Multimodal Neuromonitoring and Neurocritical Care in Swine to Enhance Translational Relevance in Brain Trauma Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Iterative Technique Development
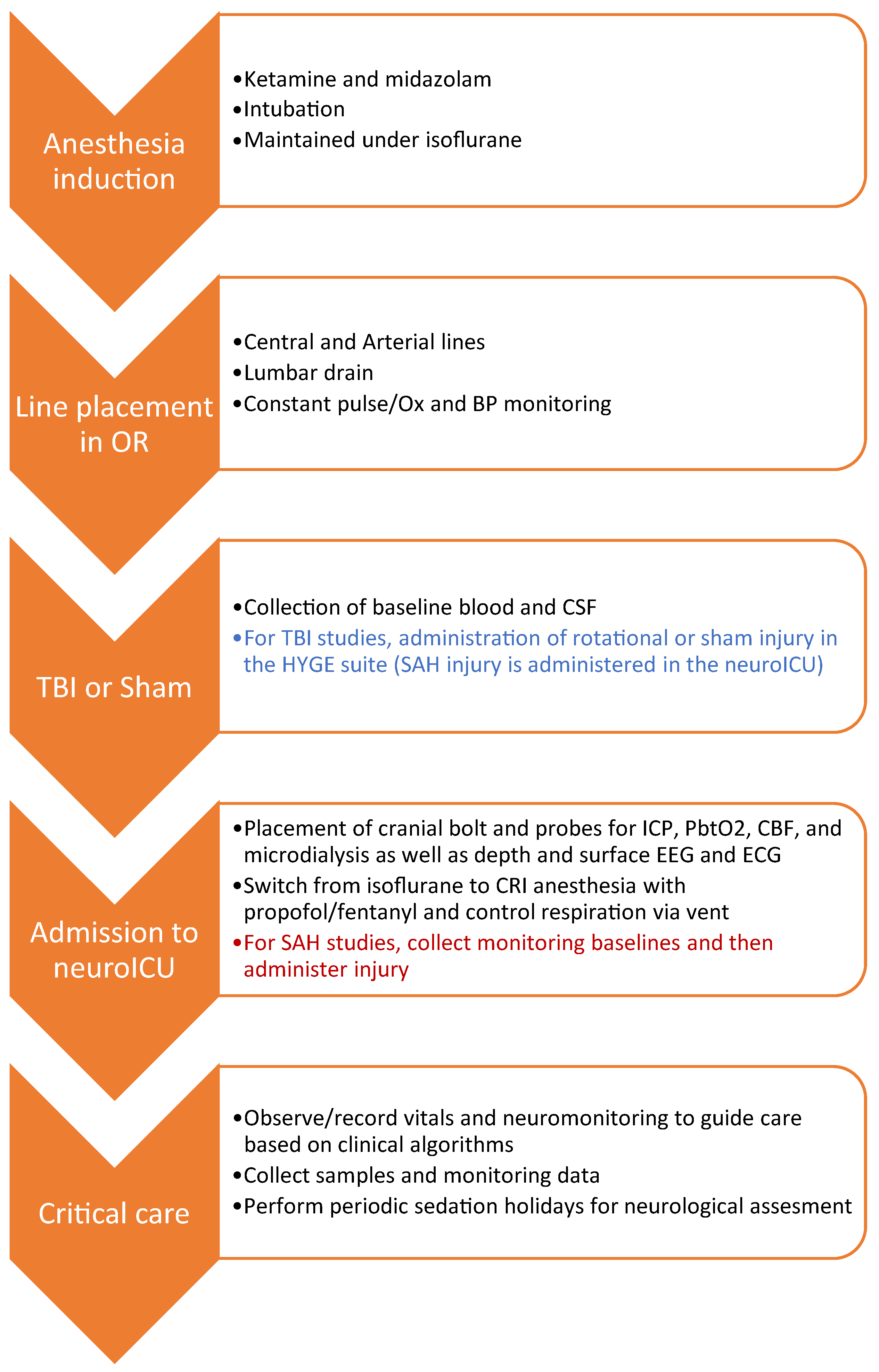
2.2. Workflow Summary
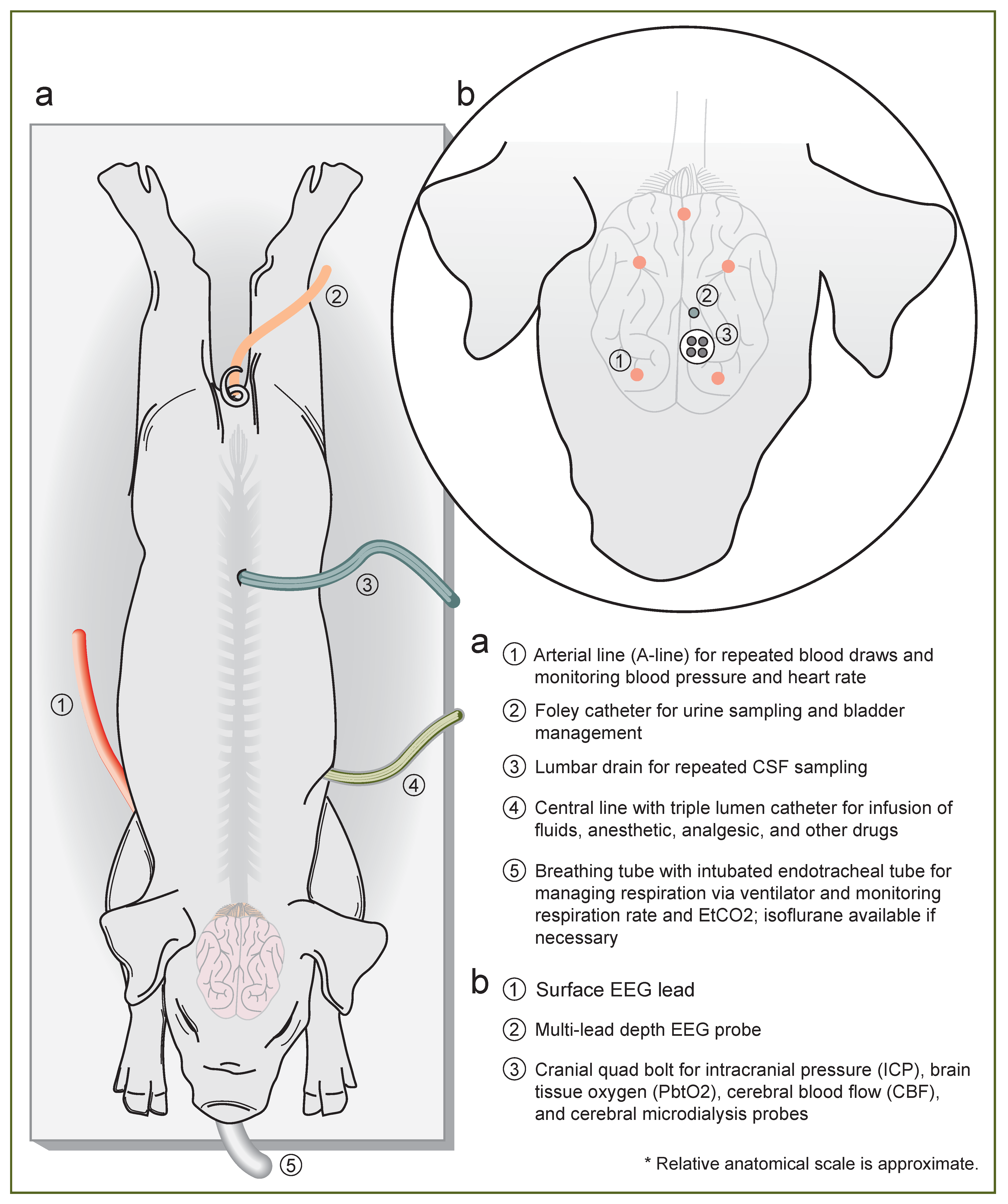
2.3. Induction and Line Placement
2.4. Rotational Traumatic Brain Injury (TBI)
2.5. Multimodal Neuromonitoring (MMNM)
2.6. Administration of Subarachnoid Hemorrhage (SAH)
2.7. Neurocritical Care
2.8. Sample Collection and Analyses
3. Results
- Medical management of sham subjects
3.1. High EtCO2
- Medical management of SAH
- Medical management of TBI with coma
3.2. Cardiovascular Distress and Post-Traumatic Epilepsy (PTE)
3.3. Coma and Wakefulness without Awareness
4. Discussion
4.1. Future Directions for Translational SAH and TBI Studies
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A-line | arterial line |
| APP | amyloid precursor protein |
| BP | blood pressure |
| CBF | cerebral blood flow |
| CPP | cerebral perfusion pressure |
| CRI | continuous rate infusion |
| CSF | cerebrospinal fluid |
| DAB | 3,3′-diaminobenzidine |
| DTI | diffusion tensor imaging |
| EEG | electroencephalography |
| EKG | electrocardiogram |
| EtCO2 | end-tidal CO2 |
| EVD | external ventricular drain |
| FFPE | formalin-fixed, paraffin-embedded |
| GCS | Glasgow Coma Scale |
| GFAP | glial fibrillary acidic protein |
| H&E | hematoxylin and eosin |
| HR | heart rate |
| IACUC | Institutional Animal Care and Use Committee |
| IBA1 | ionized calcium binding adaptor molecule 1 |
| ICP | intracranial pressure |
| IND/IDE | investigational new drug/investigational device exemption |
| LPR | lactate:pyruvate ratio |
| MAP | mean arterial pressure |
| MMNM | multimodal neuromonitoring |
| NCAF | Neurointensive Care and Assessment Facility |
| neuroICU | neurointensive care unit |
| ODC-TBI | Open Data Commons for TBI |
| PbtO2 | partial brain tissue oxygen |
| PTE | post-traumatic epilepsy |
| rad/sec | radians/second |
| SAH | subarachnoid hemorrhage |
| SpO2 | blood oxygen saturation |
| SWI | susceptibility weighted Imaging |
| TBI | traumatic brain injury |
| UCHL1 | ubiquitin carboxyl-terminal esterase L1 |
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Lucke-Wold, B.P.; Logsdon, A.F.; Robson, M.; Lee, J.M.; Bailes, J.E.; Dashnaw, M.L.; Huber, J.D.; Petraglia, A.L.; Rosen, C.L. Modeling Chronic Traumatic Encephalopathy: The Way Forward for Future Discovery. Front. Neurol. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Lacalle-Aurioles, M.; Iturria-Medina, Y. Fornix degeneration in risk factors of Alzheimer’s disease, possible trigger of cognitive decline. Cereb. Circ. Cogn. Behav. 2023, 4, 100158. [Google Scholar] [CrossRef]
- Swanson, R.L.; Acharya, N.K.; Cifu, D.X. Cerebral Microvascular Pathology Is a Common Endophenotype Between Traumatic Brain Injury, Cardiovascular Disease, and Dementia: A Hypothesis and Review. Cureus 2022, 14, e25318. [Google Scholar] [CrossRef]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Johnson, V.E.; Stewart, W.; Arena, J.D.; Smith, D.H. Traumatic Brain Injury as a Trigger of Neurodegeneration. Adv. Neurobiol. 2017, 15, 383–400. [Google Scholar] [CrossRef]
- Izzy, S.; Compton, R.; Carandang, R.; Hall, W.; Muehlschlegel, S. Self-Fulfilling Prophecies Through Withdrawal of Care: Do They Exist in Traumatic Brain Injury, Too? Neurocrit. Care 2013, 19, 347–363. [Google Scholar] [CrossRef]
- Provencio, J.J.; Hemphill, J.C.; Claassen, J.; Edlow, B.L.; Helbok, R.; Vespa, P.M.; Diringer, M.N.; Polizzotto, L.; Shutter, L.; Suarez, J.I.; et al. The Curing Coma Campaign: Framing Initial Scientific Challenges—Proceedings of the First Curing Coma Campaign Scientific Advisory Council Meeting. Neurocrit. Care 2020, 33, 1–12. [Google Scholar] [CrossRef]
