Anti-Fibrotic Efficacy of Apigenin in a Mice Model of Carbon Tetrachloride-Induced Hepatic Fibrosis by Modulation of Oxidative Stress, Inflammation, and Fibrogenesis: A Preclinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. The Chemicals and Drugs
2.2. Animals
2.3. Experimental Protocol
2.4. Evaluation of Biomarkers of Liver Functions
2.5. Histopathological Assessment of the Liver Tissues
2.6. Assessment of Oxidative Stress Biomarkers
2.7. The Assessment of Inflammatory Biomarkers
2.8. Immunohistochemical Assessment of Angiogenic Biomarkers
2.9. Protein Determination
2.10. Statistical Analysis
3. Results
3.1. Assessment of Liver Functions
3.2. Findings of Histopathological Analysis
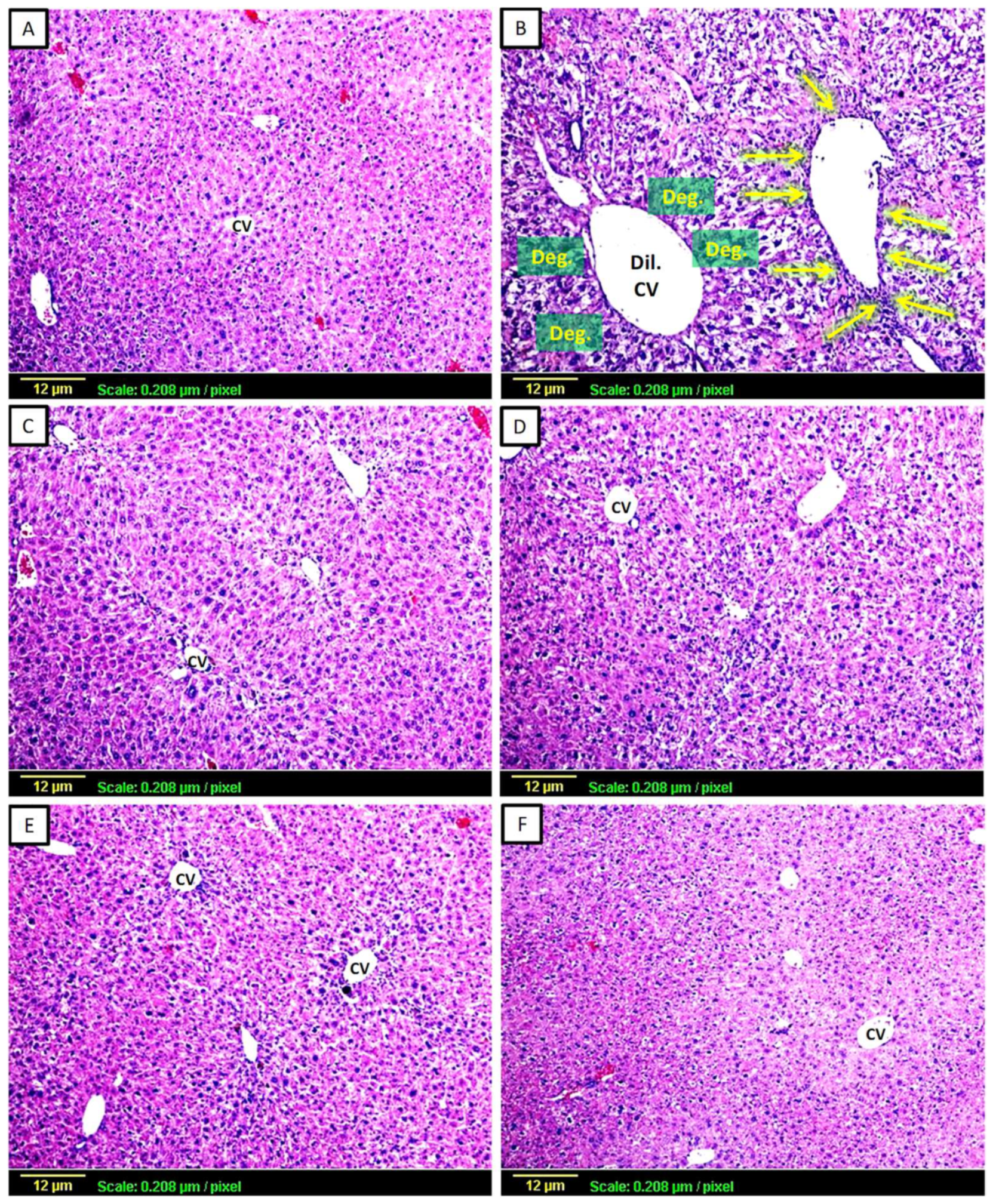
3.2.1. Haematoxylin and Eosin (H&E)
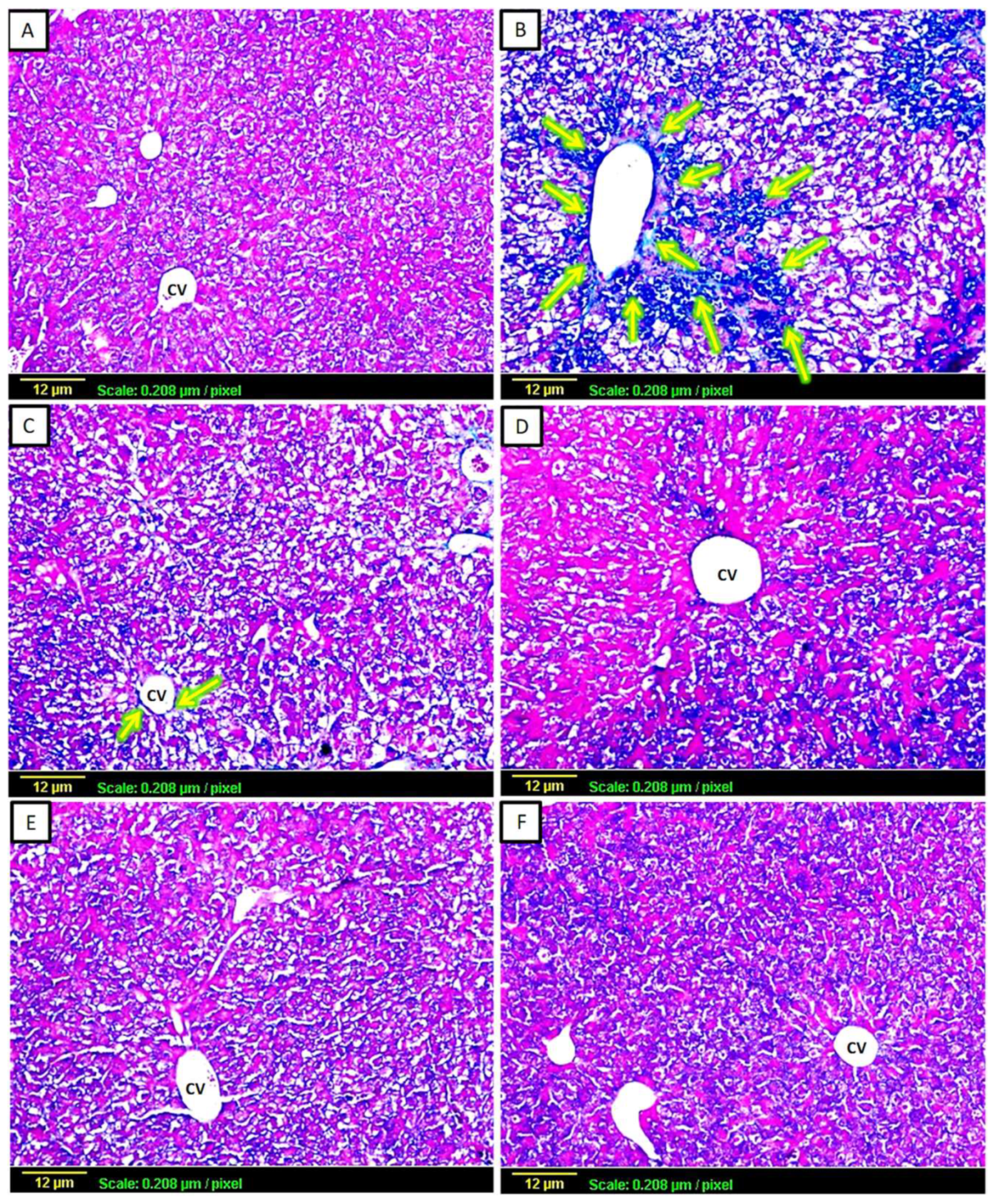
3.2.2. Masson’s Trichrome
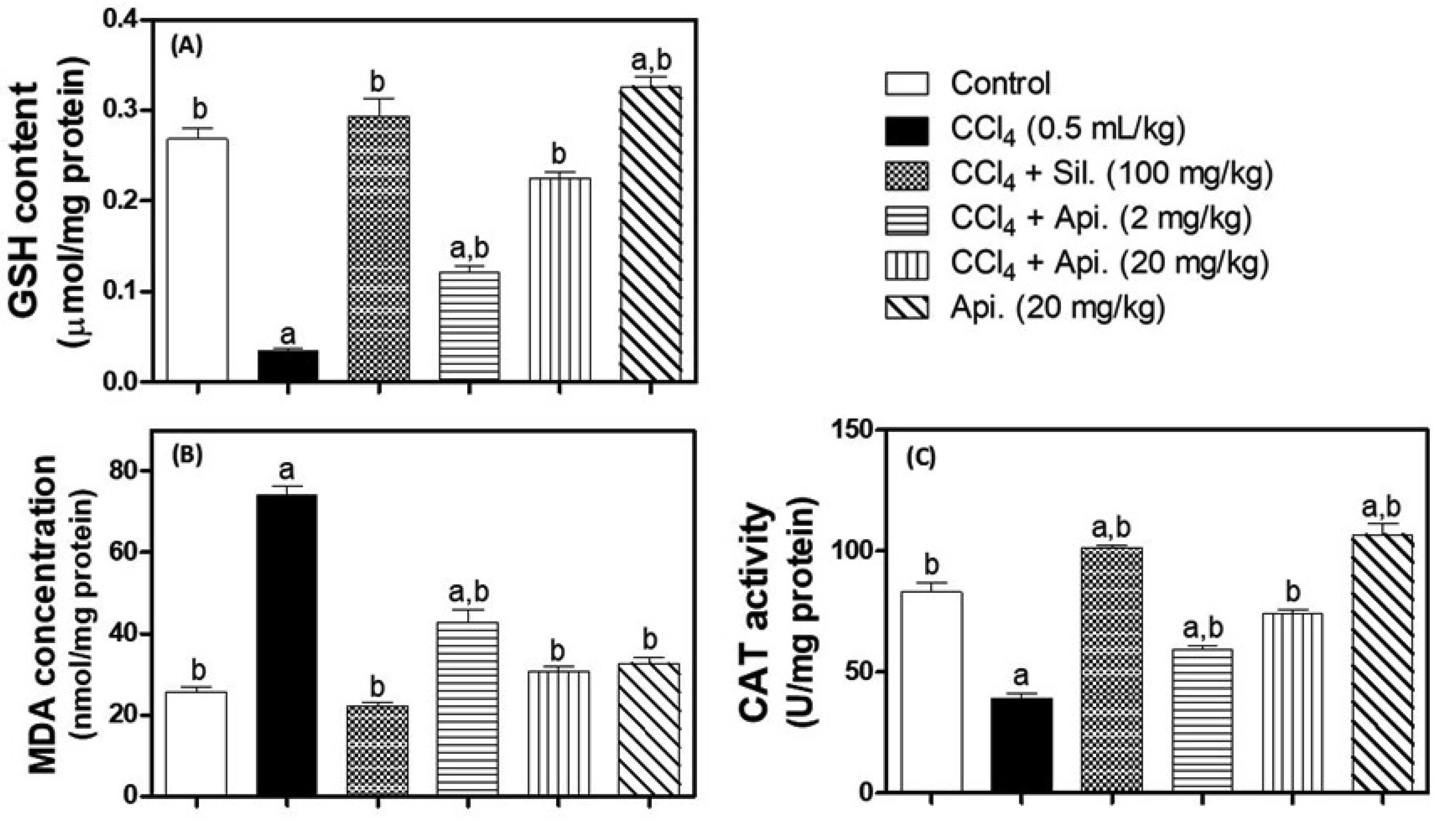
3.3. Evaluation of Oxidative Stress Biomarkers
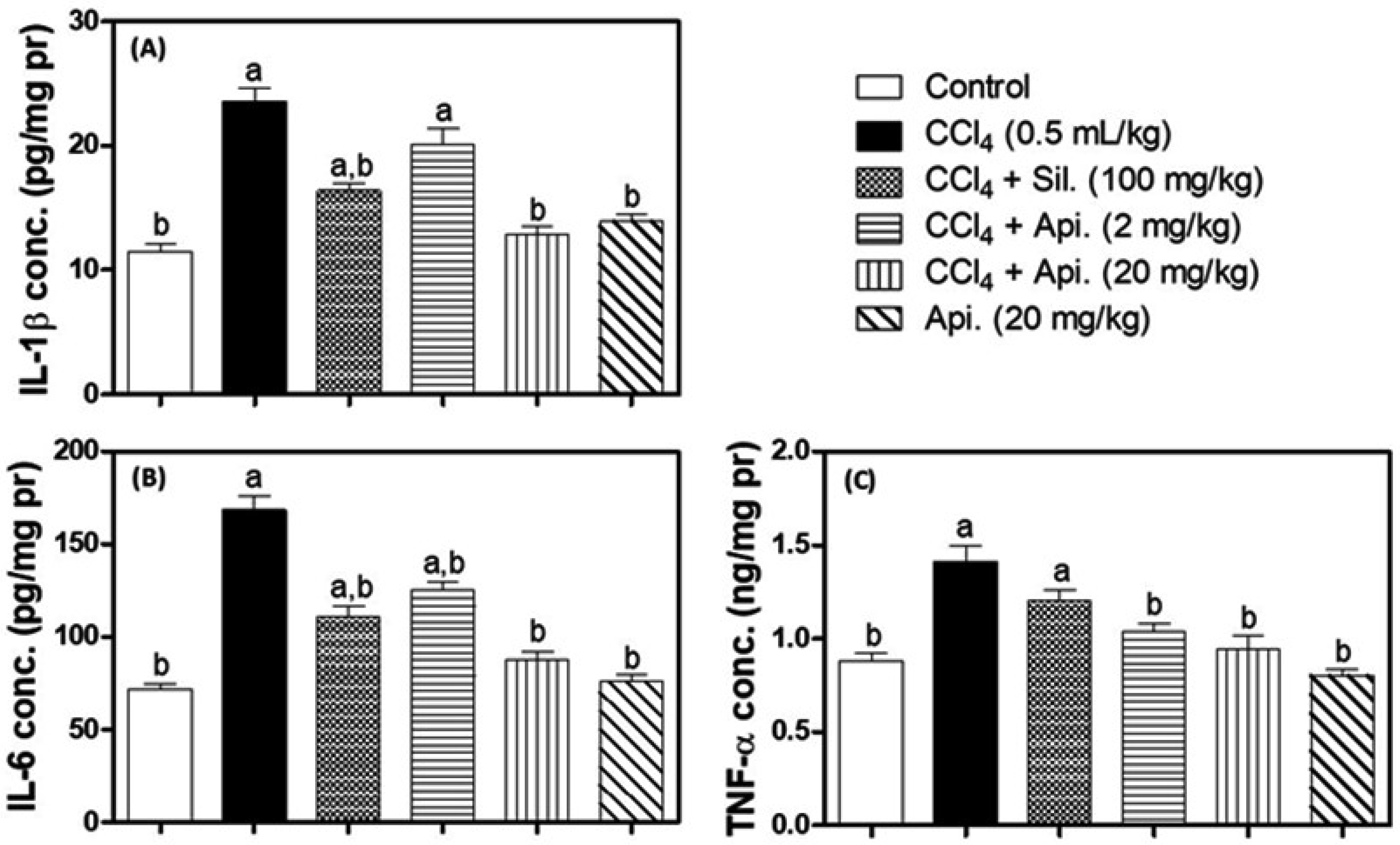
3.4. Assessment of Inflammatory Biomarkers
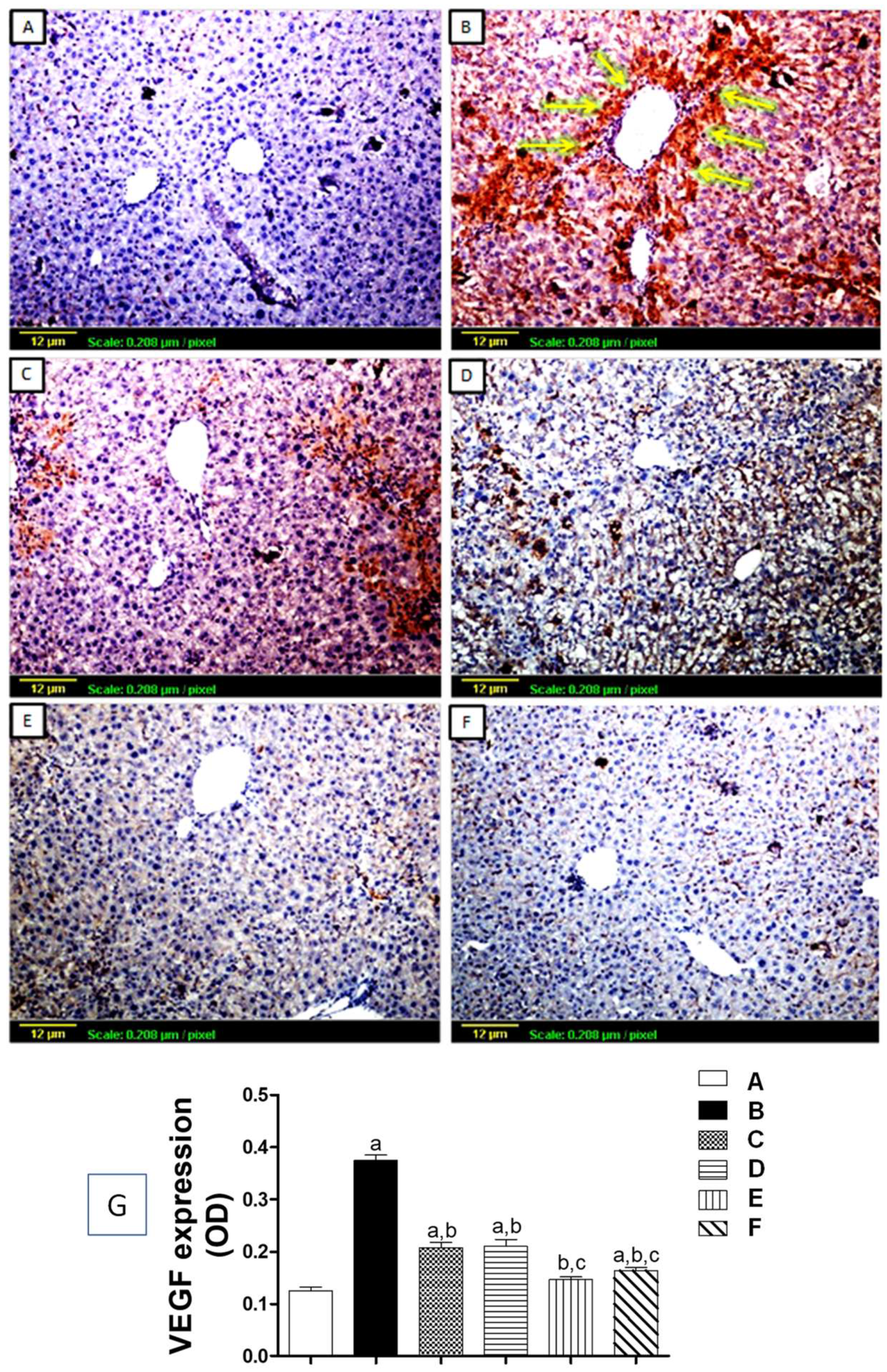
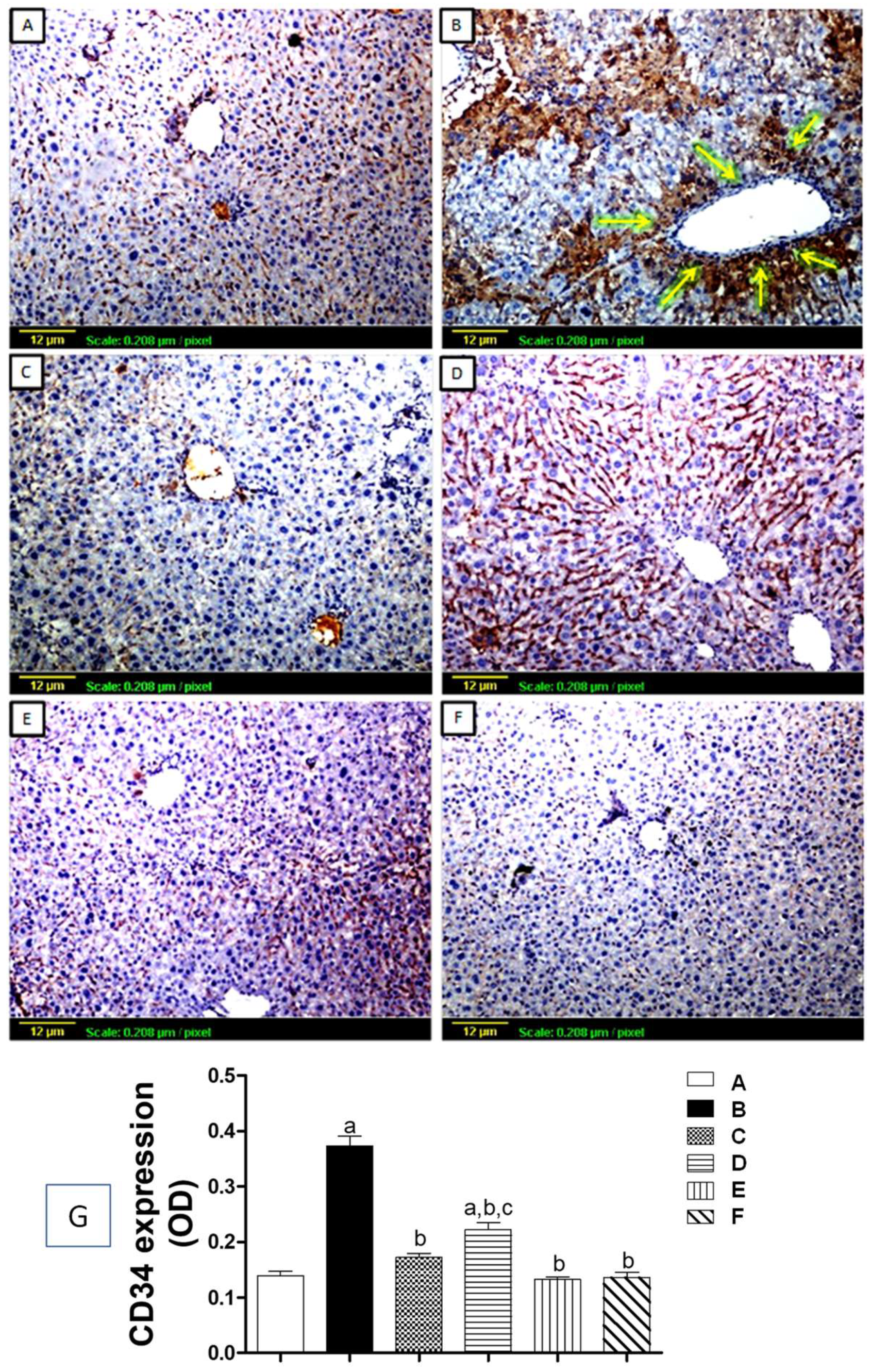
3.5. Immunohistochemical Assessment of Angiogenic Markers
3.5.1. Tissue Expression of VEGF
3.5.2. Tissue Expression of CD34
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinzani, M. Pathophysiology of liver fibrosis. Dig. Dis. 2015, 33, 492–497. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A. Recent advancement of molecular mechanisms of liver fibrosis. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 512–518. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Poulsen, K.L.; Wu, L.; Liu, S.; Miyata, T.; Song, Q.; Wei, Q.; Zhao, C.; Lin, C.; Yang, J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target Ther. 2022, 7, 287. [Google Scholar] [CrossRef]
