Neurophysiological Markers of Premotor–Motor Network Plasticity Predict Motor Performance in Young and Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Behavioral Tasks
2.3. ccPAS Procedure and Electrophysiological Recordings
2.4. Electrophysiological Recording
2.5. Data Analyses
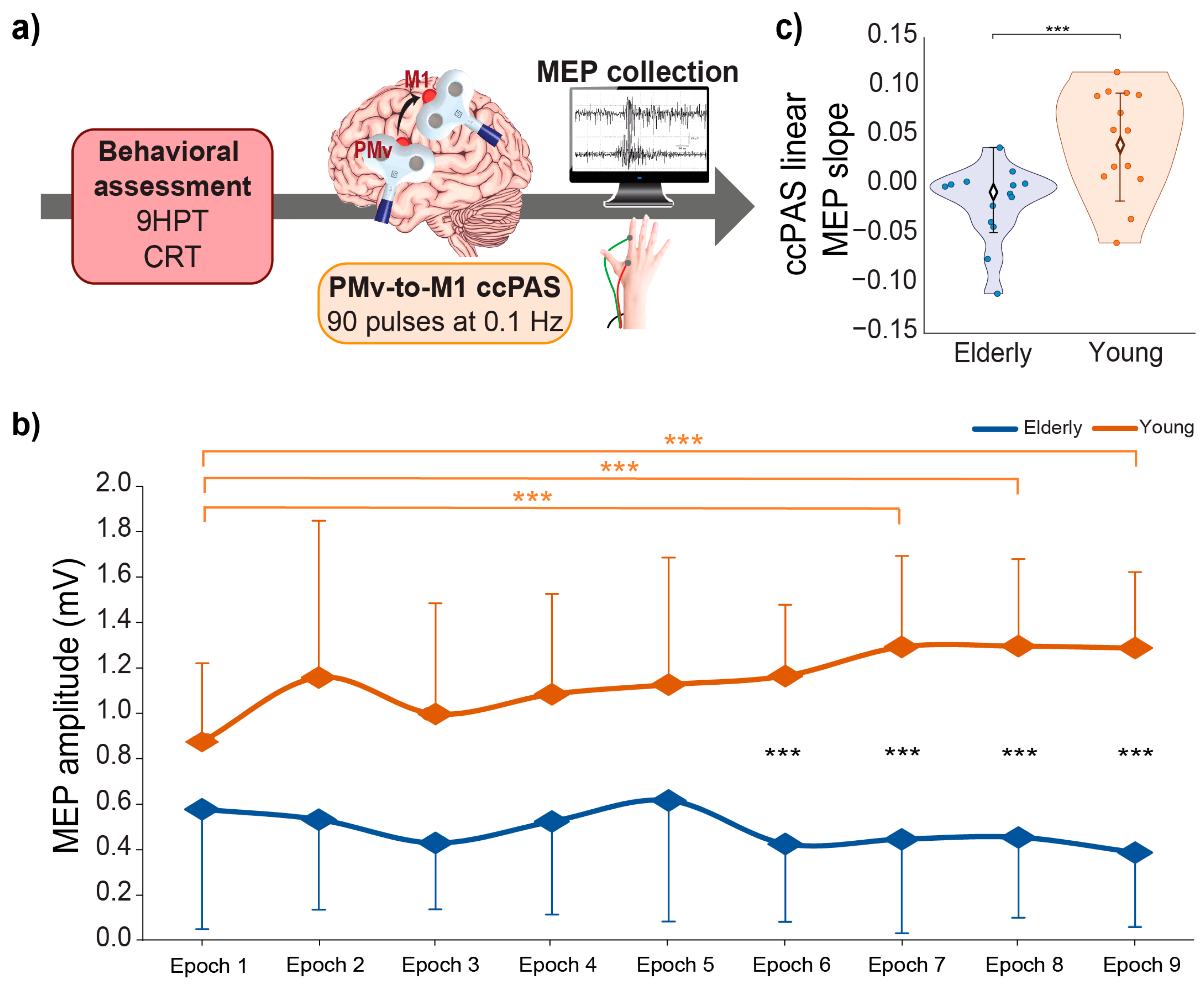
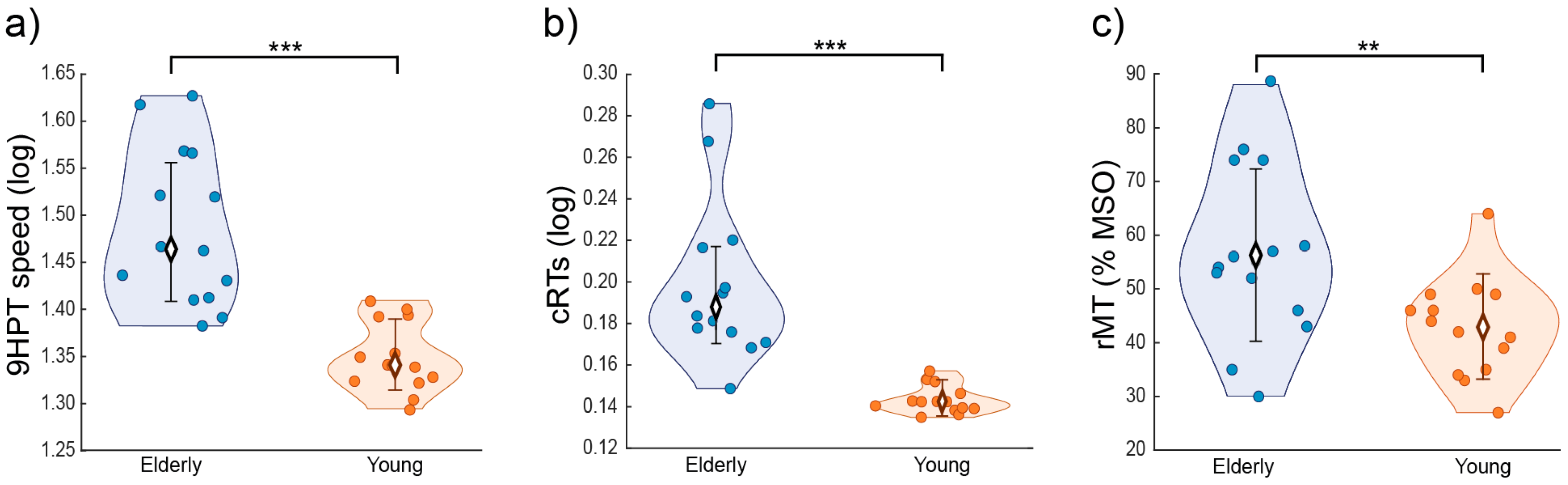
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turrini, S.; Wong, B.; Eldaief, M.; Press, D.Z.; Sinclair, D.A.; Koch, G.; Avenanti, A.; Santarnecchi, E. The Multifactorial Nature of Healthy Brain Ageing: Brain Changes, Functional Decline and Protective Factors. Ageing Res. Rev. 2023, 88, 101939. [Google Scholar] [CrossRef]
- Damoiseaux, J.S. Effects of Aging on Functional and Structural Brain Connectivity. Neuroimage 2017, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage 2001, 14, 21–36. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.R.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the Cerebral Cortex in Aging. Cereb. Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef]
- Resnick, S.M.; Pham, D.L.; Kraut, M.A.; Zonderman, A.B.; Davatzikos, C. Longitudinal Magnetic Resonance Imaging Studies of Older Adults: A Shrinking Brain. J. Neurosci. 2003, 23, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Ritchie, S.J.; Tucker-Drob, E.M.; Liewald, D.C.; Hagenaars, S.P.; Davies, G.; Wardlaw, J.M.; Gale, C.R.; Bastin, M.E.; Deary, I.J. Ageing and Brain White Matter Structure in 3,513 UK Biobank Participants. Nat. Commun. 2016, 7, 13629. [Google Scholar] [CrossRef]
- Smith, C.D.; Umberger, G.H.; Manning, E.L.; Slevin, J.T.; Wekstein, D.R.; Schmitt, F.A.; Markesbery, W.R.; Zhang, Z.; Gerhardt, G.A.; Kryscio, R.J.; et al. Critical Decline in Fine Motor Hand Movements in Human Aging. Neurology 1999, 53, 1458. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, V.K.; Siemionow, V.; Sahgal, V.; Yue, G.H. Effects of Aging on Hand Function. J. Am. Geriatr. Soc. 2001, 49, 1478–1484. [Google Scholar] [CrossRef]
- Carmeli, E.; Patish, H.; Coleman, R. The Aging Hand. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor Control and Aging: Links to Age-Related Brain Structural, Functional, and Biochemical Effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef]
- Maes, C.; Gooijers, J.; Orban de Xivry, J.-J.; Swinnen, S.P.; Boisgontier, M.P. Two Hands, One Brain, and Aging. Neurosci. Biobehav. Rev. 2017, 75, 234–256. [Google Scholar] [CrossRef]
- Nusbaum, A.O.; Tang, C.Y.; Buchsbaum, M.S.; Wei, T.C.; Atlas, S.W. Regional and Global Changes in Cerebral Diffusion with Normal Aging. Am. J. Neuroradiol. 2001, 22, 136–142. [Google Scholar] [PubMed]