- Turgeon, A.F.; Lauzier, F.; Simard, J.-F.; Scales, D.C.; Burns, K.E.; Moore, L.; Zygun, D.A.; Bernard, F.; Meade, M.O.; Dung, T.C.; et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: A Canadian multicentre cohort study. Can. Med. Assoc. J. 2011, 183, 1581–1588. [Google Scholar] [CrossRef] [Green Version]
- Egbebike, J.; Shen, Q.; Doyle, K.; Der-Nigoghossian, C.A.; Panicker, L.; Gonzales, I.J.; Grobois, L.; Carmona, J.C.; Vrosgou, A.; Kaur, A.; et al. Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: A prospective observational cohort study. Lancet Neurol. 2022, 21, 704–713. [Google Scholar] [CrossRef]
- McCrea, M.A.; Giacino, J.T.; Barber, J.; Temkin, N.R.; Nelson, L.D.; Levin, H.S.; Dikmen, S.; Stein, M.; Bodien, Y.G.; Boase, K.; et al. Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021, 78, 982. [Google Scholar] [CrossRef]
- Kowalski, R.G.; Hammond, F.M.; Weintraub, A.H.; Nakase-Richardson, R.; Zafonte, R.D.; Whyte, J.; Giacino, J.T. Recovery of Consciousness and Functional Outcome in Moderate and Severe Traumatic Brain Injury. JAMA Neurol. 2021, 78, 548. [Google Scholar] [CrossRef]
- Edlow, B.L.; Fins, J.J. Assessment of Covert Consciousness in the Intensive Care Unit: Clinical and Ethical Considerations. J. Head Trauma Rehabil. 2018, 33, 424–434. [Google Scholar] [CrossRef]
- Pham, X.; Ray, J.; Neto, A.S.; Laing, J.; Perucca, P.; Kwan, P.; O’brien, T.J.; Udy, A.A. Association of Neurocritical Care Services With Mortality and Functional Outcomes for Adults With Brain Injury: A Systematic Review and Meta-Analysis. JAMA Neurol. 2022, 79, 1049–1058. [Google Scholar] [CrossRef]
- Claassen, J.; Akbari, Y.; Alexander, S.; Bader, M.K.; Bell, K.; Bleck, T.P.; Boly, M.; Brown, J.; Chou, S.H.-Y.; Diringer, M.N.; et al. Proceedings of the First Curing Coma Campaign NIH Symposium: Challenging the Future of Research for Coma and Disorders of Consciousness. Neurocrit. Care 2021, 35, 4–23. [Google Scholar] [CrossRef]
- Luppi, A.I.; Cain, J.; Spindler, L.R.B.; Górska, U.J.; Toker, D.; Hudson, A.E.; Brown, E.N.; Diringer, M.N.; Stevens, R.D.; Massimini, M.; et al. Mechanisms Underlying Disorders of Consciousness: Bridging Gaps to Move Toward an Integrated Translational Science. Neurocrit. Care 2021, 35, 37–54. [Google Scholar] [CrossRef]
- Bailey, E.L.; McCulloch, J.; Sudlow, C.; Wardlaw, J.M. Potential Animal Models of Lacunar Stroke: A Systematic Review. Stroke 2009, 40, e451–e458. [Google Scholar] [CrossRef]
- Howells, D.W.; Porritt, M.J.; Rewell, S.S.J.; O’Collins, V.; Sena, E.S.; Van Der Worp, H.B.; Traystman, R.J.; Macleod, M.R. Different Strokes for Different Folks: The Rich Diversity of Animal Models of Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1412–1431. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Sejnowski, T.J. A universal scaling law between gray matter and white matter of cerebral cortex. Proc. Natl. Acad. Sci. USA 2000, 97, 5621–5626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, D.K.; Harris, J.P.; Browne, K.D.; Wolf, J.A.; Duda, J.E.; Meaney, D.F.; Margulies, S.S.; Smith, D.H. A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration. Methods Mol. Biol. 2016, 1462, 289–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, K.R.; Xi, G.; Hua, Y.; Kleinholz, M.; De Courten-Myers, G.M.; Myers, R.E.; Broderick, J.P.; Brott, T.G. Lobar Intracerebral Hemorrhage Model in Pigs: Rapid Edema Development in Perihematomal White Matter. Stroke 1996, 27, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Hartings, J.A.; York, J.; Carroll, C.P.; Hinzman, J.M.; Mahoney, E.; Krueger, B.; Winkler, M.K.L.; Major, S.; Horst, V.; Jahnke, P.; et al. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain 2017, 140, 2673–2690. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Khatibi, N.H.; Chen, H.; Tang, J.; Zhang, J.H. History of Preclinical Models of Intracerebral Hemorrhage; Springer: Vienna, Austria, 2019. [Google Scholar]
