Molecular Pathogenesis of Human Immunodeficiency Virus-Associated Disease of Oropharyngeal Mucosal Epithelium
Abstract
:1. Introduction
2. Role of Oropharyngeal Mucosal Epithelium in HIV-1 Transmission in Adults and Children
3. Manifestations of HIV/AIDS in the Oropharyngeal Mucosal Epithelium
3.1. HIV/AIDS Reactivates Opportunistic Infections of the Oral Mucosa
3.1.1. Synergistic Contributions of HIV-1 and EBV to the Nonmalignant Oral Lesion, Hairy Leukoplakia
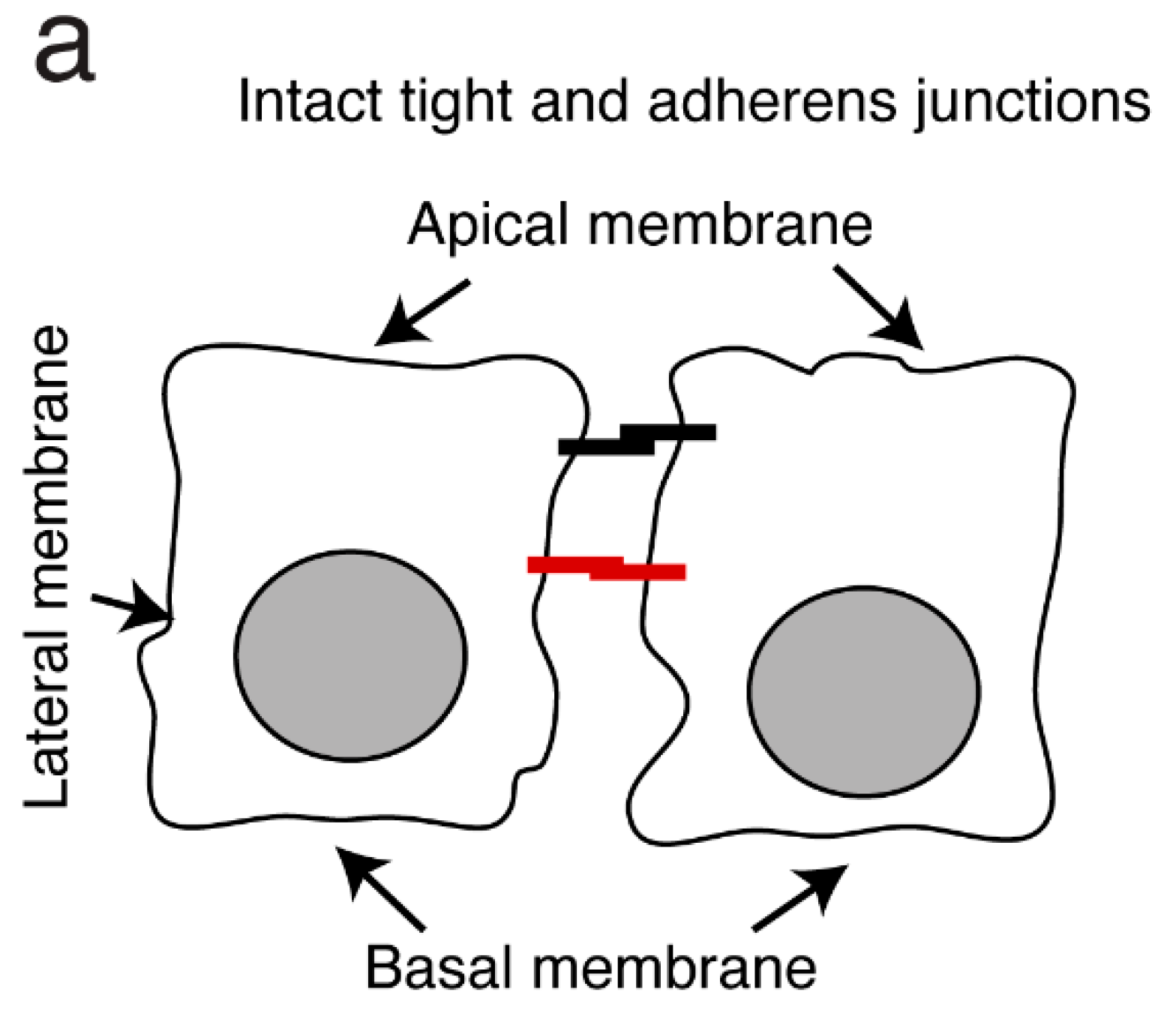
3.1.2. HIV-Induced Disruption of Tight and Adherens Junctions of Oral Epithelial Cells Facilitates the Spread of HSV-1
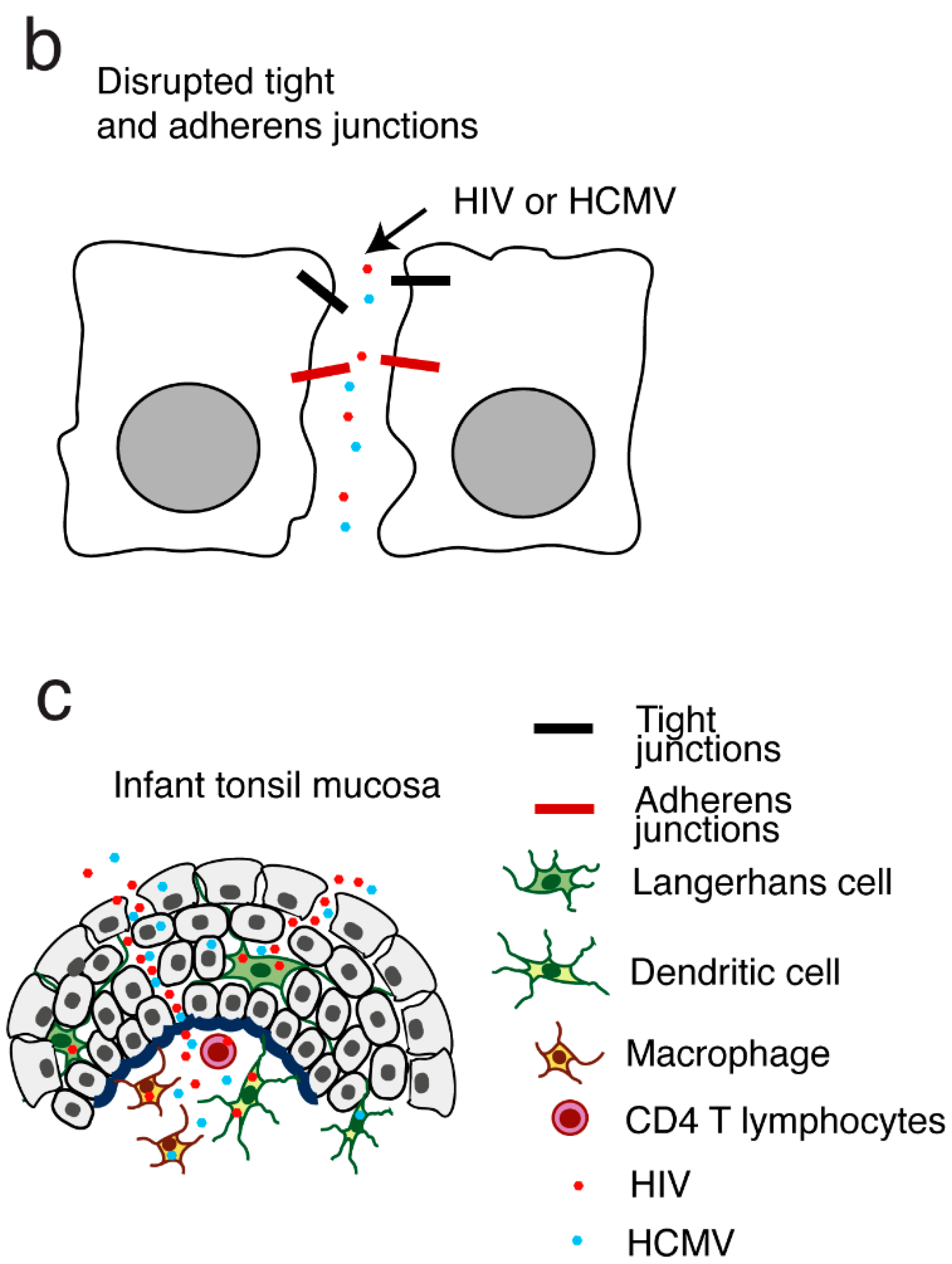
3.1.3. HIV-1 and HCMV Mother-to-Child Transmission (MTCT) through the Infant tonsillar Epithelium Synergistically Promote the Spread of Both HIV-1 and HCMV
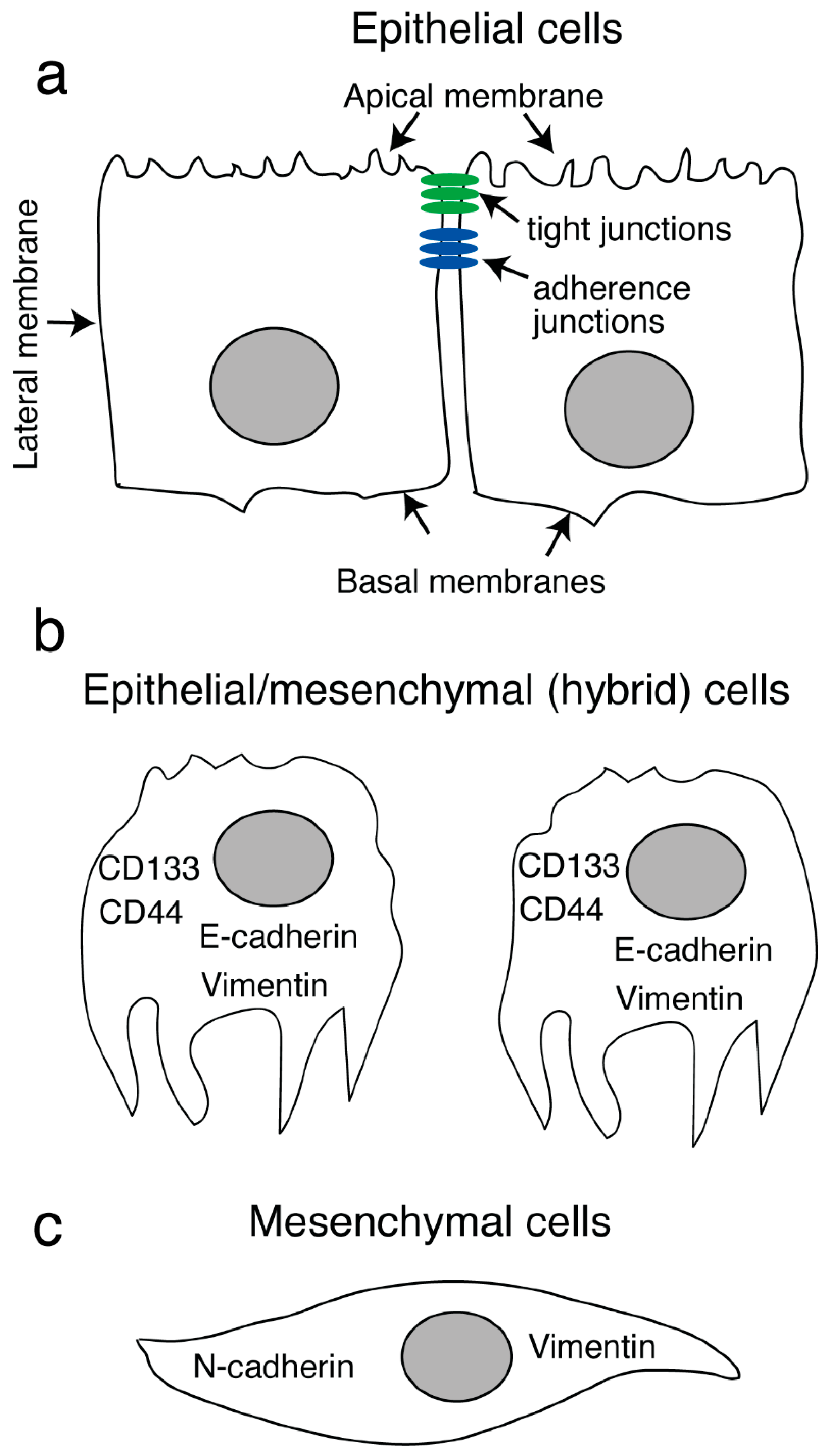
4. HIV-1 Proteins gp120 and Tat Promote Invasiveness of Both Human Papillomavirus (HPV)-Positive and HPV-Negative Neoplastic Oral and Genital Epithelial Cells
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winning, T.A.; Townsend, G.C. Oral mucosal embryology and histology. Clin. Dermatol. 2000, 18, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Soros, V.; Greene, W.C.; Levy, J.A.; Palefsky, J.M. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J. Virol. 2012, 86, 2556–2570. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S. Human immunodeficiency virus-associated disruption of mucosal barriers and its role in HIV transmission and pathogenesis of HIV/AIDS disease. Tissue Barriers 2016, 4, e1159276. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S. Virus-associated disruption of mucosal epithelial tight junctions and its role in viral transmission and spread. Tissue Barriers 2021, 9, 1943274. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M.; Herrera, R.; Chin-Hong, P.; Veluppillai, P.; Greenspan, D.; Michael Berry, J.; Pilcher, C.D.; Shiboski, C.H.; Jay, N.; Rubin, M.; et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 2013, 446, 378–388. [Google Scholar] [CrossRef]
- Jotwani, R.; Palucka, A.K.; Al-Quotub, M.; Nouri-Shirazi, M.; Kim, J.; Bell, D.; Banchereau, J.; Cutler, C.W. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: In situ, in vivo, and in vitro studies. J. Immunol. 2001, 167, 4693–4700. [Google Scholar] [CrossRef]
- Nave, H.; Gebert, A.; Pabst, R. Morphology and immunology of the human palatine tonsil. Anat. Embryol. 2001, 204, 367–373. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Savage, N.W.; Sugerman, P.B.; Walsh, L.J. Mast cell/T cell interactions in oral lichen planus. J. Oral. Pathol. Med. 2002, 31, 189–195. [Google Scholar] [CrossRef]
- Allam, J.P.; Novak, N.; Fuchs, C.; Asen, S.; Berge, S.; Appel, T.; Geiger, E.; Kochan, J.P.; Bieber, T. Characterization of dendritic cells from human oral mucosa: A new Langerhans′ cell type with high constitutive FcepsilonRI expression. J. Allergy Clin. Immunol. 2003, 112, 141–148. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Paczesny, S.; Blanco, P.; Bennett, L.; Pascual, V.; Fay, J.; Palucka, A.K. Dendritic cells: Controllers of the immune system and a new promise for immunotherapy. Ann. N. Y. Acad. Sci. 2003, 987, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, S.J.; Sweet, S.P. Oral mucosal immunity and HIV infection: Current status. Oral. Dis. 2002, 8 (Suppl. S2), 55–62. [Google Scholar] [CrossRef]
- Caputo, V.; Libera, M.; Sisti, S.; Giuliani, B.; Diotti, R.A.; Criscuolo, E. The initial interplay between HIV and mucosal innate immunity. Front. Immunol. 2023, 14, 1104423. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, S.J.; Naglik, J.R. The effects of HIV infection on oral mucosal immunity. Adv. Dent. Res. 2006, 19, 29–35. [Google Scholar] [CrossRef]
- Pelaez-Prestel, H.F.; Sanchez-Trincado, J.L.; Lafuente, E.M.; Reche, P.A. Immune Tolerance in the Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 12149. [Google Scholar] [CrossRef]
- Lombardi, T.; Hauser, C.; Budtz-Jorgensen, E. Langerhans cells: Structure, function and role in oral pathological conditions. J. Oral. Pathol. Med. 1993, 22, 193–202. [Google Scholar] [CrossRef]
- Bilsborough, J.; Viney, J.L. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology 2004, 127, 300–309. [Google Scholar] [CrossRef]
- Hon, H.; Jacob, J. Tracking dendritic cells in vivo: Insights into DC biology and function. Immunol. Res. 2004, 29, 69–80. [Google Scholar] [CrossRef]
- Wilson, N.S.; Villadangos, J.A. Lymphoid organ dendritic cells: Beyond the Langerhans cells paradigm. Immunol. Cell Biol. 2004, 82, 91–98. [Google Scholar] [CrossRef]
- Walsh, L.J. Mast cells and oral inflammation. Crit. Rev. Oral Biol. Med. 2003, 14, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Parker, L.A.; Beacock-Sharp, H.; Millington, O.R.; Chirdo, F. Oral tolerance: Overview and historical perspectives. Ann. N. Y. Acad. Sci. 2004, 1029, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ozbilgin, M.K.; Polat, S.; Mete, U.O.; Tap, O.; Kaya, M. Antigen-presenting cells in the hypertrophic pharyngeal tonsils: A histochemical, immunuhistochemical and ultrastructural study. J. Investig. Allergol. Clin. Immunol. 2004, 14, 320–328. [Google Scholar] [PubMed]
- Foti, M.; Granucci, F.; Ricciardi-Castagnoli, P. A central role for tissue-resident dendritic cells in innate responses. Trends Immunol. 2004, 25, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Sarah, S.M.; Tamilselvan, S.; Kamatchiammal, S.; Suresh, R. Expression of Toll-like receptors 2 and 4 in gingivitis and chronic periodontitis. Indian J. Dent. Res. 2006, 17, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.J.; Lasiter, J.C.; Misfeldt, M.L. Toll-like receptors in tonsillar epithelial cells. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 613–621. [Google Scholar] [CrossRef]
- Palucka, A.K.; Banchereau, J. Langerhans cells: Daughters of monocytes. Nat. Immunol. 2006, 7, 223–224. [Google Scholar] [CrossRef]