- Salat, D.H.; Tuch, D.S.; Greve, D.N.; van der Kouwe, A.J.W.; Hevelone, N.D.; Zaleta, A.K.; Rosen, B.R.; Fischl, B.; Corkin, S.; Rosas, H.D.; et al. Age-Related Alterations in White Matter Microstructure Measured by Diffusion Tensor Imaging. Neurobiol. Aging 2005, 26, 1215–1227. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Pfefferbaum, A. Diffusion Tensor Imaging and Aging. Neurosci. Biobehav. Rev. 2006, 30, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Hinder, M.R.; Fujiyama, H.; Summers, J.J. Premotor-Motor Interhemispheric Inhibition Is Released during Movement Initiation in Older but Not Young Adults. PLoS ONE 2012, 7, e52573. [Google Scholar] [CrossRef] [PubMed]
- Green, P.E.; Ridding, M.C.; Hill, K.D.; Semmler, J.G.; Drummond, P.D.; Vallence, A.-M. Supplementary Motor Area—Primary Motor Cortex Facilitation in Younger but Not Older Adults. Neurobiol. Aging 2018, 64, 85–91. [Google Scholar] [CrossRef]
- Rurak, B.K.; Rodrigues, J.P.; Power, B.D.; Drummond, P.D.; Vallence, A.-M. Reduced SMA-M1 Connectivity in Older than Younger Adults Measured Using Dual-Site TMS. Eur. J. Neurosci. 2021, 54, 6533–6552. [Google Scholar] [CrossRef]
- Verstraelen, S.; Cuypers, K.; Maes, C.; Hehl, M.; Van Malderen, S.; Levin, O.; Mikkelsen, M.; Meesen, R.L.J.; Swinnen, S.P. Neurophysiological Modulations in the (Pre)Motor-Motor Network Underlying Age-Related Increases in Reaction Time and the Role of GABA Levels—A Bimodal TMS-MRS Study. NeuroImage 2021, 243, 118500. [Google Scholar] [CrossRef]
- Caporale, N.; Dan, Y. Spike Timing-Dependent Plasticity: A Hebbian Learning Rule. Annu. Rev. Neurosci. 2008, 31, 25–46. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior; A Neuropsychological Theory; Wiley: Oxford, UK, 1949. [Google Scholar]
- Jackson, A.; Mavoori, J.; Fetz, E.E. Long-Term Motor Cortex Plasticity Induced by an Electronic Neural Implant. Nature 2006, 444, 56–60. [Google Scholar] [CrossRef]
- Markram, H.; Gerstner, W.; Sjöström, P.J. A History of Spike-Timing-Dependent Plasticity. Front. Synaptic Neurosci. 2011, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.A. Long-Term Potentiation and the Ageing Brain. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2003, 358, 765–772. [Google Scholar] [CrossRef]
- Kumar, A.; Foster, T.C. Neurophysiology of Old Neurons and Synapses. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-3818-2. [Google Scholar]
