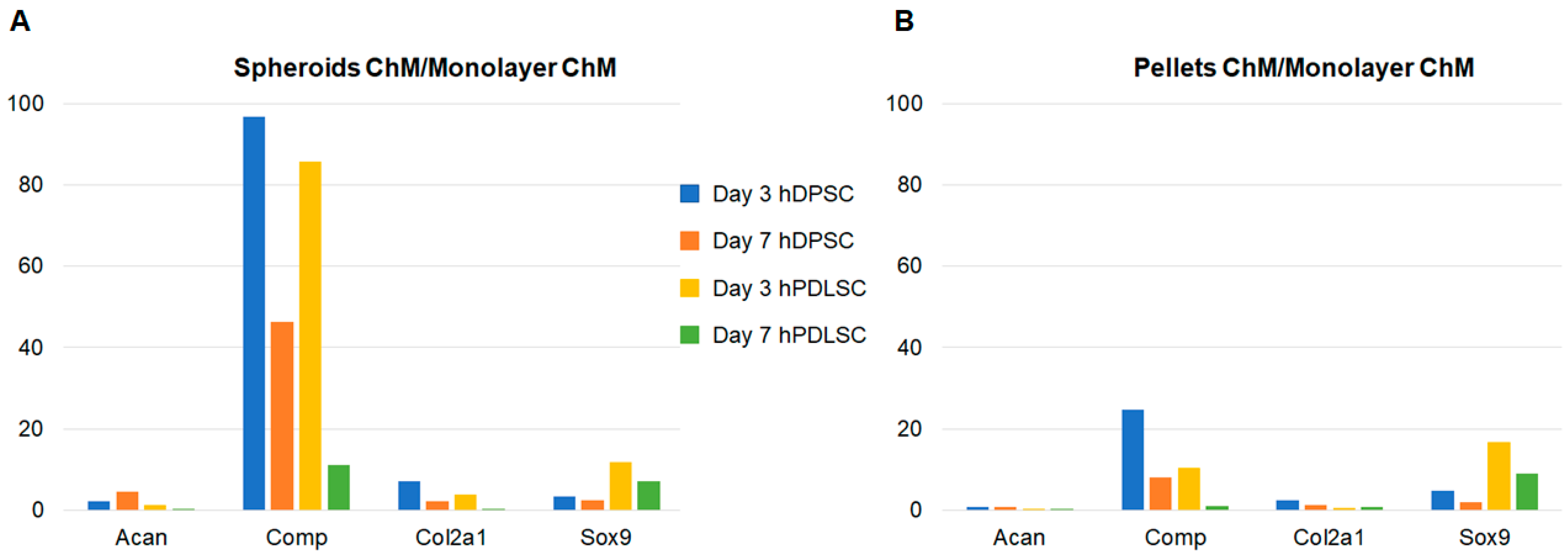
3.2. Chondrogenic Differentiation
3.2.1. Alcian Blue Staining
- Changes in cell shape;
3.2.2. Real-Time Polymerase Chain Reaction (RT-qPCR)
4. Discussion
- Aggrecan (Acan), a proteoglycan of the extracellular matrix (ECM), which is the major component of cartilage [56].
- Cartilage oligomeric matrix protein (Comp), which is a non-collagenous extracellular matrix glycoprotein that is primarily found in the human skeleton system (articular cartilage, meniscus, ligaments, tendons, and synovium) [57].
- Type II collagen (Col2a1), which is one of the main components of the ECM of hyaline articular cartilage [58].