- Wacleche, V.S.; Tremblay, C.L.; Routy, J.P.; Ancuta, P. The Biology of Monocytes and Dendritic Cells: Contribution to HIV Pathogenesis. Viruses 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Berman, J.W. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods 2003, 29, 351–361. [Google Scholar] [CrossRef]
- Muller, W.A. New mechanisms and pathways for monocyte recruitment. J. Exp. Med. 2001, 194, F47–F51. [Google Scholar] [CrossRef] [PubMed]
- Imhof, B.A.; Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004, 4, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Lundien, M.C.; Mohammed, K.A.; Nasreen, N.; Tepper, R.S.; Hardwick, J.A.; Sanders, K.L.; Van Horn, R.D.; Antony, V.B. Induction of MCP-1 expression in airway epithelial cells: Role of CCR2 receptor in airway epithelial injury. J. Clin. Immunol. 2002, 22, 144–152. [Google Scholar] [CrossRef]
- Nasreen, N.; Mohammed, K.A.; Galffy, G.; Ward, M.J.; Antony, V.B. MCP-1 in pleural injury: CCR2 mediates haptotaxis of pleural mesothelial cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 2000, 278, L591–L598. [Google Scholar] [CrossRef] [PubMed]
- Lugering, N.; Kucharzik, T.; Maaser, C.; Kraft, M.; Domschke, W. Interleukin-15 strongly inhibits interleukin-8 and monocyte chemoattractant protein-1 production in human colonic epithelial cells. Immunology 1999, 98, 504–509. [Google Scholar] [CrossRef]
- Kucharzik, T.; Lugering, N.; Pauels, H.G.; Domschke, W.; Stoll, R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin. Exp. Immunol. 1998, 111, 152–157. [Google Scholar] [CrossRef]
- Holtkamp, G.M.; De Vos, A.F.; Peek, R.; Kijlsta, A. Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells. Clin. Exp. Immunol. 1999, 118, 35–40. [Google Scholar] [CrossRef]
- Holtkamp, G.M.; Van Rossem, M.; de Vos, A.F.; Willekens, B.; Peek, R.; Kijlstra, A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin. Exp. Immunol. 1998, 112, 34–43. [Google Scholar] [CrossRef]
- Rimoldi, M.; Chieppa, M.; Vulcano, M.; Allavena, P.; Rescigno, M. Intestinal epithelial cells control dendritic cell function. Ann. N. Y. Acad. Sci. 2004, 1029, 66–74. [Google Scholar] [CrossRef]
- Takano, K.; Kojima, T.; Go, M.; Murata, M.; Ichimiya, S.; Himi, T.; Sawada, N. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J. Histochem. Cytochem. 2005, 53, 611–619. [Google Scholar] [CrossRef]
- Zen, K.; Parkos, C.A. Leukocyte-epithelial interactions. Curr. Opin. Cell Biol. 2003, 15, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Edens, H.A.; Parkos, C.A. Modulation of epithelial and endothelial paracellular permeability by leukocytes. Adv. Drug. Deliv. Rev. 2000, 41, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Rotta, G.; Valzasina, B.; Ricciardi-Castagnoli, P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology 2001, 204, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.S.; Dayton, T.; Davis, C.; Hill, S.; Jackson, T.H.; Blaschuk, O.; Symonds, M.; Okayama, N.; Kevil, C.G.; Laroux, F.S.; et al. Activated T-lymphocytes express occludin, a component of tight junctions. Inflammation 1998, 22, 573–582. [Google Scholar] [CrossRef]
- Edelblum, K.L.; Shen, L.; Weber, C.R.; Marchiando, A.M.; Clay, B.S.; Wang, Y.; Prinz, I.; Malissen, B.; Sperling, A.I.; Turner, J.R. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc. Natl. Acad. Sci. USA 2012, 109, 7097–7102. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction With the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Lefrancois, L. Intraepithelial lymphocytes: To serve and protect. Curr. Gastroenterol. Rep. 2010, 12, 513–521. [Google Scholar] [CrossRef]
- Aguirre-Urizar, J.M.; Echebarria-Goicouria, M.A.; Eguia-del-Valle, A. Acquired immunodeficiency syndrome: Manifestations in the oral cavity. Med. Oral. Patol. Oral. Cir. Bucal. 2004, 9 (Suppl. S153–S157), 148–153. [Google Scholar]
- Leigh, J.E.; Shetty, K.; Fidel, P.L., Jr. Oral opportunistic infections in HIV-positive individuals: Review and role of mucosal immunity. AIDS Patient Care STDS 2004, 18, 443–456. [Google Scholar] [CrossRef]
- Hille, J.J.; Webster-Cyriaque, J.; Palefski, J.M.; Raab-Traub, N. Mechanisms of expression of HHV8, EBV and HPV in selected HIV-associated oral lesions. Oral. Dis. 2002, 8 (Suppl. S2), 161–168. [Google Scholar] [CrossRef]
- Syrjanen, S.; Leimola-Virtanen, R.; Schmidt-Westhausen, A.; Reichart, P.A. Oral ulcers in AIDS patients frequently associated with cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections. J. Oral. Pathol. Med. 1999, 28, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Reichart, P.A. Oral ulcerations in HIV infection. Oral. Dis. 1997, 3 (Suppl. S1), S180–S182. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, D.; Greenspan, J.S. Oral manifestations of HIV infection. AIDS Clin. Care 1997, 9, 29–33. [Google Scholar] [PubMed]
- Challacombe, S. Revised classification of HIV--associated oral lesions. Br. Dent. J. 1991, 170, 305–306. [Google Scholar] [CrossRef]
- Lomeli-Martinez, S.M.; Gonzalez-Hernandez, L.A.; Ruiz-Anaya, A.J.; Lomeli-Martinez, M.A.; Martinez-Salazar, S.Y.; Mercado Gonzalez, A.E.; Andrade-Villanueva, J.F.; Varela-Hernandez, J.J. Oral Manifestations Associated with HIV/AIDS Patients. Medicina 2022, 58, 1214. [Google Scholar] [CrossRef]
- Rodriguez-Inigo, E.; Jimenez, E.; Bartolome, J.; Ortiz-Movilla, N.; Bartolome Villar, B.; Jose Arrieta, J.; Manzarbeitia, F.; Carreno, V. Detection of human immunodeficiency virus type 1 RNA by in situ hybridization in oral mucosa epithelial cells from anti-HIV-1 positive patients. J. Med. Virol. 2005, 77, 17–22. [Google Scholar] [CrossRef]
- Chou, L.L.; Epstein, J.; Cassol, S.A.; West, D.M.; He, W.; Firth, J.D. Oral mucosal Langerhans′ cells as target, effector and vector in HIV infection. J. Oral Pathol. Med. 2000, 29, 394–402. [Google Scholar] [CrossRef]
- Goto, Y.; Yeh, C.K.; Notkins, A.L.; Prabhakar, B.S. Detection of proviral sequences in saliva of patients infected with human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 1991, 7, 343–347. [Google Scholar] [CrossRef]
- Kakizawa, J.; Ushijima, H.; Oka, S.; Ikeda, Y.; Schroder, H.C.; Muller, W.E. Detection of human immunodeficiency virus-1 DNA, RNA and antibody, and occult blood in inactivated saliva: Availability of the filter paper disk method. Acta. Paediatr. Jpn. 1996, 38, 218–223. [Google Scholar] [CrossRef]