- Melià-Sorolla, M.; Castaño, C.; DeGregorio-Rocasolano, N.; Rodríguez-Esparragoza, L.; Dávalos, A.; Martí-Sistac, O.; Gasull, T. Relevance of Porcine Stroke Models to Bridge the Gap from Pre-Clinical Findings to Clinical Implementation. Int. J. Mol. Sci. 2020, 21, 6568. [Google Scholar] [CrossRef]
- Keating, C.E.; Cullen, D.K. Mechanosensation in traumatic brain injury. Neurobiol. Dis. 2020, 148, 105210. [Google Scholar] [CrossRef]
- Meaney, D.F.; Smith, D.H.; Shreiber, D.I.; Bain, A.C.; Miller, R.T.; Ross, D.T.; Gennarelli, T.A. Biomechanical Analysis of Experimental Diffuse Axonal Injury. J. Neurotrauma 1995, 12, 689–694. [Google Scholar] [CrossRef]
- Margulies, S.S.; Thibault, L.E.; Gennarelli, T.A. Physical model simulations of brain injury in the primate. J. Biomech. 1990, 23, 823–836. [Google Scholar] [CrossRef]
- Meaney, D.F.; Margulies, S.S.; Smith, D.H. Diffuse Axonal Injury. J. Neurosurg. 2001, 95, 1108–1110. [Google Scholar] [CrossRef]
- Wofford, K.L.; Grovola, M.R.; Adewole, D.O.; Browne, K.D.; Putt, M.E.; O’Donnell, J.C.; Cullen, D.K. Relationships between Injury Kinematics, Neurological Recovery, and Pathology Following Concussion. Brain Commun. 2021, fcab268. [Google Scholar] [CrossRef]
- Denny-Brown, D.E.; Russell, W.R. Experimental Concussion: (Section of Neurology). Proc. R. Soc. Med. 1941, 34, 691–692. [Google Scholar]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. J. Head Trauma Rehabil 2006, 21, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Ommaya, A.K.; Gennarelli, T.A. Cerebral Concussion and Traumatic Unconsciousness. Correlation of Experimental and Clinical Observations of Blunt Head Injuries. Brain 1974, 97, 633–654. [Google Scholar] [CrossRef] [Green Version]
- Gabrielian, L.; Willshire, L.W.; Helps, S.C.; Heuvel, C.V.D.; Mathias, J.; Vink, R. Intracranial Pressure Changes following Traumatic Brain Injury in Rats: Lack of Significant Change in the Absence of Mass Lesions or Hypoxia. J. Neurotrauma 2011, 28, 2103–2111. [Google Scholar] [CrossRef]
- O’Donnell, J.C.; Browne, K.D.; Kilbaugh, T.J.; Chen, H.I.; Whyte, J.; Cullen, D.K. Challenges and demand for modeling disorders of consciousness following traumatic brain injury. Neurosci. Biobehav. Rev. 2019, 98, 336–346. [Google Scholar] [CrossRef]
- Smith, D.H.; Nonaka, M.; Miller, R.; Leoni, M.; Chen, X.-H.; Alsop, D.; Meaney, D.F. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J. Neurosurg. 2000, 93, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.J.; Rosenfeld, J.V.; Murray, L.; Arabi, Y.M.; Davies, A.R.; D’Urso, P.; Kossmann, T.; Ponsford, J.; Seppelt, I.; Reilly, P.; et al. Decompressive Craniectomy in Diffuse Traumatic Brain Injury. N. Engl. J. Med. 2011, 364, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Kolias, A.G.; Adams, H.; Timofeev, I.S.; Corteen, E.A.; Hossain, I.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; et al. Evaluation of Outcomes Among Patients With Traumatic Intracranial Hypertension Treated With Decompressive Craniectomy vs Standard Medical Care at 24 Months: A Secondary Analysis of the RESCUEicp Randomized Clinical Trial. JAMA Neurol. 2022, 79, 664–671. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Rubiano, A.M.; Totten, A.M.; O’reilly, C.; Ullman, J.S.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; Shutter, L.; et al. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 Update of the Decompressive Craniectomy Recommendations. Neurosurgery 2020, 87, 427–434. [Google Scholar] [CrossRef]