- SRY-related HMG box-containing-9 (Sox9), which is a master transcription factor that regulates multiple events in chondrogenesis. SOX9 is involved in the transactivation of Col2a1 and Acan [58].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Xu, Z.; Shao, J.; Fu, P.; Wu, H. MicroRNA-218 promotes early chondrogenesis of mesenchymal stem cells and inhibits later chondrocyte maturation. BMC Biotechnol. 2019, 19, 6. [Google Scholar] [CrossRef]
- Danišovič, L.; Varga, I.; Polák, S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell 2012, 44, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Grevenstein, D.; Mamilos, A.; Schmitt, V.H.; Niedermair, T.; Wagner, W.; Kirkpatrick, C.J.; Brochhausen, C. Excellent histological results in terms of articular cartilage regeneration after spheroid-based autologous chondrocyte implantation (ACI). Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 417–421. [Google Scholar] [CrossRef]
- Driessen, B.J.H.; Logie, C.; Vonk, L.A. Cellular reprogramming for clinical cartilage repair. Cell Biol. Toxicol. 2017, 33, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments. Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Cooper, L.F.; Ravindran, S.; Huang, C.C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2020, 10, 1569. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, J.; Kim, K. Mesenchymal Stem Cell-Derived Exosomes for Effective Cartilage Tissue Repair and Treatment of Osteoarthritis. Biotechnol. J. 2020, 15, 2000082. [Google Scholar] [CrossRef]
- Gögele, C.; Wiltzsch, S.; Lenhart, A.; Civilleri, A.; Weiger, T.M.; Schäfer-Eckart, K.; Minnich, B.; Forchheimer, L.; Hornfeck, M.; Schulze-Tanzil, G. Highly porous novel chondro-instructive bioactive glass scaffolds tailored for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112421. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells. Sultan Qaboos Univ. Med. J. 2018, 18, e264-77. [Google Scholar] [CrossRef]
- Martellucci, S.; Manganelli, V.; Santacroce, C.; Santilli, F.; Piccoli, L.; Sorice, M.; Mattei, V. Role of Prion protein-EGFR multimolecular complex during neuronal differentiation of human dental pulp-derived stem cells. Prion 2018, 12, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Santacroce, C.; Tasciotti, V.; Martellucci, S.; Santilli, F.; Manganelli, V.; Piccoli, L.; Misasi, R.; Sorice, M.; Garofalo, T. Role of lipid rafts in neuronal differentiation of dental pulp-derived stem cells. Exp. Cell Res. 2015, 339, 231–240. [Google Scholar] [CrossRef]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.R.; Carrión, F.S.; Chaparro, A.P. Mesenchymal stem cells from the oral cavity and their potential value in tissue engineering. Periodontology 2000 2015, 67, 251–267. [Google Scholar] [CrossRef]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Martellucci, S.; Santacroce, C.; Manganelli, V.; Santilli, F.; Piccoli, L.; Cassetta, M.; Misasi, R.; Sorice, M.; Mattei, V. Isolation, Propagation, and Prion Protein Expression During Neuronal Differentiation of Human Dental Pulp Stem Cells. J. Vis. Exp. Jove 2019, 145, e59282. [Google Scholar]
- James, J.L.; Umapathy, A.; Srinivasan, S.; Barker, C.N.; Brooks, A.; Hearn, J.; Chhana, A.; EWilliams, E.; Sheppard, H.; McGlashann, S.R. The Chondrogenic Potential of First-Trimester and Term Placental Mesenchymal Stem/Stromal Cells. Cartilage 2021, 13, 544S–558S. [Google Scholar] [CrossRef]
- Gögele, C.; Müller, S.; Belov, S.; Pradel, A.; Wiltzsch, S.; Lenhart, A.; Hornfeck, M.; Kerling, V.; Rübling, A.; Kühl, H.; et al. Biodegradable Poly(D-L-lactide-co-glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering. Cells 2022, 11, 1577. [Google Scholar] [CrossRef]
- Brezulier, D.; Pellen-Mussi, P.; Tricot-Doleux, S.; Novella, A.; Sorel, O.; Jeanne, S. Development of a 3D human osteoblast cell culture model for studying mechanobiology in orthodontics. Eur. J. Orthod. 2020, 42, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Grigull, N.P.; Redeker, J.I.; Schmitt, B.; Saller, M.M.; Schönitzer, V.; Mayer-Wagner, S. Chondrogenic Potential of Pellet Culture Compared to High-Density Culture on a Bacterial Cellulose Hydrogel. Int. J. Mol. Sci. 2020, 21, 2785. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Tricot-Doleux, S.; Novella, A.; Pellen-Mussi, P.; Jeanne, S. Influence of Periodontal Ligament Stem Cell-Derived Conditioned Medium on Osteoblasts. Pharmaceutics 2022, 14, 729. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.R.; Kharat, A.H.; Kulkarni, D.G.; Kheur, S.M.; Bhonde, R.R. Long term explant culture for harvesting homogeneous population of human dental pulp stem cells. Cell Biol. Int. 2018, 42, 1602–1610. [Google Scholar] [CrossRef]
