Epidural Injection Method for Long-Term Pain Management in Rats with Spinal Stenosis
Abstract
1. Introduction
2. Materials and Methods
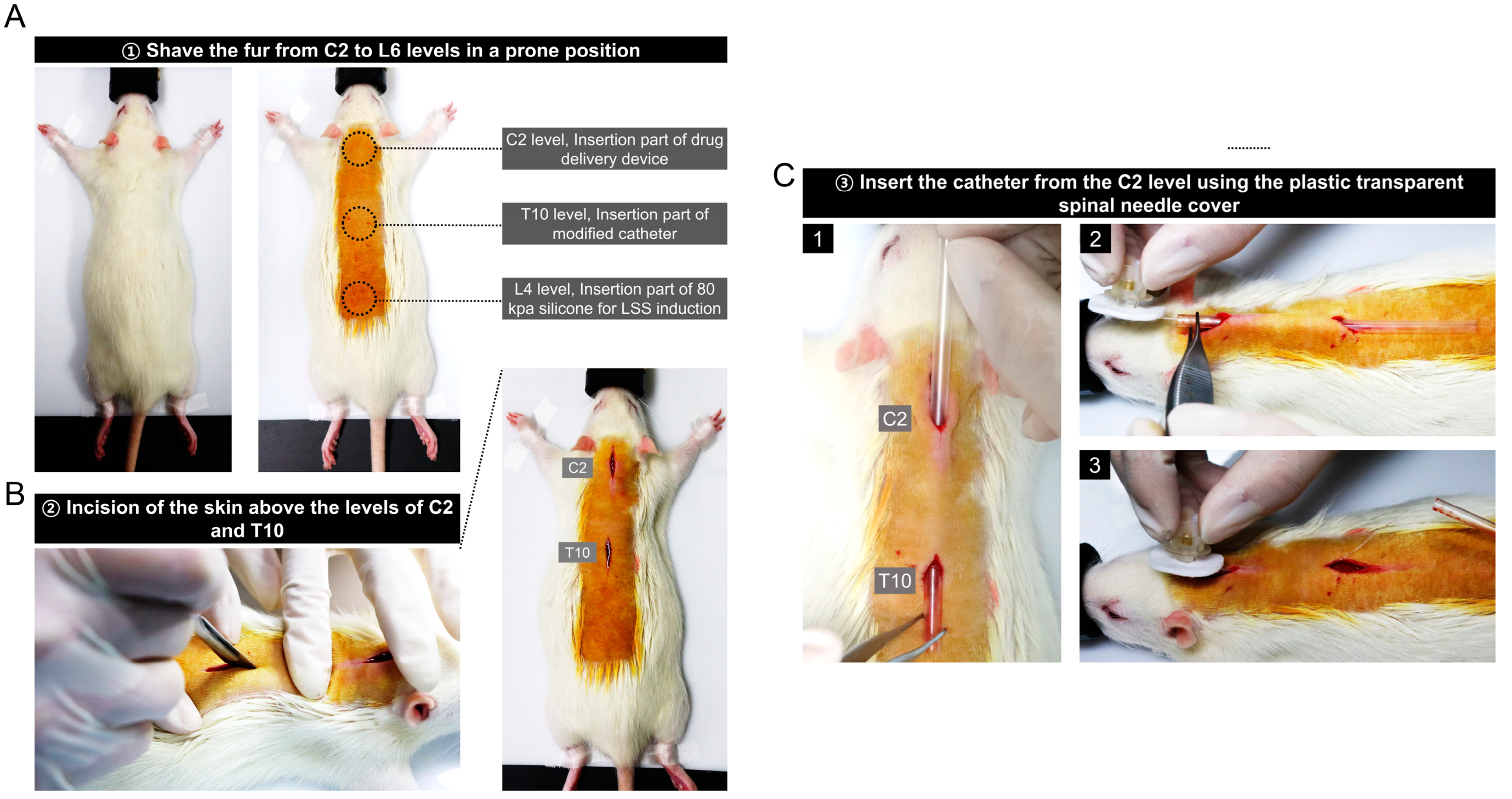
2.1. Rat Spinal Stenosis Model
2.2. Intrathecal Catheter with Drug Delivery System
2.3. Histological Analyses
2.4. Confirmation for Epidural Catheterization
2.5. Locomotor Function Assays
2.6. Statistical Analyses
3. Results
3.1. Preparation for Epidural Catheter Insertion and Stenosis Induction in Rats
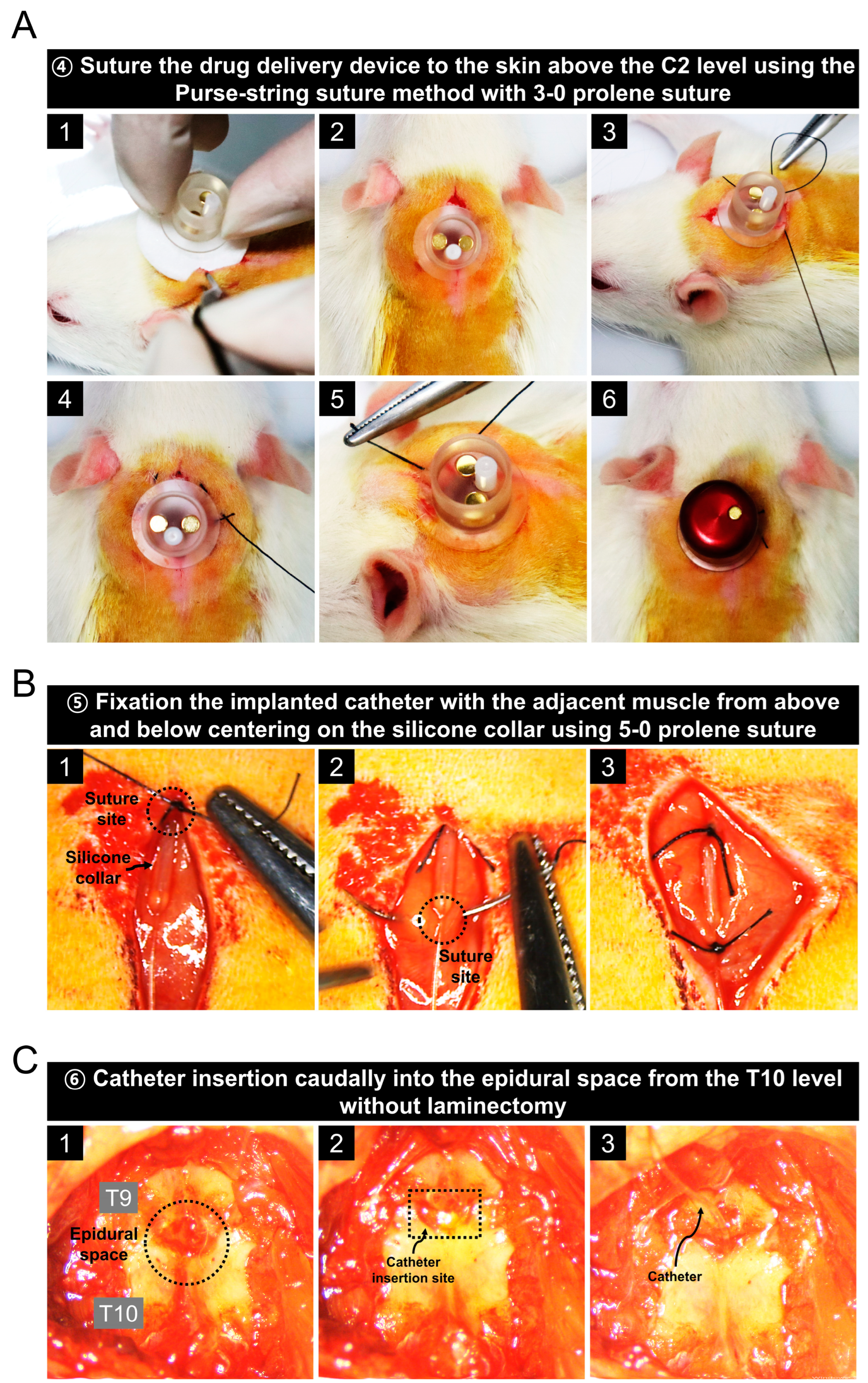
3.2. Implantation of a Drug Delivery Device into the C2 Level of Rats
3.3. Catheter Placement in the Epidural Space Starting at the T10 Level
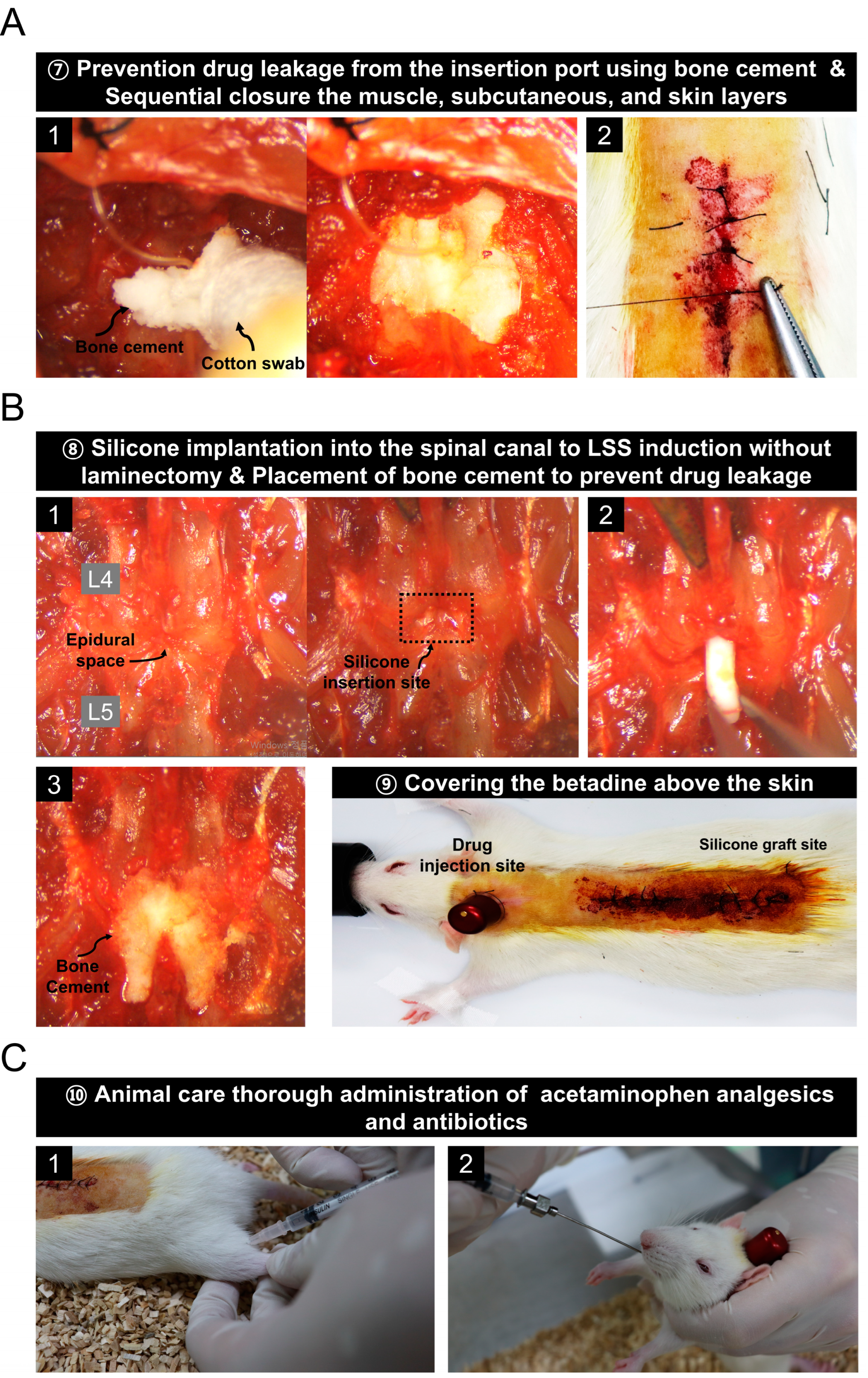
3.4. Prevention of Drug Leakage Using Bone Cement and Animal Care Procedures after Surgery
3.5. Evaluation of Epidural Catheter Placement by Evans Blue, Lidocaine Injection, Immunohistochemistry, and Functional Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carassiti, M.; Pascarella, G.; Strumia, A.; Russo, F.; Papalia, G.F.; Cataldo, R.; Gargano, F.; Costa, F.; Pierri, M.; De Tommasi, F.; et al. Epidural Steroid Injections for Low Back Pain: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Guo, J.B.; Zhang, H.W.; Zhang, Y.J.; Zhu, Y.; Zhang, J.; Hu, H.Y.; Zheng, Y.L.; Wang, X.Q. Surgical versus non-operative treatment for lumbar disc herniation: A systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Buttermann, G.R. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004, 4, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, M.; Rundell, S.D.; Gold, L.S.; Suri, P.; Friedly, J.L.; Nedeljkovic, S.S.; Deyo, R.A.; Turner, J.A.; Bresnahan, B.W.; Avins, A.L.; et al. Long-term effectiveness of epidural steroid injections after new episodes of low back pain in older adults. Eur. J. Pain 2022, 26, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. The risks of epidural and transforaminal steroid injections in the Spine: Commentary and a comprehensive review of the literature. Surg. Neurol. Int. 2013, 4, S74–S93. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Benet, L.Z. Effect of route of administration and distribution on drug action. J. Pharm. Biopharm. 1978, 6, 559–585. [Google Scholar] [CrossRef]
- Bicker, J.; Alves, G.; Falcao, A.; Fortuna, A. Timing in drug absorption and disposition: The past, present, and future of chronopharmacokinetics. Br. J. Pharm. 2020, 177, 2215–2239. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, Y.C.; Lim, Y.J.; Lee, C.J.; Lee, P.B.; Lee, S.C.; Sim, W.S.; Choi, Y.L. The neurological safety of epidural gabapentin in rats: A light microscopic examination. Anesth. Analg. 2005, 101, 1422–1426. [Google Scholar] [CrossRef]
- Suto, T.; Obata, H.; Tobe, M.; Oku, H.; Yokoo, H.; Nakazato, Y.; Saito, S. Long-term effect of epidural injection with sustained-release lidocaine particles in a rat model of postoperative pain. Br. J. Anaesth. 2012, 109, 957–967. [Google Scholar] [CrossRef]
- Yosefifard, M.; Hassanpour-Ezatti, M. Epidural administration of neostigmine-loaded nanofibers provides extended analgesia in rats. Daru 2014, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Lee, S.H.; Lee, S.J. Percutaneous transforaminal epidural injection method in an experimental rat: Minimally invasive drug delivery method to spinal epidural space. Ann. Rehabil. Med. 2012, 36, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ju, J.; Choi, E.; Nahm, F.S.; Choe, G.Y.; Lee, P.B. Effect of epidural polydeoxyribonucleotide in a rat model of lumbar foraminal stenosis. Korean J. Pain 2021, 34, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Lee, D.K.; Huh, B.K.; Choi, S.S.; Kim, H.Z.; Lim, B.G.; Kim, H.S.; Choi, Y.S.; Hur, W.S.; Lee, M.K. The effect of clonidine pretreatment on epidural resiniferatoxin in a neuropathic pain rat model. Acta Med. Okayama 2015, 69, 95–103. [Google Scholar] [CrossRef]
- Nahm, F.S.; Lee, P.B.; Choe, G.Y.; Lim, Y.J.; Kim, Y.C. Therapeutic effect of epidural hyaluronic acid in a rat model of foraminal stenosis. J. Pain Res. 2017, 10, 241–248. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Schnell, L.; Gullo, M.; Kaiser, J.; Schneider, M.P.; Mosberger, A.C.; Good, N.; Linnebank, M.; Schwab, M.E. Direct, long-term intrathecal application of therapeutics to the rodent CNS. Nat. Protoc. 2017, 12, 104–131. [Google Scholar] [CrossRef]
- Herrlich, S.; Spieth, S.; Messner, S.; Zengerle, R. Osmotic micropumps for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 1617–1627. [Google Scholar] [CrossRef]
- Lecanu, L.; Papadopoulos, V. Modeling Alzheimer’s disease with non-transgenic rat models. Alzheimers Res. 2013, 5, 17. [Google Scholar] [CrossRef]
- Ni, X.P.; Humphreys, M.H. Prevention of salt-induced hypertension by an analog of gamma-melanocyte-stimulating hormone in the rat. Am. J. Hypertens. 2007, 20, 862–865. [Google Scholar] [CrossRef][Green Version]
- Almoshari, Y. Osmotic Pump Drug Delivery Systems-A Comprehensive Review. Pharmaceuticals 2022, 15, 1430. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.Y.; Jeon, W.J.; Lee, J.; Ha, I.H. Evaluation of the effects of differences in silicone hardness on rat model of lumbar spinal stenosis. PLoS ONE 2021, 16, e0251464. [Google Scholar] [CrossRef] [PubMed]
- Abdi, S.; Datta, S.; Trescot, A.M.; Schultz, D.M.; Adlaka, R.; Atluri, S.L.; Smith, H.S.; Manchikanti, L. Epidural steroids in the management of chronic spinal pain: A systematic review. Pain Physician 2007, 10, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Balog, B.M.; Deng, K.; Askew, T.; Hanzlicek, B.; Kuang, M.; Damaser, M.S. Brain-Derived Neurotrophic Factor Is Indispensable to Continence Recovery after a Dual Nerve and Muscle Childbirth Injury Model. Int. J. Mol. Sci. 2023, 24, 4998. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.Y.; Kim, H.; Lee, J.; Jeon, W.-J.; Yeo, C.; Kim, H.; Lee, Y.J.; Ha, I.-H. Epidural Injection Method for Long-Term Pain Management in Rats with Spinal Stenosis. Biomedicines 2023, 11, 1390. https://doi.org/10.3390/biomedicines11051390
Hong JY, Kim H, Lee J, Jeon W-J, Yeo C, Kim H, Lee YJ, Ha I-H. Epidural Injection Method for Long-Term Pain Management in Rats with Spinal Stenosis. Biomedicines. 2023; 11(5):1390. https://doi.org/10.3390/biomedicines11051390
Chicago/Turabian StyleHong, Jin Young, Hyunseong Kim, Junseon Lee, Wan-Jin Jeon, Changhwan Yeo, Hyun Kim, Yoon Jae Lee, and In-Hyuk Ha. 2023. "Epidural Injection Method for Long-Term Pain Management in Rats with Spinal Stenosis" Biomedicines 11, no. 5: 1390. https://doi.org/10.3390/biomedicines11051390
APA StyleHong, J. Y., Kim, H., Lee, J., Jeon, W.-J., Yeo, C., Kim, H., Lee, Y. J., & Ha, I.-H. (2023). Epidural Injection Method for Long-Term Pain Management in Rats with Spinal Stenosis. Biomedicines, 11(5), 1390. https://doi.org/10.3390/biomedicines11051390