- Friess, S.H.; Ralston, J.; Eucker, S.A.; Helfaer, M.A.; Smith, C.; Margulies, S.S. Neurocritical Care Monitoring Correlates With Neuropathology in a Swine Model of Pediatric Traumatic Brain Injury. Neurosurgery 2011, 69, 1139–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friess, S.H.; Smith, C.; Kilbaugh, T.; Frangos, S.G.; Ralston, J.; Helfaer, M.A.; Margulies, S.S. Early cerebral perfusion pressure augmentation with phenylephrine after traumatic brain injury may be neuroprotective in a pediatric swine model. Crit. Care Med. 2012, 40, 2400–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friess, S.H.; Bruins, B.; Kilbaugh, T.; Smith, C.; Margulies, S.S. Differing Effects when Using Phenylephrine and Norepinephrine To Augment Cerebral Blood Flow after Traumatic Brain Injury in the Immature Brain. J. Neurotrauma 2015, 32, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyberg, C.; Karlsson, T.; Hillered, L.; Engström, E.R. Metabolic Pattern of the Acute Phase of Subarachnoid Hemorrhage in a Novel Porcine Model: Studies with Cerebral Microdialysis with High Temporal Resolution. PLoS ONE 2014, 9, e99904. [Google Scholar] [CrossRef]
- Datzmann, T.; Kapapa, T.; Scheuerle, A.; McCook, O.; Merz, T.; Unmuth, S.; Hoffmann, A.; Mathieu, R.; Mayer, S.; Mauer, U.M.; et al. In-depth characterization of a long-term, resuscitated model of acute subdural hematoma–induced brain injury. J. Neurosurg. 2019, 134, 223–234. [Google Scholar] [CrossRef]
- Weenink, R.P.; Vrijdag, X.C.; van Putten, M.J.; Hollmann, M.W.; Stevens, M.F.; van Gulik, T.M.; van Hulst, R.A. Quantitative electroencephalography in a swine model of cerebral arterial gas embolism. Clin. Neurophysiol. 2012, 123, 411–417. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, C.; Cavanaugh, J.M.; Kallakuri, S.; Desai, A.; Zhang, L.; King, A.I. Quantitative electroencephalography in a swine model of blast-induced brain injury. Brain Inj. 2017, 31, 120–126. [Google Scholar] [CrossRef]
- Mader, M.M.; Leidorf, A.; Hecker, A.; Heimann, A.; Mayr, P.S.M.; Kempski, O.; Alessandri, B.; Wöbker, G. Evaluation of a New Multiparameter Brain Probe for Simultaneous Measurement of Brain Tissue Oxygenation, Cerebral Blood Flow, Intracranial Pressure, and Brain Temperature in a Porcine Model. Neurocrit. Care 2018, 29, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Nwafor, D.C.; Brichacek, A.L.; Foster, C.H.; Lucke-Wold, B.P.; Ali, A.; Colantonio, M.A.; Brown, C.M.; Qaiser, R. Pediatric Traumatic Brain Injury: An Update on Preclinical Models, Clinical Biomarkers, and the Implications of Cerebrovascular Dysfunction. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221098124. [Google Scholar] [CrossRef]
- Cralley, A.L.; Moore, E.E.; Kissau, D.; Coleman, J.R.M.; Vigneshwar, N.; DeBot, M.; Schaid, T.R.J.; Moore, H.B.M.; Cohen, M.J.; Hansen, K.; et al. A combat casualty relevant dismounted complex blast injury model in swine. J. Trauma Inj. Infect. Crit. Care 2022, 93, S110–S118. [Google Scholar] [CrossRef]
- Adedipe, A.; John, A.S.; Krishnamoorthy, V.; Wang, X.; Steck, D.T.; Ferreira, R.; White, N.; Stern, S. Left Ventricular Function in the Initial Period After Severe Traumatic Brain Injury in Swine. Neurocrit. Care 2022, 37, 200–208. [Google Scholar] [CrossRef]
- Abdou, H.; Edwards, J.; Patel, N.; Stonko, D.P.; Elansary, N.; Lang, E.; Richmond, M.J.; Ptak, T.; White, J.M.; Scalea, T.M.; et al. Characterizing Brain Perfusion in a Swine Model of Raised Intracranial Pressure. J. Surg. Res. 2022, 278, 64–69. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals Guide for the Care and Use of Laboratory Animals. In The National Academies Collection: Reports Funded by National Institutes of Health, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0.