- Ramadori, G.; Moriconi, F.; Malik, I.; Dudas, J. Physiology and pathophysiology of liver inflammation, damage and repair. J. Physiol. Pharmacol. 2008, 59, 107–117. [Google Scholar]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 145. [Google Scholar]
- Li, W.Q.; Liu, W.H.; Qian, D.; Liu, J.; Zhou, S.Q.; Zhang, L.; Peng, W.; Su, L.; Zhang, H. Traditional Chinese medicine: An important source for discovering candidate agents against hepatic fibrosis. Front. Pharmacol. 2022, 13, 962525. [Google Scholar] [CrossRef]
- Sun, M.; Kisseleva, T. Reversibility of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S60–S63. [Google Scholar] [CrossRef] [PubMed]
- Atta, H.M. Reversibility and heritability of liver fibrosis: Implications for research and therapy. World J. Gastroenterol. 2015, 21, 5138. [Google Scholar] [CrossRef]
- Novo, E.; Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair 2008, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Batusic, D.S.; von Bargen, A.; Blaschke, S.; Dudas, J.; Ramadori, G. Different physiology of interferon-α/-γ in models of liver regeneration in the rat. Histochem. Cell Biol. 2011, 136, 131–144. [Google Scholar] [CrossRef]
- Song, K.; Kwon, H.; Han, C.; Chen, W.; Zhang, J.; Ma, W.; Dash, S.; Gandhi, C.R.; Wu, T. Yes-Associated Protein in Kupffer Cells Enhances the Production of Proinflammatory Cytokines and Promotes the Development of Nonalcoholic Steatohepatitis. Hepatology 2020, 72, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Rosmorduc, O.; Housset, C. Hypoxia: A link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin. Liver Dis. 2010, 30, 258–270. [Google Scholar] [CrossRef]
- Nath, B.; Szabo, G. Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases. Hepatology 2012, 55, 622–633. [Google Scholar] [CrossRef]