- Liuzzi, G.; Chirianni, A.; Clementi, M.; Bagnarelli, P.; Valenza, A.; Cataldo, P.T.; Piazza, M. Analysis of HIV-1 load in blood, semen and saliva: Evidence for different viral compartments in a cross-sectional and longitudinal study. Aids 1996, 10, F51–F56. [Google Scholar] [CrossRef]
- Maticic, M.; Poljak, M.; Kramar, B.; Tomazic, J.; Vidmar, L.; Zakotnik, B.; Skaleric, U. Proviral HIV-1 DNA in gingival crevicular fluid of HIV-1-infected patients in various stages of HIV disease. J. Dent. Res. 2000, 79, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.N.; Barr, C.E.; Hewlitt, I.; Boorstein, R.; Kong, F.; Bagasra, O.; Bobroski, L.E.; Joshi, B. Detection of HIV in oral mucosal cells. Oral. Dis. 1997, 3 (Suppl. S1), S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.N.; Barr, C.E.; Seshamma, T.; Reidy, J.; Pomerantz, R.J.; Bagasra, O. Infection of oral mucosal cells by human immunodeficiency virus type 1 in seropositive persons. J. Infect. Dis. 1995, 171, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, R.A.; Whittington, W.L.; Celum, C.L.; Collis, T.; Lucchetti, A.; Sanchez, J.L.; Hughes, J.P.; Coombs, R.W. Factors associated with oropharyngeal human immunodeficiency virus shedding. J. Infect. Dis. 2003, 188, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, P.; Berger, I.; Autschbach, F.; Weinstein, M.; Funke, B.; Verdin, E.; Goldsmith, M.A.; Keppler, O.T. Tissue-resident macrophages are productively infected ex vivo by primary X4 isolates of human immunodeficiency virus type 1. J. Virol. 2005, 79, 5220–5226. [Google Scholar] [CrossRef]
- del Romero, J.; Marincovich, B.; Castilla, J.; Garcia, S.; Campo, J.; Hernando, V.; Rodriguez, C. Evaluating the risk of HIV transmission through unprotected orogenital sex. Aids 2002, 16, 1296–1297. [Google Scholar] [CrossRef]
- Page-Shafer, K.; Shiboski, C.H.; Osmond, D.H.; Dilley, J.; McFarland, W.; Shiboski, S.C.; Klausner, J.D.; Balls, J.; Greenspan, D.; Greenspan, J.S. Risk of HIV infection attributable to oral sex among men who have sex with men and in the population of men who have sex with men. Aids 2002, 16, 2350–2352. [Google Scholar] [CrossRef]
- Tudor-Williams, G.; Lyall, E.G. Mother to infant transmission of HIV. Curr. Opin. Infect Dis. 1999, 12, 21–26. [Google Scholar] [CrossRef]
- Semba, R.D.; Kumwenda, N.; Hoover, D.R.; Taha, T.E.; Quinn, T.C.; Mtimavalye, L.; Biggar, R.J.; Broadhead, R.; Miotti, P.G.; Sokoll, L.J.; et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J. Infect. Dis. 1999, 180, 93–98. [Google Scholar] [CrossRef]
- Kuhn, L.; Kim, H.Y.; Walter, J.; Thea, D.M.; Sinkala, M.; Mwiya, M.; Kankasa, C.; Decker, D.; Aldrovandi, G.M. HIV-1 concentrations in human breast milk before and after weaning. Sci. Transl. Med. 2013, 5, 181ra151. [Google Scholar] [CrossRef]
- Satomi, M.; Shimizu, M.; Shinya, E.; Watari, E.; Owaki, A.; Hidaka, C.; Ichikawa, M.; Takeshita, T.; Takahashi, H. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J. Infect. Dis. 2005, 191, 174–181. [Google Scholar] [CrossRef]
- Lewis, P.; Nduati, R.; Kreiss, J.K.; John, G.C.; Richardson, B.A.; Mbori-Ngacha, D.; Ndinya-Achola, J.; Overbaugh, J. Cell-free human immunodeficiency virus type 1 in breast milk. J. Infect. Dis. 1998, 177, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Koulinska, I.N.; Villamor, E.; Chaplin, B.; Msamanga, G.; Fawzi, W.; Renjifo, B.; Essex, M. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J. Acquir. Immune. Defic. Syndr. 2006, 41, 93–99. [Google Scholar] [CrossRef] [PubMed]
- De Cock, K.M.; Fowler, M.G.; Mercier, E.; de Vincenzi, I.; Saba, J.; Hoff, E.; Alnwick, D.J.; Rogers, M.; Shaffer, N. Prevention of mother-to-child HIV transmission in resource-poor countries: Translating research into policy and practice. JAMA 2000, 283, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, K. Mother-to-child Transmission of HIV: A Global Perspective. Curr. Infect Dis. Rep. 2007, 9, 511–517. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Full Report—In Danger: UNAIDS Global AIDS Update 2022. Available online: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update (accessed on 13 March 2023).
- Bobardt, M.D.; Chatterji, U.; Selvarajah, S.; Van der Schueren, B.; David, G.; Kahn, B.; Gallay, P.A. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 2007, 81, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.L.; Asin, S.N.; Yeaman, G.R.; Wira, C.R. HIV-1 infection of the female reproductive tract. Curr. HIV/AIDS Rep. 2005, 2, 35–38. [Google Scholar] [CrossRef]
- Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Soros, V.; Greene, W.C.; Levy, J.A.; Palefsky, J.M. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 2011, 409, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Dwinell, M.B.; Eckmann, L.; Leopard, J.D.; Varki, N.M.; Kagnoff, M.F. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 1999, 117, 359–367. [Google Scholar] [CrossRef]
- Liu, X.; Zha, J.; Chen, H.; Nishitani, J.; Camargo, P.; Cole, S.W.; Zack, J.A. Human immunodeficiency virus type 1 infection and replication in normal human oral keratinocytes. J. Virol. 2003, 77, 3470–3476. [Google Scholar] [CrossRef]
- Herrera, R.; Morris, M.; Rosbe, K.; Feng, Z.; Weinberg, A.; Tugizov, S. Human beta-defensins 2 and -3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology 2016, 487, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles. Virology 2018, 515, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.B.; Maher, D.M.; Herzberg, M.C.; Southern, P.J. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithelium: Implications for HIV binding and primary oral infection. Virol. J. 2006, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Rosbe, K.; Tugizov, S.M. Inactivation of HIV-1 in Polarized Infant Tonsil Epithelial Cells by Human Beta-Defensins 2 and 3 Tagged with the Protein Transduction Domain of HIV-1 Tat. Viruses 2021, 13, 2043. [Google Scholar] [CrossRef] [PubMed]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLoS Pathog 2017, 13, e1006247. [Google Scholar] [CrossRef]