- Rex, C.S.; Kramár, E.A.; Colgin, L.L.; Lin, B.; Gall, C.M.; Lynch, G. Long-Term Potentiation Is Impaired in Middle-Aged Rats: Regional Specificity and Reversal by Adenosine Receptor Antagonists. J. Neurosci. 2005, 25, 5956–5966. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Teyler, T.J.; Robbins, N. Aging Differentially Alters Forms of Long-Term Potentiation in Rat Hippocampal Area CA1. J. Neurophysiol. 1998, 79, 334–341. [Google Scholar] [CrossRef]
- Vouimba, R.M.; Foy, M.R.; Foy, J.G.; Thompson, R.F. 17beta-Estradiol Suppresses Expression of Long-Term Depression in Aged Rats. Brain Res. Bull. 2000, 53, 783–787. [Google Scholar] [CrossRef]
- Mahncke, H.W.; Bronstone, A.; Merzenich, M.M. Brain Plasticity and Functional Losses in the Aged: Scientific Bases for a Novel Intervention. In Progress in Brain Research; Møller, A.R., Ed.; Reprogramming of the Brain; Elsevier: Amsterdam, The Netherlands, 2006; Volume 157, pp. 81–109. [Google Scholar]
- Bhandari, A.; Radhu, N.; Farzan, F.; Mulsant, B.H.; Rajji, T.K.; Daskalakis, Z.J.; Blumberger, D.M. A Meta-Analysis of the Effects of Aging on Motor Cortex Neurophysiology Assessed by Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2016, 127, 2834–2845. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural Plasticity in the Ageing Brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Sel, A.; Hibbard, P.B.; Avenanti, A.; Romei, V. Increasing Interhemispheric Connectivity between Human Visual Motion Areas Uncovers Asymmetric Sensitivity to Horizontal Motion. Curr. Biol. 2022, 32, 4064–4070.e3. [Google Scholar] [CrossRef]
- Chiappini, E.; Silvanto, J.; Hibbard, P.B.; Avenanti, A.; Romei, V. Strengthening Functionally Specific Neural Pathways with Transcranial Brain Stimulation. Curr. Biol. 2018, 28, R735–R736. [Google Scholar] [CrossRef] [PubMed]
- Di Luzio, P.; Tarasi, L.; Silvanto, J.; Avenanti, A.; Romei, V. Human Perceptual and Metacognitive Decision-Making Rely on Distinct Brain Networks. PLoS Biol. 2022, 20, e3001750. [Google Scholar] [CrossRef]
- Koch, G.; Ponzo, V.; Lorenzo, F.D.; Caltagirone, C.; Veniero, D. Hebbian and Anti-Hebbian Spike-Timing-Dependent Plasticity of Human Cortico-Cortical Connections. J. Neurosci. 2013, 33, 9725–9733. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; Siebner, H.S.; Morgante, F.; Mastroeni, C.; Girlanda, P.; Quartarone, A. Paired Associative Stimulation of Left and Right Human Motor Cortex Shapes Interhemispheric Motor Inhibition Based on a Hebbian Mechanism. Cereb. Cortex 2009, 19, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Romei, V.; Chiappini, E.; Hibbard, P.B.; Avenanti, A. Empowering Reentrant Projections from V5 to V1 Boosts Sensitivity to Motion. Curr. Biol. 2016, 26, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; Bove, M.; Naro, A.; Tacchino, A.; Mastroeni, C.; Avanzino, L.; Crupi, D.; Morgante, F.; Siebner, H.R.; Quartarone, A. Associative Cortico-Cortical Plasticity May Affect Ipsilateral Finger Opposition Movements. Behav. Brain Res. 2011, 216, 433–439. [Google Scholar] [CrossRef]