- Zadorozhna, M.; Di Gioia, S.; Conese, M.; Mangieri, D. Neovascularization is a key feature of liver fibrosis progression: Anti-angiogenesis as an innovative way of liver fibrosis treatment. Mol. Biol. Rep. 2020, 47, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, F.; Lu, Y.; Zheng, S. Update on implications and mechanisms of angiogenesis in liver fibrosis. Hepatol. Res. 2015, 45, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Foglia, B.; Novo, E.; Protopapa, F.; Maggiora, M.; Bocca, C.; Cannito, S.; Parola, M. Hypoxia, Hypoxia-Inducible Factors and Liver Fibrosis. Cells 2021, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Novo, E.; Miglietta, A.; Parola, M. Angiogenesis and Fibrogenesis in Chronic Liver Diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 477–488. [Google Scholar]
- Hiraganahalli, B.D.; Chinampudur, V.C.; Dethe, S.; Mundkinajeddu, D.; Pandre, M.K.; Balachandran, J.; Agarwal, A. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharm. Mag. 2012, 8, 116–123. [Google Scholar]
- Latief, U.; Ahmad, R. Herbal remedies for liver fibrosis: A review on the mode of action of fifty herbs. J. Tradit. Complement. Med. 2018, 8, 352–360. [Google Scholar] [PubMed]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, J.; Jeong, N.Y.; Chung, H.J. The natural plant flavonoid apigenin is a strong antioxidant that effectively delays peripheral neurodegenerative processes. Anat. Sci. Int. 2019, 94, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahn, M.-R. Apigenin Suppresses Angiogenesis by Inhibiting Tube Formation and Inducing Apoptosis. Nat. Prod. Commun. 2016, 11, 1934578X1601101005. [Google Scholar]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Venigalla, M.; Gyengesi, E.; Münch, G. Curcumin and Apigenin—Novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015, 10, 1181–1185. [Google Scholar] [PubMed]
- Park, S.; Kim, J.W.; Kim, J.H.; Lim, C.W.; Kim, B. Differential Roles of Angiogenesis in the Induction of Fibrogenesis and the Resolution of Fibrosis in Liver. Biol. Pharm. Bull. 2015, 38, 980–985. [Google Scholar] [CrossRef]