- Kyffin, J.A.; Sharma, P.; Leedale, J.; Colley, H.E.; Murdoch, C.; Harding, A.L.; Mistry, P.; Webb, S.D. Characterisation of a functional rat hepatocyte spheroid model. Toxicol. Vitr. 2019, 55, 160–172. [Google Scholar] [CrossRef]
- Schon, B.S.; Schrobback, K.; van der Ven, M.; Stroebel, S.; Hooper, G.J.; Woodfield, T.B.F. Validation of a high-throughput microtissue fabrication process for 3D assembly of tissue engineered cartilage constructs. Cell Tissue Res. 2012, 347, 629–642. [Google Scholar] [CrossRef]
- Taylor, S.C.; Mrkusich, E.M. The state of RT-quantitative PCR: Firsthand observations of implementation of minimum information for the publication of quantitative real-time PCR experiments (MIQE). J. Mol. Microbiol. Biotechnol. 2014, 24, 46–52. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Fraile, M.; González-Jubete, A.; González, L.O.; Vizoso, F.J. Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges. Int. J. Mol. Sci. 2022, 23, 8905. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef]
- Maumus, M.; Pers, Y.M.; Ruiz, M.; Jorgensen, C.; Noël, D. Cellules souches mésenchymateuses et médecine régénératrice: Quel avenir pour l’arthrose? Médecine/Sciences 2018, 34, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Alt, E.; Yan, Y.; Gehmert, S.; Song, Y.H.; Altman, A.; Gehmert, S.; Vykoukal, D.; Bai, X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol. Cell. 2011, 103, 197–208. [Google Scholar] [CrossRef]
- Lo Monaco, M.; Gervois, P.; Beaumont, J.; Clegg, P.; Bronckaers, A.; Vandeweerd, J.M.; Lambrichts, I. Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis. Cells 2020, 9, 980. [Google Scholar] [CrossRef]
- Maldonado, D.C.; Nicoliche, T.; Faber, J.; Kerkis, I.; Saez, D.M.; Sasaki, R.T.; Cavenaghi Pereira da Silva, M. Intra-articular human deciduous dental pulp stem cell administration vs. pharmacological therapy in experimental osteoarthritis rat model. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3546–3556. [Google Scholar]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2018, 93, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019, 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise Reviews: Characteristics and Potential Applications of Human Dental Tissue-Derived Mesenchymal Stem Cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Mark Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, D.; Park, M.; Kim, S.; Choi, Y.J.; Moon, S.J.; Shin, D.M.; Lee, J.H.; Kim, E. Single-Cell RNA Sequencing Analysis of Human Dental Pulp Stem Cell and Human Periodontal Ligament Stem Cell. J. Endod. 2022, 48, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Freyria, A.M.; Courtes, S.; Mallein-Gerin, F. Différenciation des cellules souches mésenchymateuses adultes humaines: Effet chondrogénique de la BMP-2. Pathol. Biol. 2008, 56, 326–333. [Google Scholar] [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Hazrati, A.; Malekpour, K.; Soudi, S.; Hashemi, S.M. Mesenchymal stromal/stem cells spheroid culture effect on the therapeutic efficacy of these cells and their exosomes: A new strategy to overcome cell therapy limitations. Biomed Pharmacother. 2022, 152, 113211. [Google Scholar] [CrossRef]
- Staubli, F.; Stoddart, M.J.; D’Este, M.; Schwab, A. Pre-culture of human mesenchymal stromal cells in spheroids facilitates chondrogenesis at a low total cell count upon embedding in biomaterials to generate cartilage microtissues. Acta Biomater. 2022, 143, 253–265. [Google Scholar] [CrossRef]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef]
- Tsvetkova, A.V.; Vakhrushev, I.V.; Basok, Y.B.; Grigor’Ev, A.M.; Kirsanova, L.A.; Lupatov, A.Y.; Sevastianov, V.I.; Yarygin, K.N. Chondrogeneic Potential of MSC from Different Sources in Spheroid Culture. Bull. Exp. Biol. Med. 2021, 170, 528–536. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lee, Y.C.; Hung, C.Y.; Yang, P.J.; Lai, P.C.; Feng, S.W. Three-dimensional Spheroid Culture Enhances Multipotent Differentiation and Stemness Capacities of Human Dental Pulp-derived Mesenchymal Stem Cells by Modulating MAPK and NF-kB Signaling Pathways. Stem Cell Rev. Rep. 2021, 17, 1810–1826. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.U.; Lee, H.S.; Lee, B.N.; Hwang, Y.C.; Kim, S.Y.; Chang, S.W.; Choi, K.K.; Kim, D.S.; Jang, J.H. In Vitro Characterization of Dental Pulp Stem Cells Cultured in Two Microsphere-Forming Culture Plates. J. Clin. Med. 2020, 9, 242. [Google Scholar] [CrossRef]