- Koganemaru, S.; Mima, T.; Nakatsuka, M.; Ueki, Y.; Fukuyama, H.; Domen, K. Human Motor Associative Plasticity Induced by Paired Bihemispheric Stimulation. J. Physiol. 2009, 587, 4629–4644. [Google Scholar] [CrossRef]
- Johnen, V.M.; Neubert, F.X.; Buch, E.R.; Verhagen, L.; O’Reilly, J.X.; Mars, R.B.; Rushworth, M.F.S. Causal Manipulation of Functional Connectivity in a Specific Neural Pathway During Behaviour and at Rest. Elife 2015, 4, e04585. [Google Scholar] [CrossRef]
- Tübing, J.; Gigla, B.; Brandt, V.C.; Verrel, J.; Weissbach, A.; Beste, C.; Münchau, A.; Bäumer, T. Associative Plasticity in Supplementary Motor Area—Motor Cortex Pathways in Tourette Syndrome. Sci. Rep. 2018, 8, 11984. [Google Scholar] [CrossRef]
- Sel, A.; Verhagen, L.; Angerer, K.; David, R.; Klein-Flügge, M.C.; Rushworth, M.F. Increasing and Decreasing Interregional Brain Coupling Increases and Decreases Oscillatory Activity in the Human Brain. Proc. Natl. Acad. Sci. USA 2021, 118, 1–9. [Google Scholar] [CrossRef]
- Lazari, A.; Salvan, P.; Verhagen, L.; Cottaar, M.; Papp, D.; van der Werf, O.J.; Gavine, B.; Kolasinski, J.; Webster, M.; Stagg, C.J.; et al. A Macroscopic Link between Interhemispheric Tract Myelination and Cortico-Cortical Interactions during Action Reprogramming. Nat. Commun. 2022, 13, 4253. [Google Scholar] [CrossRef]
- Buch, E.R.; Johnen, V.M.; Nelissen, N.; O’Shea, J.; Rushworth, M.F.S. Noninvasive Associative Plasticity Induction in a Corticocortical Pathway of the Human Brain. J. Neurosci. 2011, 31, 17669–17679. [Google Scholar] [CrossRef]
- Casarotto, A.; Dolfini, E.; Cardellicchio, P.; Fadiga, L.; D’Ausilio, A.; Koch, G. Mechanisms of Hebbian-like Plasticity in the Ventral Premotor—Primary Motor Network. J. Physiol. 2022, 601, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Borgomaneri, S.; Marangon, M.; Turrini, S.; Romei, V.; Avenanti, A. Driving Associative Plasticity in Premotor-Motor Connections through a Novel Paired Associative Stimulation Based on Long-Latency Cortico-Cortical Interactions. Brain Stimul. 2020, 13, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Fiori, F.; Chiappini, E.; Avenanti, A. Enhanced Action Performance Following TMS Manipulation of Associative Plasticity in Ventral Premotor-Motor Pathway. NeuroImage 2018, 183, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Turrini, S.; Fiori, F.; Chiappini, E.; Lucero, B.; Santarnecchi, E.; Avenanti, A. Cortico-Cortical Paired Associative Stimulation (CcPAS) over Premotor-Motor Areas Affects Local Circuitries in the Human Motor Cortex via Hebbian Plasticity. NeuroImage 2023, 21, 120027. [Google Scholar] [CrossRef] [PubMed]