- Scholten, D.; Trebicka, J.; Liedtke, C.; Weiskirchen, R. The carbon tetrachloride model in mice. Lab Anim. 2015, 49, 4–11. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, S.K.; Noel, S.; Sharma, S.; Rath, S.K. Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS ONE 2012, 7, e31964. [Google Scholar] [CrossRef]
- Shan, L.; Liu, Z.; Ci, L.; Shuai, C.; Lv, X.; Li, J. Research progress on the anti-hepatic fibrosis action and mechanism of natural products. Int. Immunopharmacol. 2019, 75, 105765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Oh, D.S.; Oh, J.Y.; Son, T.G.; Yuk, D.Y.; Jung, Y.S. Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response. Molecules 2016, 21, 443. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J. Comprehensive investigation of hydroxypropyl methylcellulose, propylene glycol, polysorbate 80, and hydroxypropyl-beta-cyclodextrin for use in general toxicology studies. Toxicol. Sci. 2010, 117, 485–492. [Google Scholar]
- Gad, S.C.; Spainhour, C.B.; Shoemake, C.; Pallman, D.R.; Stricker-Krongrad, A.; Downing, P.A.; Seals, R.E.; Eagle, L.A.; Polhamus, K.; Daly, J. Tolerable Levels of Nonclinical Vehicles and Formulations Used in Studies by Multiple Routes in Multiple Species with Notes on Methods to Improve Utility. Int. J. Toxicol. 2016, 35, 95–178. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Amir, M.; Khan, M.A.; Ahmad, S.; Akhtar, M.; Mujeeb, M.; Ahmad, A.; Khan, S.A.; Al-Abbasi, F.A. Ameliorating effects of Tamarindus indica fruit extract on anti-tubercular drugs induced liver toxicity in rats. Nat. Prod. Res. 2016, 30, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Buchwalow, I.B.; Böcker, W. Immunohistochemistry: Basics and Methods; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Ravichandra, A.; Schwabe, R.F. Mouse Models of Liver Fibrosis. Methods Mol Biol. 2021, 2299, 339–356. [Google Scholar] [PubMed]