- Prideaux, M.; Wright, C.S.; Noonan, M.L.; Yi, X.; Clinkenbeard, E.L.; Mevel, E.; Wheeler, J.A.; Byers, S.; Wijenayaka, A.R.; Gronthos, S.; et al. Generation of two multipotent mesenchymal progenitor cell lines capable of osteogenic, mature osteocyte, adipogenic, and chondrogenic differentiation. Sci. Rep. 2021, 11, 22593. [Google Scholar] [CrossRef]
- Watanabe, H. Aggrecan and versican: Two brothers close or apart. Am. J. Physiol. Cell Physiol. 2022, 322, C967–C976. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 9253. [Google Scholar] [CrossRef]
- Song, H.; Park, K.H. Regulation and function of SOX9 during cartilage development and regeneration. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020; Volume 67, pp. 12–23. [Google Scholar]
- Zhang, L.; Su, P.; Xu, C.; Yang, J.; Yu, W.; Huang, D. Chondrogenic differentiation of human mesenchymal stem cells: A comparison between micromass and pellet culture systems. Biotechnol. Lett. 2010, 32, 1339–1346. [Google Scholar] [CrossRef]
- Ko, J.; Park, J.; Kim, J.; Im, G. Characterization of adipose-derived stromal/stem cell spheroids versus single-cell suspension in cell survival and arrest of osteoarthritis progression. J. Biomed. Mater. Res. A 2021, 109, 869–878. [Google Scholar] [CrossRef] [PubMed]





| Primer | Sequence 5′-3′ | Exon Position | Product Size (bp) | Primer Efficiency, Ep (%) | R2 |
|---|---|---|---|---|---|
| 18S | F: ATTAAGGGTGTGGGCCGAAG R: GGTGATCACACGTTCCACCT | F: E1/2 R: E2/3 | 111 | 110.1 | 1 |
| GAPDH | F: AAGGTGAAGGTCGGAGTCAAC R: GGGGTCATTGATGGCAACA | F: E2 R: E3 | 102 | 90.4 | 1 |
| ACAN | F: GCACAGCCACCACCTACAAAC R: AGCGACAAGAAGAGGACACCG | F: E15/16 R: E16 | 175 | 101.4 | 0.91 |
| COL2A1 | F: GGCAATAGCAGGTTCACGTACA R: CGATAACAGTCTTGCCCCACTT | F: E52 R: E53 | 79 | 113.8 | 0.93 |
| SOX9 | F: GAAGCTCGCGGACCAGTA R: TCTCGCTCTCGTTCAGAAGT | F: E1 R: E2 | 89 | 96 | 0.95 |
| COMP | F: AGGGTACCCAACTCAGACCA R: AGTTGTCCCGAGAGTCCTGA | F: E11 R: E13 | 178 | 93 | 1 |
| Monolayer | Spheroids | Pellets | ||
|---|---|---|---|---|
| Day 3 | Acan | 12.36 | 38.49 | 2.03 |
| Comp | 14.72 | 128.02 | 293.75 | |
| Col2a1 | 1.32 | 0.43 | 0.39 | |
| Sox9 | 0.54 | 1.07 | 0.79 | |
| Day 7 | Acan | 22.31 | 57.71 | 2.79 |
| Comp | 513.74 | 910.12 | 1046.70 | |
| Col2a1 | 2.51 | 0.57 | 1.19 | |
| Sox9 | 0.55 | 0.68 | 0.62 |
| Monolayer | Spheroids | Pellets | ||
|---|---|---|---|---|
| Day 3 | Acan | 4.73 | 3.64 | 1.27 |
| Comp | 25.26 | 1536.67 | 278.17 | |
| Col2a1 | 1.20 | 0.33 | 0.28 | |
| Sox9 | 0.53 | 0.96 | 1.22 | |
| Day 7 | Acan | 4.24 | 59.33 | 1.40 |
| Comp | 2122.08 | 1144.45 | 257.64 | |
| Col2a1 | 4.28 | 0.17 | 0.56 | |
| Sox9 | 0.40 | 0.63 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mélou, C.; Pellen-Mussi, P.; Novello, S.; Brézulier, D.; Novella, A.; Tricot, S.; Bellaud, P.; Chauvel-Lebret, D. Spheroid Culture System, a Promising Method for Chondrogenic Differentiation of Dental Mesenchymal Stem Cells. Biomedicines 2023, 11, 1314. https://doi.org/10.3390/biomedicines11051314
Mélou C, Pellen-Mussi P, Novello S, Brézulier D, Novella A, Tricot S, Bellaud P, Chauvel-Lebret D. Spheroid Culture System, a Promising Method for Chondrogenic Differentiation of Dental Mesenchymal Stem Cells. Biomedicines. 2023; 11(5):1314. https://doi.org/10.3390/biomedicines11051314
Chicago/Turabian StyleMélou, Caroline, Pascal Pellen-Mussi, Solen Novello, Damien Brézulier, Agnès Novella, Sylvie Tricot, Pascale Bellaud, and Dominique Chauvel-Lebret. 2023. "Spheroid Culture System, a Promising Method for Chondrogenic Differentiation of Dental Mesenchymal Stem Cells" Biomedicines 11, no. 5: 1314. https://doi.org/10.3390/biomedicines11051314
APA StyleMélou, C., Pellen-Mussi, P., Novello, S., Brézulier, D., Novella, A., Tricot, S., Bellaud, P., & Chauvel-Lebret, D. (2023). Spheroid Culture System, a Promising Method for Chondrogenic Differentiation of Dental Mesenchymal Stem Cells. Biomedicines, 11(5), 1314. https://doi.org/10.3390/biomedicines11051314