- Pope, M. Mucosal dendritic cells and immunodeficiency viruses. J. Infect. Dis. 1999, 179 (Suppl. S3), S427–S430. [Google Scholar] [CrossRef]
- Steinman, R.M.; Granelli-Piperno, A.; Pope, M.; Trumpfheller, C.; Ignatius, R.; Arrode, G.; Racz, P.; Tenner-Racz, K. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top Microbiol. Immunol. 2003, 276, 1–30. [Google Scholar]
- Turville, S.; Wilkinson, J.; Cameron, P.; Dable, J.; Cunningham, A.L. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 2003, 2, 2. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; van Kooyk, Y. DC-SIGN: A novel HIV receptor on DCs that mediates HIV-1 transmission. Curr. Top Microbiol. Immunol. 2003, 276, 31–54. [Google Scholar]
- Gurney, K.B.; Elliott, J.; Nassanian, H.; Song, C.; Soilleux, E.; McGowan, I.; Anton, P.A.; Lee, B. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J. Virol. 2005, 79, 5762–5773. [Google Scholar] [CrossRef]
- Arrighi, J.F.; Pion, M.; Garcia, E.; Escola, J.M.; van Kooyk, Y.; Geijtenbeek, T.B.; Piguet, V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 2004, 200, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Liu, Q.H.; Tomkowicz, B.; Yi, Y.; Freedman, B.D.; Collman, R.G. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J. Leukoc. Biol. 2003, 74, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Del Corno, M.; Liu, Q.H.; Schols, D.; de Clercq, E.; Gessani, S.; Freedman, B.D.; Collman, R.G. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood 2001, 98, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Dayanithi, G.; Yahi, N.; Baghdiguian, S.; Fantini, J. Intracellular calcium release induced by human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein in human intestinal epithelial cells: A putative mechanism for HIV-1 enteropathy. Cell Calcium 1995, 18, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sufiawati, I.; Herrera, R.; Mayer, W.; Cai, X.; Borkakoti, J.; Lin, V.; Rosbe, K.; Tugizov, S.M. Human Immunodeficiency Virus (HIV) and Human Cytomegalovirus (HCMV) Coinfection of Infant Tonsil Epithelium May Synergistically Promote both HIV-1 and HCMV Spread and Infection. J. Virol. 2021, 95, e0092121. [Google Scholar] [CrossRef]
- Sufiawati, I.; Tugizov, S.M. HIV-Associated Disruption of Tight and Adherens Junctions of Oral Epithelial Cells Facilitates HSV-1 Infection and Spread. PLoS ONE 2014, 9, e88803. [Google Scholar] [CrossRef]
- Sufiawati, I.; Tugizov, S.M. HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. J. Gen. Virol. 2018, 99, 937–947. [Google Scholar] [CrossRef]
- Vacharaksa, A.; Asrani, A.C.; Gebhard, K.H.; Fasching, C.E.; Giacaman, R.A.; Janoff, E.N.; Ross, K.F.; Herzberg, M.C. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology 2008, 5, 66. [Google Scholar] [CrossRef]
- Kohli, A.; Islam, A.; Moyes, D.L.; Murciano, C.; Shen, C.; Challacombe, S.J.; Naglik, J.R. Oral and vaginal epithelial cell lines bind and transfer cell-free infectious HIV-1 to permissive cells but are not productively infected. PLoS ONE 2014, 9, e98077. [Google Scholar] [CrossRef]
- Maher, D.M.; Zhang, Z.Q.; Schacker, T.W.; Southern, P.J. Ex vivo modeling of oral HIV transmission in human palatine tonsil. J. Histochem. Cytochem. 2005, 53, 631–642. [Google Scholar] [CrossRef]
- Maher, D.; Wu, X.; Schacker, T.; Larson, M.; Southern, P. A model system of oral HIV exposure, using human palatine tonsil, reveals extensive binding of HIV infectivity, with limited progression to primary infection. J. Infect. Dis. 2004, 190, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, A.E.; Kajava, A.V.; Steinert, P.M. Epithelial barrier function: Assembly and structural features of the cornified cell envelope. Bioessays 2002, 24, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Ericsson, M.; Krantz, E.; Selke, S.; Corey, L. Oral shedding of herpes simplex virus type 2. Sex. Transm. Infect. 2004, 80, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Roizman, B.; Kenkins, F.J.; Kristie, T.M. Herpesviruses: Biology, gene regulation, latency, and genetic engineering. In The Molecular Basis of Viral Replication; Bercoff, R.P., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1987; pp. 517–546. [Google Scholar]
- Muller, W.J.; Jones, C.A.; Koelle, D.M. Immunobiology of herpes simplex virus and cytomegalovirus infections of the fetus and newborn. Curr. Immunol. Rev. 2010, 6, 38–55. [Google Scholar] [CrossRef]
- Gianella, S.; Massanella, M.; Wertheim, J.O.; Smith, D.M. The Sordid Affair Between Human Herpesvirus and HIV. J. Infect. Dis. 2015, 212, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Berger, J.R.; Mootoor, Y.; Avdiushko, S.A.; Zhu, H.; Kryscio, R.J. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J. Clin. Microbiol. 2006, 44, 2409–2415. [Google Scholar] [CrossRef]
- Carvalho, K.S.; Silvestre Ede, A.; Maciel Sda, S.; Lira, H.I.; Galvao, R.A.; Soares, M.J.; Costa, C.H.; Malaquias, L.C.; Coelho, L.F. PCR detection of multiple human herpesvirus DNA in saliva from HIV-infected individuals in Teresina, State of Piaui, Brazil. Rev. Soc. Bras. Med. Trop. 2010, 43, 620–623. [Google Scholar] [CrossRef]
- Gantt, S.; Carlsson, J.; Shetty, A.K.; Seidel, K.D.; Qin, X.; Mutsvangwa, J.; Musingwini, G.; Woelk, G.; Zijenah, L.S.; Katzenstein, D.A.; et al. Cytomegalovirus and Epstein-Barr virus in breast milk are associated with HIV-1 shedding but not with mastitis. AIDS 2008, 22, 1453–1460. [Google Scholar] [CrossRef]
- Munawwar, A.; Singh, S. Human Herpesviruses as Copathogens of HIV Infection, Their Role in HIV Transmission, and Disease Progression. J. Lab. Physicians. 2016, 8, 5–18. [Google Scholar] [CrossRef]
- Jiang, R.; Scott, R.S.; Hutt-Fletcher, L.M. Epstein-Barr virus shed in saliva is high in B-cell-tropic glycoprotein gp42. J. Virol. 2006, 80, 7281–7283. [Google Scholar] [CrossRef] [PubMed]
- Kieff, E.; Rickinson, A. Epstein-Barr virus and its replication. In Fields Virology, 5th ed.; Lippincott Williams and Willkins: New York, NY, USA, 2007; pp. 2602–2654. [Google Scholar]