- Grovola, M.R.; Paleologos, N.; Brown, D.P.; Tran, N.; Wofford, K.L.; Harris, J.P.; Browne, K.D.; Shewokis, P.A.; Wolf, J.A.; Cullen, D.K.; et al. Diverse changes in microglia morphology and axonal pathology during the course of 1 year after mild traumatic brain injury in pigs. Brain Pathol. 2021, 31, e12953. [Google Scholar] [CrossRef]
- Menon, D.K.; Coles, J.P.; Gupta, A.K.; Fryer, T.D.; Smielewski, P.; Chatfield, D.A.; Aigbirhio, F.; Skepper, J.N.; Minhas, P.S.; Hutchinson, P.J.; et al. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004, 32, 1384–1390. [Google Scholar] [CrossRef]
- Vespa, P.M.; O’phelan, K.; McArthur, D.; Miller, C.; Eliseo, M.; Hirt, D.; Glenn, T.; Hovda, D.A. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit. Care Med. 2007, 35, 1153–1160. [Google Scholar] [CrossRef]
- van Santbrink, H.; Maas, A.I.; Avezaat, C.J. Continuous Monitoring of Partial Pressure of Brain Tissue Oxygen in Patients with Severe Head Injury. Neurosurgery 1996, 38, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Brink, W.A.V.D.; van Santbrink, H.; Steyerberg, E.W.; Avezaat, C.J.J.; Suazo, J.A.C.; Hogesteeger, C.; Jansen, W.J.; Kloos, L.M.H.; Vermeulen, J.; Maas, A.I.R. Brain Oxygen Tension in Severe Head Injury. Neurosurgery 2000, 46, 868–878. [Google Scholar] [CrossRef]
- Artru, F.; Jourdan, C.; Perret-Liaudet, A.; Charlot, M.; Mottolese, C. Low brain tissue oxygen pressure: Incidence and corrective therapies. Neurol. Res. 1998, 20 (Suppl. 1), S48–S51. [Google Scholar] [CrossRef]
- Valadka, A.B.; Goodman, J.C.; Gopinath, S.P.; Uzura, M.; Robertson, C.S. Comparison of Brain Tissue Oxygen Tension to Microdialysis-Based Measures of Cerebral Ischemia in Fatally Head-Injured Humans. J. Neurotrauma 1998, 15, 509–519. [Google Scholar] [CrossRef]
- Bardt, T.F.; Unterberg, A.W.; Härtl, R.; Kiening, K.L.; Schneider, G.H.; Lanksch, W.R. Monitoring of Brain Tissue PO2 in Traumatic Brain Injury: Effect of Cerebral Hypoxia on Outcome. Acta Neurochir. Suppl. 1998, 71, 153–156. [Google Scholar] [CrossRef]
- Kiening, K.; Härtl, R.; Unterberg, A.; Schneider, G.-H.; Bardt, T.; Lanksch, W. Brain tissue pO2-monitoring in comatose patients: Implications for therapy. Neurol. Res. 1997, 19, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Bohman, L.-E.; Heuer, G.G.; Macyszyn, L.; Maloney-Wilensky, E.; Frangos, S.; Le Roux, P.D.; Kofke, A.; Levine, J.M.; Stiefel, M.F. Medical Management of Compromised Brain Oxygen in Patients with Severe Traumatic Brain Injury. Neurocrit. Care 2011, 14, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.L.; Georgoff, P.; Maloney-Wilensky, E.; Sims, C.; Sarani, B.; Stiefel, M.F.; LeRoux, P.D.; Schwab, C.W. Reduced Brain Tissue Oxygen in Traumatic Brain Injury: Are Most Commonly Used Interventions Successful? J. Trauma Inj. Infect. Crit. Care 2011, 70, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Nangunoori, R.; Maloney-Wilensky, E.; Stiefel, M.; Park, S.; Kofke, W.A.; Levine, J.M.; Yang, W.; Le Roux, P.D. Brain Tissue Oxygen-Based Therapy and Outcome After Severe Traumatic Brain Injury: A Systematic Literature Review. Neurocrit. Care 2012, 17, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, D.O.; Shutter, L.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef]
- Barsan, W. Brain Oxygen Optimization in Severe TBI (BOOST3): A Comparative Effectiveness Study to Test the Efficacy of a Prescribed Treatment Protocol Based on Monitoring the Partial Pressure of Brain Tissue Oxygen; 2021. Available online: clinicaltrials.gov (accessed on 20 December 2021).