- Turrini, S.; Fiori, F.; Chiappini, E.; Santarnecchi, E.; Romei, V.; Avenanti, A. Gradual Enhancement of Corticomotor Excitability during Cortico-Cortical Paired Associative Stimulation. Sci. Rep. 2022, 12, 14670. [Google Scholar] [CrossRef] [PubMed]
- Turrini, S.; Bevacqua, N.; Cataneo, A.; Chiappini, E.; Fiori, F.; Candidi, M.; Avenanti, A. Transcranial Cortico-Cortical Paired Associative Stimulation (CcPAS) over Ventral Premotor-Motor Pathways Enhances Action Performance and Corticomotor Excitability in Young Adults More than in Elderly Adults. Front. Aging Neurosci. 2023, 15, 9508. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Ponzo, V.; Motta, C.; Bonnì, S.; Picazio, S.; Caltagirone, C.; Bozzali, M.; Martorana, A.; Koch, G. Impaired Spike Timing Dependent Cortico-Cortical Plasticity in Alzheimer’s Disease Patients. J. Alzheimers Dis. 2018, 66, 983–991. [Google Scholar] [CrossRef]
- Bonnì, S.; Lupo, F.; Lo Gerfo, E.; Martorana, A.; Perri, R.; Caltagirone, C.; Koch, G. Altered Parietal-Motor Connections in Alzheimer’s Disease Patients. J. Alzheimers Dis. 2013, 33, 525–533. [Google Scholar] [CrossRef]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and Recommendations for TMS Use in Healthy Subjects and Patient Populations, with Updates on Training, Ethical and Regulatory Issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Bikson, M.; Hanlon, C.A.; Woods, A.J.; Gillick, B.T.; Charvet, L.; Lamm, C.; Madeo, G.; Holczer, A.; Almeida, J.; Antal, A.; et al. Guidelines for TMS/TES Clinical Services and Research through the COVID-19 Pandemic. Brain Stimul. 2020, 13, 1124–1149. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Kashman, N.; Volland, G. Adult Norms for the Nine Hole Peg Test of Finger Dexterity. Occup. Ther. J. Res. 1985, 5, 24–38. [Google Scholar] [CrossRef]
- Oxford Grice, K.; Vogel, K.A.; Le, V.; Mitchell, A.; Muniz, S.; Vollmer, M.A. Adult Norms for a Commercially Available Nine Hole Peg Test for Finger Dexterity. Am. J. Occup. Ther. 2003, 57, 570–573. [Google Scholar] [CrossRef]
- Hamzei, F.; Läppchen, C.H.; Glauche, V.; Mader, I.; Rijntjes, M.; Weiller, C. Functional Plasticity Induced by Mirror Training: The Mirror as the Element Connecting Both Hands to One Hemisphere. Neurorehabil. Neural. Rep. 2012, 26, 484–496. [Google Scholar] [CrossRef]