- Rickinson, A.B.; Kieff, E. Epstein-Barr Virus. In Fields Virology; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Lippincott-Williams and Wilkins: Philadelphia, PA, USA, 2001; Volume 2, pp. 2575–2627. [Google Scholar]
- Daud, I.I.; Coleman, C.B.; Smith, N.A.; Ogolla, S.; Simbiri, K.; Bukusi, E.A.; Ng′ang′a, Z.W.; Sumba, P.O.; Vulule, J.; Ploutz-Snyder, R.; et al. Breast Milk as a Potential Source of Epstein-Barr Virus Transmission Among Infants Living in a Malaria-Endemic Region of Kenya. J. Infect. Dis. 2015, 212, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, L.; Gantt, S.; Mayer, B.T.; Krantz, E.M.; Orem, J.; Wald, A.; Corey, L.; Schiffer, J.T.; Casper, C. Virus and host-specific differences in oral human herpesvirus shedding kinetics among Ugandan women and children. Sci. Rep. 2017, 7, 13105. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, R.; Manji, K.; Matee, M.; Naburi, H.; Bisimba, J.; Martinez, R.; Wieland-Alter, W.; Kim, F.; von Reyn, C.F.; Palumbo, P. HSV oropharyngeal shedding among HIV-infected children in Tanzania. Int. J. STD AIDS 2015, 26, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Slyker, J.A. Cytomegalovirus and paediatric HIV infection. J. Virus Erad. 2016, 2, 208–214. [Google Scholar] [CrossRef]
- Greenspan, D.; Greenspan, J.S.; Conant, M.; Petersen, V.; Silverman, S., Jr.; de Souza, Y. Oral "hairy" leucoplakia in male homosexuals: Evidence of association with both papillomavirus and a herpes-group virus. Lancet 1984, 2, 831–834. [Google Scholar] [CrossRef]
- Greenspan, J.S.; Greenspan, D.; Lennette, E.T.; Abrams, D.I.; Conant, M.A.; Petersen, V.; Freese, U.K. Replication of Epstein-Barr virus within the epithelial cells of oral "hairy" leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 1985, 313, 1564–1571. [Google Scholar] [CrossRef]
- Greenspan, D.; Greenspan, J.S.; Hearst, N.G.; Pan, L.Z.; Conant, M.A.; Abrams, D.I.; Hollander, H.; Levy, J.A. Relation of oral hairy leukoplakia to infection with the human immunodeficiency virus and the risk of developing AIDS. J. Infect. Dis. 1987, 155, 475–481. [Google Scholar] [CrossRef]
- Walling, D.M.; Flaitz, C.M.; Hosein, F.G.; Montes-Walters, M.; Nichols, C.M. Effect of Epstein-Barr virus replication on Langerhans cells in pathogenesis of oral hairy leukoplakia. J. Infect. Dis. 2004, 189, 1656–1663. [Google Scholar] [CrossRef]
- Walling, D.M.; Flaitz, C.M.; Nichols, C.M. Epstein-Barr virus replication in oral hairy leukoplakia: Response, persistence, and resistance to treatment with valacyclovir. J. Infect. Dis. 2003, 188, 883–890. [Google Scholar] [CrossRef]
- Daniels, T.E.; Greenspan, D.; Greenspan, J.S.; Lennette, E.; Schiodt, M.; Petersen, V.; de Souza, Y. Absence of Langerhans cells in oral hairy leukoplakia, an AIDS-associated lesion. J. Investig. Dermatol. 1987, 89, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.; Herrera, R.; Veluppillai, P.; Greenspan, J.; Greenspan, D.; Palefsky, J.M. Epstein-Barr Virus (EBV)-Infected Monocytes Facilitate Dissemination of EBV within the Oral Mucosal Epithelium. J. Virol. 2007, 81, 5484–5496. [Google Scholar] [CrossRef] [PubMed]
- Walling, D.M.; Ray, A.J.; Nichols, J.E.; Flaitz, C.M.; Nichols, C.M. Epstein-barr virus infection of langerhans cell precursors as a mechanism of oral epithelial entry, persistence, and reactivation. J. Virol. 2007, 81, 7249–7268. [Google Scholar] [CrossRef] [PubMed]
- Sonza, S.; Mutimer, H.P.; Oelrichs, R.; Jardine, D.; Harvey, K.; Dunne, A.; Purcell, D.F.; Birch, C.; Crowe, S.M. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. Aids 2001, 15, 17–22. [Google Scholar] [CrossRef]
- Zhu, T.; Muthui, D.; Holte, S.; Nickle, D.; Feng, F.; Brodie, S.; Hwangbo, Y.; Mullins, J.I.; Corey, L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 2002, 76, 707–716. [Google Scholar] [CrossRef]
- Collman, R.G.; Perno, C.F.; Crowe, S.M.; Stevenson, M.; Montaner, L.J. HIV and cells of macrophage/dendritic lineage and other non-T cell reservoirs: New answers yield new questions. J. Leukoc. Biol. 2003, 74, 631–634. [Google Scholar] [CrossRef]
- Crowe, S.; Zhu, T.; Muller, W.A. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J. Leukoc. Biol. 2003, 74, 635–641. [Google Scholar] [CrossRef]
- Smith, P.D.; Meng, G.; Salazar-Gonzalez, J.F.; Shaw, G.M. Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J. Leukoc. Biol. 2003, 74, 642–649. [Google Scholar] [CrossRef]
- Savard, M.; Belanger, C.; Tardif, M.; Gourde, P.; Flamand, L.; Gosselin, J. Infection of primary human monocytes by Epstein-Barr virus. J. Virol. 2000, 74, 2612–2619. [Google Scholar] [CrossRef]
- Knol, A.C.; Quereux, G.; Pandolfino, M.C.; Khammari, A.; Dreno, B. Presence of Epstein-Barr virus in Langerhans cells of CTCL lesions. J. Investig. Dermatol. 2005, 124, 280–282. [Google Scholar] [CrossRef]
- Sonza, S.; Crowe, S.M. Reservoirs for HIV infection and their persistence in the face of undetectable viral load. AIDS Patient Care STDS 2001, 15, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Shimakage, M.; Kimura, M.; Yanoma, S.; Ibe, M.; Yokota, S.; Tsujino, G.; Kozuka, T.; Dezawa, T.; Tamura, S.; Ohshima, A.; et al. Expression of latent and replicative-infection genes of Epstein-Barr virus in macrophage. Arch. Virol. 1999, 144, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rossen, R.D.; Smith, C.W.; Laughter, A.H.; Noonan, C.A.; Anderson, D.C.; McShan, W.M.; Hurvitz, M.Y.; Orson, F.M. HIV-1-stimulated expression of CD11/CD18 integrins and ICAM-1: A possible mechanism for extravascular dissemination of HIV-1-infected cells. Trans. Assoc. Am. Physicians. 1989, 102, 117–130. [Google Scholar] [PubMed]