- Sayed, E.A.; Badr, G.; Hassan, K.A.; Waly, H.; Ozdemir, B.; Mahmoud, M.H.; Alamery, S. Induction of liver fibrosis by CCl4 mediates pathological alterations in the spleen and lymph nodes: The potential therapeutic role of propolis. Saudi J. Biol. Sci. 2021, 28, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Cicek, M.; Sabancilar, İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev. Environ. Health 2020, 36, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Ali, F.; Rahul Naz, F.; Jyoti, S.; Siddique, Y.H. Protective effect of apigenin against N-nitrosodiethylamine (NDEA)-induced hepatotoxicity in albino rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 767, 13–20. [Google Scholar] [CrossRef]
- Zhou, R.J.; Ye, H.; Wang, F.; Wang, J.L.; Xie, M.L. Apigenin inhibits d-galactosamine/LPS-induced liver injury through upregulation of hepatic Nrf-2 and PPARγ expressions in mice. Biochem. Biophys. Res. Commun. 2017, 493, 625–630. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Phar-macother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Luangmonkong, T.; Suriguga, S.; Mutsaers, H.A.M.; Groothuis, G.M.M.; Olinga, P.; Boersema, M. Targeting Oxidative Stress for the Treatment of Liver Fibrosis. Rev. Physiol. Biochem. Pharmacol. 2018, 175, 71–102. [Google Scholar]
- Goudarzi, M.; Kalantar, M.; Sadeghi, E.; Karamallah, M.H.; Kalantar, H. Protective effects of apigenin on altered lipid peroxidation, inflammation, and antioxidant factors in methotrexate-induced hepatotoxicity. Naunyn-Schmiedeberg’s Arch Pharm. 2021, 394, 523–531. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Teng, Y.; Yao, C.; Li, X.; Cao, M.; Li, X.; Pan, G.; Lu, K.; Galons, H.; Yu, P. Antioxidant properties of flavonoid derivatives and their hepatoprotective effects on CCl4 induced acute liver injury in mice. RSC Adv. 2018, 8, 15366–15371. [Google Scholar] [CrossRef]