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow Coma Scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Platt, S.R.; Radaelli, S.T.; McDonnell, J.J. The Prognostic Value of the Modified Glasgow Coma Scale in Head Trauma in Dogs. J. Vet. Intern. Med. 2001, 15, 581–584. [Google Scholar] [CrossRef]
- Sharma, D.; Holowaychuk, M.K. Retrospective evaluation of prognostic indicators in dogs with head trauma: 72 cases (January-March 2011). J. Vet. Emerg. Crit. Care 2015, 25, 631–639. [Google Scholar] [CrossRef]
- Shores, A. Craniocerebral Trauma. In Current Veterinary Therapy X; Kirk, R.W., Ed.; WB Saunders: Philadelphia, PA, USA, 1983; pp. 847–854. [Google Scholar]
- Wofford, K.L.; Harris, J.P.; Browne, K.D.; Brown, D.P.; Grovola, M.R.; Mietus, C.J.; Wolf, J.A.; Duda, J.E.; Putt, M.E.; Spiller, K.L.; et al. Rapid neuroinflammatory response localized to injured neurons after diffuse traumatic brain injury in swine. Exp. Neurol. 2017, 290, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Grovola, M.; Paleologos, N.; Wofford, K.L.; Harris, J.P.; Browne, K.D.; Johnson, V.; Duda, J.E.; Wolf, J.A.; Cullen, D.K. Mossy cell hypertrophy and synaptic changes in the hilus following mild diffuse traumatic brain injury in pigs. J. Neuroinflamm. 2020, 17, 44. [Google Scholar] [CrossRef]
- Grovola, M.R.; von Reyn, C.; Loane, D.J.; Cullen, D.K. Understanding microglial responses in large animal models of traumatic brain injury: An underutilized resource for preclinical and translational research. J. Neuroinflamm. 2023, 20, 67. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Brolinson, P.G.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef]
- Papa, L.; Brophy, G.M.; Welch, R.D.; Lewis, L.M.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Lopez, M.A.; Haeussler, C.A.; Giordano, D.I.M.; et al. Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients With and Without Mild Traumatic Brain Injury. JAMA Neurol. 2016, 73, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Korley, F.K.; Jain, S.; Sun, X.; Puccio, A.M.; Yue, J.K.; Gardner, R.C.; Wang, K.K.W.; Okonkwo, D.O.; Yuh, E.L.; Mukherjee, P.; et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: An observational cohort study. Lancet Neurol. 2022, 21, 803–813. [Google Scholar] [CrossRef]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood Biomarkers for Traumatic Brain Injury: A Quantitative Assessment of Diagnostic and Prognostic Accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Papa, L.; Ladde, J.G.; O’brien, J.F.; Thundiyil, J.G.; Tesar, J.; Leech, S.; Cassidy, D.D.; Roa, J.; Hunter, C.; Miller, S.; et al. Evaluation of Glial and Neuronal Blood Biomarkers Compared With Clinical Decision Rules in Assessing the Need for Computed Tomography in Patients With Mild Traumatic Brain Injury. JAMA Netw. Open 2022, 5, e221302. [Google Scholar] [CrossRef]
- Graham, N.S.N.; Zimmerman, K.A.; Moro, F.; Heslegrave, A.; Maillard, S.A.; Bernini, A.; Miroz, J.-P.; Donat, C.K.; Lopez, M.Y.; Bourke, N.; et al. Axonal marker neurofilament light predicts long-term outcomes and progressive neurodegeneration after traumatic brain injury. Sci. Transl. Med. 2021, 13, eabg9922. [Google Scholar] [CrossRef]
- Lind, N.M.; Moustgaard, A.; Jelsing, J.; Vajta, G.; Cumming, P.; Hansen, A.K. The use of pigs in neuroscience: Modeling brain disorders. Neurosci. Biobehav. Rev. 2007, 31, 728–751. [Google Scholar] [CrossRef]
- Østergaard, K.; Holm, I.E.; Zimmer, J. Tyrosine hydroxylase and acetylcholinesterase in the domestic pig mesencephalon: An immunocytochemical and histochemical study. J. Comp. Neurol. 1992, 322, 149–166. [Google Scholar] [CrossRef]
- Namjoshi, D.R.; Cheng, W.H.; McInnes, K.A.; Martens, K.M.; Carr, M.; Wilkinson, A.; Fan, J.; Robert, J.; Hayat, A.; Cripton, P.A.; et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): A novel, surgery-free model of traumatic brain injury. Mol. Neurodegener. 2014, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerbeck, A.D.; Fanizzi, C.; Kim, J.H.; Gangolli, M.; Bayly, P.V.; Wellington, C.L.; Brody, D.L.; Kummer, T.T. modCHIMERA: A novel murine closed-head model of moderate traumatic brain injury. Sci. Rep. 2018, 8, 7677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadian, A.; Mizzi, A.; Banasiak, M.; Downes, K.; Camporesi, E.M.; Thompson Sullebarger, J.; Vasan, R.; Mangar, D.; Van Loveren, H.R.; Agazzi, S. Cardiac manifestations of subarachnoid hemorrhage. Heart Lung Vessel. 2013, 5, 168–178. [Google Scholar] [PubMed]
- Koza, Y.; Aydin, N.; Aydin, M.D.; Koza, E.A.; Bayram, E.; Atalay, C.; Altas, E.; Kursad, H.; Kabalar, M.E. Neurogenic Stress Cardiomyopathy Following Subarachnoid Hemorrhage Is Associated with Vagal Complex Degeneration: First Experimental Study. World Neurosurg. 2019, 129, e741–e748. [Google Scholar] [CrossRef]
- Norberg, E.; Odenstedt-Herges, H.; Rydenhag, B.; Oras, J. Impact of Acute Cardiac Complications After Subarachnoid Hemorrhage on Long-Term Mortality and Cardiovascular Events. Neurocrit. Care 2018, 29, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Feldstein, E.; Dominguez, J.F.; Kaur, G.; Patel, S.D.; Dicpinigaitis, A.J.; Semaan, R.; Fuentes, L.E.; Ogulnick, J.; Ng, C.; Rawanduzy, C.; et al. Cardiac arrest in spontaneous subarachnoid hemorrhage and associated outcomes. Neurosurg. Focus 2022, 52, E6. [Google Scholar] [CrossRef]