- Weeks, B.S.; Klotman, M.E.; Dhawan, S.; Kibbey, M.; Rappaport, J.; Kleinman, H.K.; Yamada, K.M.; Klotman, P.E. HIV-1 infection of human T lymphocytes results in enhanced alpha 5 beta 1 integrin expression. J. Cell Biol. 1991, 114, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.; Zeroli, C.; Martegani, R.; Pugliese, A.; Basilico, C.; Speranza, F. Levels of the bcl-2 protein, fibronectin and alpha(5)beta(1) fibronectin receptor in HIV-1-infected patients with Kaposi′s sarcoma. Microb. Infect. 2000, 2, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.; Ferrario, G.; Issi, M.; Pugliese, A.; Speranza, F. Expression of the alpha 5 beta 1 fibronectin receptor on T lymphocytes of patients with HIV-1 infection. J. Clin. Pathol. 1996, 49, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Lafrenie, R.M.; Lee, S.F.; Hewlett, I.K.; Yamada, K.M.; Dhawan, S. Involvement of integrin alphavbeta3 in the pathogenesis of human immunodeficiency virus type 1 infection in monocytes. Virology 2002, 297, 31–38. [Google Scholar] [CrossRef]
- Tugizov, S.M.; Berline, J.W.; Palefsky, J.M. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 2003, 9, 307–314. [Google Scholar] [CrossRef]
- Xiao, J.; Palefsky, J.M.; Herrera, R.; Berline, J.; Tugizov, S.M. The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology 2008, 370, 430–442. [Google Scholar] [CrossRef]
- Guerreiro-Cacais, A.O.; Li, L.; Donati, D.; Bejarano, M.T.; Morgan, A.; Masucci, M.G.; Hutt-Fletcher, L.; Levitsky, V. Capacity of Epstein-Barr virus to infect monocytes and inhibit their development into dendritic cells is affected by the cell type supporting virus replication. J. Gen. Virol. 2004, 85, 2767–2778. [Google Scholar] [CrossRef]
- Dhawan, S.; Puri, R.K.; Kumar, A.; Duplan, H.; Masson, J.M.; Aggarwal, B.B. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood 1997, 90, 1535–1544. [Google Scholar] [PubMed]
- Matzen, K.; Dirkx, A.E.; oude Egbrink, M.G.; Speth, C.; Gotte, M.; Ascherl, G.; Grimm, T.; Griffioen, A.W.; Sturzl, M. HIV-1 Tat increases the adhesion of monocytes and T-cells to the endothelium in vitro and in vivo: Implications for AIDS-associated vasculopathy. Virus Res. 2004, 104, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lafrenie, R.M.; Wahl, L.M.; Epstein, J.S.; Hewlett, I.K.; Yamada, K.M.; Dhawan, S. HIV-1-Tat modulates the function of monocytes and alters their interactions with microvessel endothelial cells. A mechanism of HIV pathogenesis. J. Immunol. 1996, 156, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Palefsky, J.M.; Herrera, R.; Berline, J.; Tugizov, S.M. EBV BMRF-2 facilitates cell-to-cell spread of virus within polarized oral epithelial cells. Virology 2009, 388, 335–343. [Google Scholar] [CrossRef]
- Walston, J.J.; Hayman, I.R.; Gore, M.; Ferguson, M.; Temple, R.M.; Liao, J.; Alam, S.; Meyers, C.; Tugizov, S.M.; Hutt-Fletcher, L.; et al. The Epstein-Barr Virus Glycoprotein BDLF2 Is Essential for Efficient Viral Spread in Stratified Epithelium. J. Virol. 2023, 97, e0152822. [Google Scholar] [CrossRef]
- Dawson, C.W.; Eliopoulos, A.G.; Dawson, J.; Young, L.S. BHRF1, a viral homologue of the Bcl-2 oncogene, disturbs epithelial cell differentiation. Oncogene 1995, 10, 69–77. [Google Scholar] [PubMed]
- Fantuzzi, L.; Belardelli, F.; Gessani, S. Monocyte/macrophage-derived CC chemokines and their modulation by HIV-1 and cytokines: A complex network of interactions influencing viral replication and AIDS pathogenesis. J. Leukoc. Biol. 2003, 74, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, L.; Conti, L.; Gauzzi, M.C.; Eid, P.; Del Corno, M.; Varano, B.; Canini, I.; Belardelli, F.; Gessani, S. Regulation of chemokine/cytokine network during in vitro differentiation and HIV-1 infection of human monocytes: Possible importance in the pathogenesis of AIDS. J. Leukoc. Biol. 2000, 68, 391–399. [Google Scholar] [CrossRef]
- Muthumani, K.; Hwang, D.S.; Choo, A.Y.; Mayilvahanan, S.; Dayes, N.S.; Thieu, K.P.; Weiner, D.B. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int. Immunol. 2005, 17, 103–116. [Google Scholar] [CrossRef]
- Fantuzzi, L.; Purificato, C.; Donato, K.; Belardelli, F.; Gessani, S. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: A novel mechanism for AIDS pathogenesis. J. Virol. 2004, 78, 9763–9772. [Google Scholar] [CrossRef]
- Conti, L.; Fantuzzi, L.; Del Corno, M.; Belardelli, F.; Gessani, S. Immunomodulatory effects of the HIV-1 gp120 protein on antigen presenting cells: Implications for AIDS pathogenesis. Immunobiology 2004, 209, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, R.; Pimpinelli, N.; Ficarra, G.; Borgognoni, L.; Gaglioti, D.; Milo, D.; Romagnoli, P. Morphology and membrane antigens of nonlymphoid accessory cells in oral hairy leukoplakia. Hum. Pathol. 1990, 21, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, D.; Canchola, A.J.; MacPhail, L.A.; Cheikh, B.; Greenspan, J.S. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet 2001, 357, 1411–1412. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, J.S.; Greenspan, D.; Webster-Cyriaque, J. Hairy leukoplakia; lessons learned: 30-plus years. Oral. Dis. 2016, 22 (Suppl. S1), 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Chen, H.; Secours, R.; Lee, A.; Webster-Cyriaque, J.; Ghannoum, M.; Evans, S.; Bernard, D.; Reznik, D.; Dittmer, D.P.; et al. High Accuracy of Common HIV-Related Oral Disease Diagnoses by Non-Oral Health Specialists in the AIDS Clinical Trial Group. PLoS ONE 2015, 10, e0131001. [Google Scholar] [CrossRef]
- Posavad, C.M.; Wald, A.; Kuntz, S.; Huang, M.L.; Selke, S.; Krantz, E.; Corey, L. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J. Infect. Dis. 2004, 190, 693–696. [Google Scholar] [CrossRef]
- Tan, D.H.; Raboud, J.M.; Kaul, R.; Walmsley, S.L. Antiretroviral therapy is not associated with reduced herpes simplex virus shedding in HIV coinfected adults: An observational cohort study. BMJ Open 2014, 4, e004210. [Google Scholar] [CrossRef]
- Nazli, A.; Chan, O.; Dobson-Belaire, W.N.; Ouellet, M.; Tremblay, M.J.; Gray-Owen, S.D.; Arsenault, A.L.; Kaushic, C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010, 6, e1000852. [Google Scholar] [CrossRef]
- Nazli, A.; Kafka, J.K.; Ferreira, V.H.; Anipindi, V.; Mueller, K.; Osborne, B.J.; Dizzell, S.; Chauvin, S.; Mian, M.F.; Ouellet, M.; et al. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J. Immunol. 2013, 191, 4246–4258. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [PubMed]