- Zou, L.; Chen, S.; Li, L.; Wu, T. The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp. Toxicol. Pathol. 2017, 69, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, R.; Xu, S.; Li, Y.; Qin, Y.; Wu, Q.; Xiao, Z. Xiaochaihutang attenuates liver fibrosis by activation of Nrf2 pathway in rats. Biomed. Pharmacother. 2017, 96, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Wang, X.M.; Xu, D.Y.; Sang, L.X.; Han, Y.; Jiang, L.Y. Morin enhances hepatic Nrf2 expression in a liver fibrosis rat model. World J. Gastroenterol. 2017, 23, 8334–8344. [Google Scholar] [CrossRef]
- Robinson, M.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- McQuitty, C.E.; Williams, R.; Chokshi, S.; Urbani, L. Immunomodulatory Role of the Extracellular Matrix Within the Liver Disease Microenvironment. Front. Immunol. 2020, 11, 574276. [Google Scholar] [CrossRef] [PubMed]
- Suou, K.; Taniguchi, F.; Tagashira, Y.; Kiyama, T.; Terakawa, N.; Harada, T. Apigenin inhibits tumor necrosis factor α-induced cell proliferation and prostaglandin E2 synthesis by inactivating NFκB in endometriotic stromal cells. Fertil. Steril. 2011, 95, 1518–1521. [Google Scholar] [CrossRef]
- Said, M.M.; Azab, S.S.; Saeed, N.M.; El-Demerdash, E. Antifibrotic Mechanism of Pinocembrin: Impact on Oxidative Stress, Inflammation and TGF-β /Smad Inhibition in Rats. Ann. Hepatol. 2018, 17, 307–317. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Mo, W.; Feng, J.; Li, S.; Li, J.; Liu, T.; Xu, S.; Wang, W.; Lu, X.; et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci. Rep. 2017, 7, 9289. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Yao, Z.; Wang, L.; Zhang, F.; Shao, J.; Chen, A.; Zheng, S. Activation of autophagy is required for Oroxylin A to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. Int. Immunopharmacol. 