- Kerro, A.; Woods, T.; Chang, J.J. Neurogenic stunned myocardium in subarachnoid hemorrhage. J. Crit. Care 2017, 38, 27–34. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Morris, N.; Lahiri, S.; Roth, W.; Witsch, J.; Machado, I.; Agarwal, S.; Park, S.; Meyers, P.M.; Connolly, E.S.; et al. Use of Intra-aortic- Balloon Pump Counterpulsation in Patients with Symptomatic Vasospasm Following Subarachnoid Hemorrhage and Neurogenic Stress Cardiomyopathy. J. Vasc. Interv. Neurol. 2016, 9, 28–34. [Google Scholar]
- Frontera, J.A. Clinical Trials in Cardiac Arrest and Subarachnoid Hemorrhage: Lessons from the Past and Ideas for the Future. Stroke Res. Treat. 2013, 2013, 263974. [Google Scholar] [CrossRef]
- Zachariah, J.; Stanich, J.A.; Braksick, S.A.; Wijdicks, E.F.; Campbell, R.L.; Bell, M.R.; White, R. Indicators of Subarachnoid Hemorrhage as a Cause of Sudden Cardiac Arrest. Clin. Pract. Cases Emerg. Med. 2016, 1, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Kim, K.; Lim, Y.S.; Lee, H.J.; Lee, S.J.; Jung, E.; Kim, J.; Yang, H.J.; Kim, J.J.; Hwang, S.Y. Incidence and clinical features of intracranial hemorrhage causing out-of-hospital cardiac arrest: A multicenter retrospective study. Am. J. Emerg. Med. 2016, 34, 2326–2330. [Google Scholar] [CrossRef]
- Levin, H.S.; Meyers, C.A.; Grossman, R.G.; Sarwar, M. Ventricular Enlargement After Closed Head Injury. Arch. Neurol. 1981, 38, 623–629. [Google Scholar] [CrossRef]
- Levine, B.; Fujiwara, E.; O’Connor, C.; Richard, N.; Kovacevic, N.; Mandic, M.; Restagno, A.; Easdon, C.; Robertson, I.H.; Graham, S.J.; et al. In Vivo Characterization of Traumatic Brain Injury Neuropathology with Structural and Functional Neuroimaging. J. Neurotrauma 2006, 23, 1396–1411. [Google Scholar] [CrossRef] [Green Version]
- Meyers, C.A.; Levin, H.S.; Eisenberg, H.M.; Guinto, F.C. Early versus late lateral ventricular enlargement following closed head injury. J. Neurol. Neurosurg. Psychiatry 1983, 46, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Poca, M.A.; Sahuquillo, J.; Mataro, M.; Benejam, B.; Arikan, F.; Baguena, M. Ventricular Enlargement after Moderate or Severe Head Injury: A Frequent and Neglected Problem. J. Neurotrauma 2005, 22, 1303–1310. [Google Scholar] [CrossRef]
- Oishi, K.; Lyketsos, C.G. Alzheimer’s Disease and the Fornix. Front. Aging Neurosci. 2014, 6, 241. [Google Scholar] [CrossRef] [Green Version]
- Mielke, M.M.; Okonkwo, O.C.; Oishi, K.; Mori, S.; Tighe, S.; Miller, M.I.; Ceritoglu, C.; Brown, T.; Albert, M.; Lyketsos, C.G. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Douet, V.; Chang, L. Fornix as an Imaging Marker for Episodic Memory Deficits in Healthy Aging and in Various Neurological Disorders. Front. Aging Neurosci. 2015, 6, 343–362. [Google Scholar] [CrossRef] [Green Version]
- Metzler-Baddeley, C.; Jones, D.K.; Belaroussi, B.; Aggleton, J.P.; O’Sullivan, M.J. Frontotemporal Connections in Episodic Memory and Aging: A Diffusion MRI Tractography Study. J. Neurosci. 2011, 31, 13236–13245. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, E.; Raman, M.; Huebner, P.; Liu, A.; Mungas, D.; Carmichael, O.; DeCarli, C. Loss of Fornix White Matter Volume as a Predictor of Cognitive Impairment in Cognitively Normal Elderly Individuals. JAMA Neurol. 2013, 70, 1389–1395. [Google Scholar] [CrossRef]
- Chou, A.; Torres-Espín, A.; Huie, J.R.; Krukowski, K.; Lee, S.; Nolan, A.; Guglielmetti, C.; Hawkins, B.E.; Chaumeil, M.M.; Manley, G.T.; et al. Empowering Data Sharing and Analytics through the Open Data Commons for Traumatic Brain Injury Research. Neurotrauma Rep. 2022, 3, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.D.; Chen, X.-H.; Meaney, D.; Smith, D.H. Mild Traumatic Brain Injury and Diffuse Axonal Injury in Swine. J. Neurotrauma 2011, 28, 1747–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, J.C.; Swanson, R.L.; Wofford, K.L.; Grovola, M.R.; Purvis, E.M.; Petrov, D.; Cullen, D.K. Emerging Approaches for Regenerative Rehabilitation Following Traumatic Brain Injury. In Regenerative Rehabilitation: From Basic Science to the Clinic; Greising, S.M., Call, J.A., Eds.; Physiology in Health and Disease; Springer International Publishing: Cham, Switzerland, 2022; pp. 409–459. ISBN 978-3-030-95884-8. [Google Scholar]






| Authors, Year | Title | Injury | Time | Anesthesia | Neuromonitoring Modalities | EEG | Other | A Line | Central Line | Lumbar Drain |
|---|---|---|---|---|---|---|---|---|---|---|
| Friess et al., 2011 [42] | “Neurocritical Care Monitoring Correlates with Neuropathology in a Swine Model of Pediatric Traumatic Brain Injury” | rotational TBI | 6 h | isoflurane and CRI fentanyl | ICP, PbtO2, microdialysis for LPR | N | IHC | Y | Y | N |
| Friess et al., 2012 [43] | “Early cerebral perfusion pressure augmentation with phenylephrine after traumatic brain injury may be neuroprotective in a pediatric swine model” | rotational TBI | 6 h | isoflurane and CRI fentanyl | ICP, PbtO2, CBF, microdialysis for LPR | N | IHC | Y | Y | N |
| Weenink et al., 2012 [47] | “Quantitative electroencephalography in a swine model of cerebral arterial gas embolism” | arterial gas embolism | 4 h | IV ketamine, sufentanil, midazolam, and pancuronium bromide | ICP, PbtO2, microdialysis for lactate and glucose | Y (surface) | n/a | Y | Y | N |