- Krummenacher, C.; Nicola, A.V.; Whitbeck, J.C.; Lou, H.; Hou, W.; Lambris, J.D.; Geraghty, R.J.; Spear, P.G.; Cohen, G.H.; Eisenberg, R.J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 1998, 72, 7064–7074. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Spear, P.G. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J. Virol. 2002, 76, 7203–7208. [Google Scholar] [CrossRef] [PubMed]
- Grinde, B. Herpesviruses: Latency and reactivation—Viral strategies and host response. J. Oral. Microbiol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- WHO. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Vrazo, A.C.; Sullivan, D.; Ryan Phelps, B. Eliminating Mother-to-Child Transmission of HIV by 2030: 5 Strategies to Ensure Continued Progress. Glob. Health Sci. Pract. 2018, 6, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Yudin, M.H. HIV Infection in Pregnant Women: A 2020 Update. Prenat. Diagn. 2020, 40, 1715–1721. [Google Scholar] [CrossRef]
- Phillips, T.K.; Teasdale, C.A.; Geller, A.; Ng′eno, B.; Mogoba, P.; Modi, S.; Abrams, E.J. Approaches to transitioning women into and out of prevention of mother-to-child transmission of HIV services for continued ART: A systematic review. J. Int. AIDS Soc. 2021, 24, e25633. [Google Scholar] [CrossRef]
- Tugizov, S.M.; Herrera, R.; Palefsky, J.M. Epstein-Barr Virus Transcytosis through Polarized Oral Epithelial Cells. J. Virol. 2013, 87, 8179–8194. [Google Scholar] [CrossRef]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Drew, W.L. Cytomegalovirus infection in patients with AIDS. Clin. Infect Dis. 1992, 14, 608–615. [Google Scholar] [CrossRef]
- Kovacs, A.; Schluchter, M.; Easley, K.; Demmler, G.; Shearer, W.; La Russa, P.; Pitt, J.; Cooper, E.; Goldfarb, J.; Hodes, D.; et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N. Engl. J. Med. 1999, 341, 77–84. [Google Scholar] [CrossRef]
- Nigro, G.; Krzysztofiak, A.; Gattinara, G.C.; Mango, T.; Mazzocco, M.; Porcaro, M.A.; Provvedi, S.; Booth, J.C. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. AIDS 1996, 10, 1127–1133. [Google Scholar] [PubMed]
- Chandwani, S.; Kaul, A.; Bebenroth, D.; Kim, M.; John, D.D.; Fidelia, A.; Hassel, A.; Borkowsky, W.; Krasinski, K. Cytomegalovirus infection in human immunodeficiency virus type 1-infected children. Pediatr. Infect. Dis. J. 1996, 15, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Gie, R.P.; Goussard, P. CMV pneumonia in HIV-infected and HIV-uninfected infants: A neglected disease? Int. J. Tuberc. Lung. Dis. 2017, 21, 1209–1210. [Google Scholar] [CrossRef] [PubMed]
- Gianella, S.; Letendre, S. Cytomegalovirus and HIV: A Dangerous Pas de Deux. J. Infect. Dis. 2016, 214 (Suppl. S2), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Pass, R.F.; Anderson, B. Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. J. Pediatr. Infect. Dis. Soc. 2014, 3 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Schmink, S.; Kruszon-Moran, D.; Dollard, S.C.; Lanzieri, T.M. Effect of Breastfeeding and Additional Household Children on Cytomegalovirus Seroprevalence among U.S. Children 1 to 5 Years of Age. Clin. Vaccine Immunol. 2017, 24, e00243-17. [Google Scholar] [CrossRef]
- Meier, J.; Lienicke, U.; Tschirch, E.; Kruger, D.H.; Wauer, R.R.; Prosch, S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J. Clin. Microbiol. 2005, 43, 1318–1324. [Google Scholar] [CrossRef]
- Hamprecht, K.; Witzel, S.; Maschmann, J.; Dietz, K.; Baumeister, A.; Mikeler, E.; Goelz, R.; Speer, C.P.; Jahn, G. Rapid detection and quantification of cell free cytomegalovirus by a high-speed centrifugation-based microculture assay: Comparison to longitudinally analyzed viral DNA load and pp67 late transcript during lactation. J. Clin. Virol. 2003, 28, 303–316. [Google Scholar] [CrossRef]
- Yasuda, A.; Kimura, H.; Hayakawa, M.; Ohshiro, M.; Kato, Y.; Matsuura, O.; Suzuki, C.; Morishima, T. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics 2003, 111, 1333–1336. [Google Scholar] [CrossRef]
- Viljoen, J.; Tuaillon, E.; Nagot, N.; Danaviah, S.; Peries, M.; Padayachee, P.; Foulongne, V.; Bland, R.; Rollins, N.; Newell, M.L.; et al. Cytomegalovirus, and possibly Epstein-Barr virus, shedding in breast milk is associated with HIV-1 transmission by breastfeeding. AIDS 2015, 29, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Leister, E.; Jacobsen, D.L.; Boucoiran, I.; Huang, M.L.; Jerome, K.R.; Jourdain, G.; Ngo-Giang-Huong, N.; Burchett, S.; Frenkel, L. Risk of congenital cytomegalovirus infection among HIV-exposed uninfected infants is not decreased by maternal nelfinavir use during pregnancy. J. Med. Virol. 2016, 88, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Slyker, J.A.; Lohman-Payne, B.L.; John-Stewart, G.C.; Maleche-Obimbo, E.; Emery, S.; Richardson, B.; Dong, T.; Iversen, A.K.; Mbori-Ngacha, D.; Overbaugh, J.; et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS 2009, 23, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Wiener, J.; Dollard, S.C.; Amin, M.M.; Ellington, S.; Chasela, C.; Kayira, D.; Tegha, G.; Kamwendo, D.; Jamieson, D.J.; et al. Effect of cytomegalovirus infection on breastfeeding transmission of HIV and on the health of infants born to HIV-infected mothers. AIDS 2015, 29, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.Y.; Sheth, P.M.; Persad, D.; Kovacs, C.; Kain, T.; Diong, C.; Su, D.; Ostrowski, M.; Raboud, J.M.; Kaul, R. Impact of CMV therapy with valganciclovir on immune activation and the HIV viral load in semen and blood: An observational clinical study. J. Acquir. Immune. Defic. Syndr. 2014, 65, 251–258. [Google Scholar] [CrossRef]
- Adland, E.; Klenerman, P.; Goulder, P.; Matthews, P.C. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front. Microbiol. 2015, 6, 1016. [Google Scholar] [CrossRef]
- DeMeritt, I.B.; Milford, L.E.; Yurochko, A.D. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J. Virol. 2004, 78, 4498–4507. [Google Scholar] [CrossRef]
- Chan, G.; Bivins-Smith, E.R.; Smith, M.S.; Yurochko, A.D. Transcriptome analysis of NF-kappaB- and phosphatidylinositol 3-kinase-regulated genes in human cytomegalovirus-infected monocytes. J. Virol. 2008, 82, 1040–1046. [Google Scholar] [CrossRef]