2018, 56, 148–155. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, A.; Baig, M.W.; Ullah, N.; Ahmed, N.; Tipu, M.K.; Ali, H.; Khane, S. Poncirin attenuates CCL4-induced liver injury through inhibition of oxidative stress and inflammatory cytokines in mice. BMC Complement Med. Ther. 2020, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Kukla, M. Angiogenesis: A phenomenon which aggravates chronic liver disease progression. Hepatol. Int. 2013, 7, 4–12. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, M.Q.; Liu, Z.M.; Xu, M.; Huang, Z.H.; Liu, H.J.; Gao, Y.; Zhou, W.J. A strategy of vascular-targeted therapy for liver fibrosis. Hepatology 2022, 76, 660–675. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, W.; Chen, M.; Huang, L.; Li, S.; Li, Z.; Pan, Q.; Lv, S.; Yang, X.; Wang, Y.; et al. Apigenin suppresses tumor angiogenesis and growth via inhibiting. Chem. Biol. Interact. 2022, 361, 109966. [Google Scholar] [CrossRef] [PubMed]
- Enciso, N.; Amiel, J.; Fabián-Domínguez, F.; Pando, J.; Rojas, N.; Cisneros-Huamaní, C.; Nava, E.; Enciso, J. Model of Liver Fibrosis Induction by Thioacetamide in Rats for Regenerative Therapy Studies. Anal. Cell Pathol. 2022, 2022, 2841894. [Google Scholar] [CrossRef]

| Groups | Treatments | ALT (U/L) | AST (U/L) | TC (mg/dL) | TG (mg/dL) | TB (mg/dL) |
|---|---|---|---|---|---|---|
| Group-1 | Normal Control | 19.83 ± 2.76 | 33.92 ± 5.67 | 82.17 ± 7.64 | 97.27 ± 5.93 | 0.31 ± 0.04 |
| Group-2 | CCl4 Control | 120.67 a ± 8.94 | 148.08 a ± 9.85 | 159.2 a ± 10.02 | 204.1 a ± 12.48 | 1.72 a ± 0.14 |
| Group-3 | CCl4 + Silymarin (100 mg/kg) | 35.16 a,b ± 5.49 | 56.32 a,b ± 5.76 | 92.55 b ± 7.50 | 120.72 a,b ± 6.5 | 0.59 a,b ± 0.08 |
| Group-4 | CCl4 + Apigenin (2 mg/Kg) | 86.03 a,b,c ± 9.62 | 97.98 a,b,c ± 6.25 | 158.15 a,c ± 7.62 | 202.4 a,c ± 10.2 | 1.06 a,b,c ± 0.09 |