| Nyberg et al., 2014 [45] | “Metabolic Pattern of the Acute Phase of Subarachnoid Hemorrhage in a Novel Porcine Model: Studies with Cerebral Microdialysis with High Temporal Resolution” | SAH | 135 min | CRI ketamine, morphine, and rocuronium bromide | ICP, microdialysis for glucose and LPR | N | CT scan after experiment | Y | Y | N |
| Friess et al., 2015 [44] | “Differing effects when using phenylephrine and norepinephrine to augment cerebral blood flow after traumatic brain injury in the immature brain” | rotational TBI | 6 h | CRI midazolam and fentanyl | ICP, PbtO2, CBF, microdialysis for LPR | N | IHC | Y | Y | N |
| Chen et al., 2017 [48] | “Quantitative electroencephalography in a swine model of blast induced brain injury” | blast TBI | 2 h | IV propofol | none | Y (surface) | n/a | N | N | N |
| Mader et al., 2018 [49] | “Evaluation of a New Multiparameter Brain Probe for Simultaneous Measurement of Brain Tissue Oxygenation, Cerebral Blood Flow, Intracranial Pressure, and Brain Temperature in a Porcine Model” | CCI, physiological challenges | ~5 h | CRI thiopental and piritramide | testing single probe for ICP, PbtO2, CBF | N | n/a | Y | Y | N |
| Datzman et al., 2019 [46] | “In-depth characterization of a long-term, resuscitated model of acute subdural hematoma–induced brain injury” | ASDH | 54 h | CRI propofol and fentanyl | ICP, PbtO2, microdialysis for lactate and glucose | N | mGCS; IHC; brain tissue mitochondrial respiration (Oroboros); plasma GFAP and NSE | Y | Y | N |
| Cralley et al., 2022 [51] | “Zone 1 REBOA in a combat DCBI swine model does not worsen brain injury” | dismounted complex blast injury (DCBI) | 6 h | CRI propofol and fentanyl | ICP | N | brain water content, MAP | Y | Y | N |
| Adedipe et al., 2022 [52] | “Left Ventricular Function in the Initial Period After Severe Traumatic Brain Injury in Swine” | fluid percussion injury | 8 h | isoflurane | ICP | N | transesophageal echocardiography, coagulation, blood flow | Y | Y | N |
| Abdou et al., 2022 [53] | “Characterizing Brain Perfusion in a Swine Model of Raised Intracranial Pressure” | raised ICP via intracranial Fogarty balloon | 2 h | isoflurane | ICP | N | computed tomography perfusion for CBF | Y | Y | N |
| To maximize arousal (5 min after stopping propofol): grip and roll between fingers; first cheek, then neck (sternocleidomastoid), then shoulder/back (trapezious) | ||||||
| Swine Coma Scale - MAXIMIZE AROUSAL PRIOR TO EXAM - | At each timepoint, indicate the score for each category. | |||||
| Score | EYE BLINK | Baseline | 2 h | 4 h | 8 h | 12 h |
| 3 | Blinks spontaneously (wait 3 min) | 3 | 3 | 3 | ||
| 2 | Blinks upon stimulation (e.g. pinch or ear tickle; not near eye) | 2 | 2 | |||
| 1 | No blinking (without directly touching the eye) | |||||
| MOTOR ACTIVITY | ||||||
| 6 | Voluntary walking | 6 | ||||
| 5 | Sitting | |||||
| 4 | Isolated spontaneous movements (e.g. limbs or head) | 4 | 4 | 4 | 4 | |
| 3 | Withdraws forepaw and/or hindpaw in response to noxious stimulation | |||||
| 2 | Muscle contractions in response to noxious stimulation of the limbs | |||||
| 1 | Absence of motor response to noxious stimulation | |||||
| AUDITORY RESPONSE | ||||||
| 2 | Auditory startle | 2 | 2 | 2 | ||
| 1 | None | 1 | 1 | |||
| BRAIN STEM REFLEXES (score 3 and skip if motor score is 6) | ||||||
| 3 | Both palpebral AND pinna reflexes present | 3 | 3 | 3 | 3 | 3 |
| 2 | Palpebral OR pinna reflex present | |||||
| 1 | Absence of palpebral and pinna reflexes | |||||
| RESPIRATION | ||||||
| 4 | Not on ventilator, breathes with a regular pattern | 4 | 4 | 4 | ||
| 3 | Not on ventilator, breathes with an irregular pattern | 3 | ||||
| 2 | Breathes above ventilator rate (initiates spontaneous breaths) | 2 | ||||
| 1 | Breathes at ventilator rate or apnea (no spontaneous breaths) | |||||
| TOTAL | 18 | 12 | 13 | 16 | 16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Donnell, J.C.; Browne, K.D.; Kvint, S.; Makaron, L.; Grovola, M.R.; Karandikar, S.; Kilbaugh, T.J.; Cullen, D.K.; Petrov, D. Multimodal Neuromonitoring and Neurocritical Care in Swine to Enhance Translational Relevance in Brain Trauma Research. Biomedicines 2023, 11, 1336. https://doi.org/10.3390/biomedicines11051336
O’Donnell JC, Browne KD, Kvint S, Makaron L, Grovola MR, Karandikar S, Kilbaugh TJ, Cullen DK, Petrov D. Multimodal Neuromonitoring and Neurocritical Care in Swine to Enhance Translational Relevance in Brain Trauma Research. Biomedicines. 2023; 11(5):1336. https://doi.org/10.3390/biomedicines11051336
Chicago/Turabian StyleO’Donnell, John C., Kevin D. Browne, Svetlana Kvint, Leah Makaron, Michael R. Grovola, Saarang Karandikar, Todd J. Kilbaugh, D. Kacy Cullen, and Dmitriy Petrov. 2023. "Multimodal Neuromonitoring and Neurocritical Care in Swine to Enhance Translational Relevance in Brain Trauma Research" Biomedicines 11, no. 5: 1336. https://doi.org/10.3390/biomedicines11051336
APA StyleO’Donnell, J. C., Browne, K. D., Kvint, S., Makaron, L., Grovola, M. R., Karandikar, S., Kilbaugh, T. J., Cullen, D. K., & Petrov, D. (2023). Multimodal Neuromonitoring and Neurocritical Care in Swine to Enhance Translational Relevance in Brain Trauma Research. Biomedicines, 11(5), 1336. https://doi.org/10.3390/biomedicines11051336