- Yurochko, A.D.; Kowalik, T.F.; Huong, S.M.; Huang, E.S. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J. Virol. 1995, 69, 5391–5400. [Google Scholar] [CrossRef]
- Johnson, R.A.; Ma, X.L.; Yurochko, A.D.; Huang, E.S. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 2001, 82, 493–497. [Google Scholar] [CrossRef]
- Bushara, O.; Krogh, K.; Weinberg, S.E.; Finkelman, B.S.; Sun, L.; Liao, J.; Yang, G.Y. Human Immunodeficiency Virus Infection Promotes Human Papillomavirus-Mediated Anal Squamous Carcinogenesis: An Immunologic and Pathobiologic Review. Pathobiology 2022, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R.V. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Palefsky, J.M. Human papillomavirus-associated anal and cervical cancers in HIV-infected individuals: Incidence and prevention in the antiretroviral therapy era. Curr. Opin. HIV AIDS 2017, 12, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjose, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Sathish, N.; Wang, X.; Yuan, Y. Human Papillomavirus (HPV)-associated Oral Cancers and Treatment Strategies. J. Dent. Res. 2014, 93, 29S–36S. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Wang, H.; Jiang, T.; Zhang, M.; Zhang, Y.; Su, B.; Tian, Y. Renal Cell Carcinoma Associated With HIV/AIDS: A Review of the Epidemiology, Risk Factors, Diagnosis, and Treatment. Front. Oncol. 2022, 12, 872438. [Google Scholar] [CrossRef]
- McLemore, M.S.; Haigentz, M., Jr.; Smith, R.V.; Nuovo, G.J.; Alos, L.; Cardesa, A.; Brandwein-Gensler, M. Head and neck squamous cell carcinomas in HIV-positive patients: A preliminary investigation of viral associations. Head Neck Pathol. 2010, 4, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Lee, J.E.; Lee, S.; Lee, S.H.; Kang, J.S.; Son, H.; Lee, H.; Kim, J. Nationwide population-based incidence of cancer among patients with HIV/AIDS in South Korea. Sci. Rep. 2022, 12, 9974. [Google Scholar] [CrossRef] [PubMed]
- Negri, F.; Missale, G.; Antoni, A.D.; Porta, C. Hepatocellular cancer therapy in patients with HIV infection: Disparities in cancer care, trials enrolment, and cancer-related research. Transl. Oncol. 2021, 14, 101153. [Google Scholar] [CrossRef]
- Isaguliants, M.; Bayurova, E.; Avdoshina, D.; Kondrashova, A.; Chiodi, F.; Palefsky, J.M. Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind. Cancers 2021, 13, 305. [Google Scholar] [CrossRef]
- Lien, K.; Mayer, W.; Herrera, R.; Rosbe, K.; Tugizov, S.M. HIV-1 proteins gp120 and tat induce the epithelial-mesenchymal transition in oral and genital mucosal epithelial cells. PLoS ONE 2019, 14, e0226343. [Google Scholar] [CrossRef] [PubMed]
- Ocana, O.H.; Nieto, M.A. Epithelial plasticity, stemness and pluripotency. Cell Res. 2010, 20, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Barillari, G.; Bei, R.; Manzari, V.; Modesti, A. Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events? Int. J. Mol. Sci. 2021, 22, 13543. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem. Cell 2019, 24, 65–78. [Google Scholar] [CrossRef]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef]
- Jolly, M.K.; Murphy, R.J.; Bhatia, S.; Whitfield, H.J.; Redfern, A.; Davis, M.J.; Thompson, E.W. Measuring and Modelling the Epithelial- Mesenchymal Hybrid State in Cancer: Clinical Implications. Cells Tissues Organs 2022, 211, 110–133. [Google Scholar] [CrossRef]
- Grosse-Wilde, A.; Fouquier d′Herouel, A.; McIntosh, E.; Ertaylan, G.; Skupin, A.; Kuestner, R.E.; del Sol, A.; Walters, K.A.; Huang, S. Stemness of the hybrid Epithelial/Mesenchymal State in Breast Cancer and Its Association with Poor Survival. PLoS ONE 2015, 10, e0126522. [Google Scholar] [CrossRef]
- Ruscetti, M.; Quach, B.; Dadashian, E.L.; Mulholland, D.J.; Wu, H. Tracking and Functional Characterization of Epithelial-Mesenchymal Transition and Mesenchymal Tumor Cells during Prostate Cancer Metastasis. Cancer Res. 2015, 75, 2749–2759. [Google Scholar] [CrossRef]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef]
- Wendt, M.K.; Tian, M.; Schiemann, W.P. Deconstructing the mechanisms and consequences of TGF-beta-induced EMT during cancer progression. Cell Tissue Res. 2012, 347, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.; Mayer, W.; Herrera, R.; Padilla, N.T.; Cai, X.; Lin, V.; Pholcharoenchit, R.; Palefsky, J.; Tugizov, S.M. HIV-1 Proteins gp120 and Tat Promote Epithelial-Mesenchymal Transition and Invasiveness of HPV-Positive and HPV-Negative Neoplastic Genital and Oral Epithelial Cells. Microbiol. Spectr. 2022, 10, e0362222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Chen, C.H.; Yen, C.H.; Tung, C.W.; Chen, C.J.; Chen, Y.A.; Huang, M.S. Human immunodeficiency virus Tat-TIP30 interaction promotes metastasis by enhancing the nuclear translocation of Snail in lung cancer cell lines. Cancer Sci. 2018, 109, 3105–3114. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.A.; Ferreira, F.; Mandke, P.; Chau, E.; Aggarwal, N.R.; D’Alessio, F.R.; Lambert, A.A.; Kirk, G.; Blankson, J.; Drummond, M.B.; et al. HIV Impairs Lung Epithelial Integrity and Enters the Epithelium to Promote Chronic Lung Inflammation. PLoS ONE 2016, 11, e0149679. [Google Scholar] [CrossRef]
- Chung, C.Y.; Alden, S.L.; Funderburg, N.T.; Fu, P.; Levine, A.D. Progressive proximal-to-distal reduction in expression of the tight junction complex in colonic epithelium of virally-suppressed HIV+ individuals. PLoS Pathog. 2014, 10, e1004198. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tugizov, S.M. Molecular Pathogenesis of Human Immunodeficiency Virus-Associated Disease of Oropharyngeal Mucosal Epithelium. Biomedicines 2023, 11, 1444. https://doi.org/10.3390/biomedicines11051444
Tugizov SM. Molecular Pathogenesis of Human Immunodeficiency Virus-Associated Disease of Oropharyngeal Mucosal Epithelium. Biomedicines. 2023; 11(5):1444. https://doi.org/10.3390/biomedicines11051444
Chicago/Turabian StyleTugizov, Sharof M. 2023. "Molecular Pathogenesis of Human Immunodeficiency Virus-Associated Disease of Oropharyngeal Mucosal Epithelium" Biomedicines 11, no. 5: 1444. https://doi.org/10.3390/biomedicines11051444