| Group-5 | CCl4 + Apigenin (20 mg/Kg) | 46.15 a,b ± 4.97 | 73.55 a,b,c ± 5.28 | 111.6 a,b,c ± 8.65 | 157.8 a,b,c ± 7.68 | 0.76 a,b,c ± 0.10 |
| Group-6 | Apigenin Alone (20 mg/Kg) | 21.30 b,c ± 3.92 | 32.50 b,c ± 6.63 | 80.25 b ± 4.04 | 98.42 b,c ± 8.35 | 0.31 b,c ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melaibari, M.; Alkreathy, H.M.; Esmat, A.; Rajeh, N.A.; Shaik, R.A.; Alghamdi, A.A.; Ahmad, A. Anti-Fibrotic Efficacy of Apigenin in a Mice Model of Carbon Tetrachloride-Induced Hepatic Fibrosis by Modulation of Oxidative Stress, Inflammation, and Fibrogenesis: A Preclinical Study. Biomedicines 2023, 11, 1342. https://doi.org/10.3390/biomedicines11051342
Melaibari M, Alkreathy HM, Esmat A, Rajeh NA, Shaik RA, Alghamdi AA, Ahmad A. Anti-Fibrotic Efficacy of Apigenin in a Mice Model of Carbon Tetrachloride-Induced Hepatic Fibrosis by Modulation of Oxidative Stress, Inflammation, and Fibrogenesis: A Preclinical Study. Biomedicines. 2023; 11(5):1342. https://doi.org/10.3390/biomedicines11051342
Chicago/Turabian StyleMelaibari, Maryam, Huda M. Alkreathy, Ahmed Esmat, Nisreen A. Rajeh, Rasheed A. Shaik, Anwar A. Alghamdi, and Aftab Ahmad. 2023. "Anti-Fibrotic Efficacy of Apigenin in a Mice Model of Carbon Tetrachloride-Induced Hepatic Fibrosis by Modulation of Oxidative Stress, Inflammation, and Fibrogenesis: A Preclinical Study" Biomedicines 11, no. 5: 1342. https://doi.org/10.3390/biomedicines11051342
APA StyleMelaibari, M., Alkreathy, H. M., Esmat, A., Rajeh, N. A., Shaik, R. A., Alghamdi, A. A., & Ahmad, A. (2023). Anti-Fibrotic Efficacy of Apigenin in a Mice Model of Carbon Tetrachloride-Induced Hepatic Fibrosis by Modulation of Oxidative Stress, Inflammation, and Fibrogenesis: A Preclinical Study. Biomedicines, 11(5), 1342. https://doi.org/10.3390/biomedicines11051342