- Avenanti, A.; Coccia, M.; Ladavas, E.; Provinciali, L.; Ceravolo, M.G. Low-Frequency RTMS Promotes Use-Dependent Motor Plasticity in Chronic Stroke: A Randomized Trial. Neurology 2012, 78, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hutchinson, S.; Théoret, H.; Schlaug, G.; Pascual-Leone, A. Repetitive TMS of the Motor Cortex Improves Ipsilateral Sequential Simple Finger Movements. Neurology 2004, 62, 91–98. [Google Scholar] [CrossRef]
- Koch, G.; Olmo, M.F.D.; Cheeran, B.; Schippling, S.; Caltagirone, C.; Driver, J.; Rothwell, J.C. Functional Interplay between Posterior Parietal and Ipsilateral Motor Cortex Revealed by Twin-Coil Transcranial Magnetic Stimulation during Reach Planning toward Contralateral Space. J. Neurosci. 2008, 28, 5944–5953. [Google Scholar] [CrossRef]
- Mansur, C.G.; Fregni, F.; Boggio, P.S.; Riberto, M.; Gallucci-Neto, J.; Santos, C.M.; Wagner, T.; Rigonatti, S.P.; Marcolin, M.A.; Pascual-Leone, A. A Sham Stimulation-Controlled Trial of RTMS of the Unaffected Hemisphere in Stroke Patients. Neurology 2005, 64, 1802–1804. [Google Scholar] [CrossRef]
- Davare, M.; Lemon, R.; Olivier, E. Selective Modulation of Interactions between Ventral Premotor Cortex and Primary Motor Cortex during Precision Grasping in Humans. J. Physiol. 2008, 586, 2735–2742. [Google Scholar] [CrossRef]
- Fiori, F.; Chiappini, E.; Candidi, M.; Romei, V.; Borgomaneri, S.; Avenanti, A. Long-Latency Interhemispheric Interactions between Motor-Related Areas and the Primary Motor Cortex: A Dual Site TMS Study. Sci. Rep. 2017, 7, 14936. [Google Scholar] [CrossRef]
- Fiori, F.; Chiappini, E.; Soriano, M.; Paracampo, R.; Romei, V.; Borgomaneri, S.; Avenanti, A. Long-Latency Modulation of Motor Cortex Excitability by Ipsilateral Posterior Inferior Frontal Gyrus and Pre-Supplementary Motor Area. Sci. Rep. 2016, 6, 38396. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Iorio, R.D.; Lazzaro, V.D.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application: An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Avenanti, A.; Annela, L.; Serino, A. Suppression of Premotor Cortex Disrupts Motor Coding of Peripersonal Space. NeuroImage 2012, 63, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Avenanti, A.; Paracampo, R.; Annella, L.; Tidoni, E.; Aglioti, S.M. Boosting and Decreasing Action Prediction Abilities Through Excitatory and Inhibitory TDCS of Inferior Frontal Cortex. Cereb. Cortex 2018, 28, 1282–1296. [Google Scholar] [CrossRef]
- Dafotakis, M.; Sparing, R.; Eickhoff, S.B.; Fink, G.R.; Nowak, D.A. On the Role of the Ventral Premotor Cortex and Anterior Intraparietal Area for Predictive and Reactive Scaling of Grip Force. Brain Res. 2008, 1228, 73–80. [Google Scholar] [CrossRef]
- Davare, M.; Andres, M.; Cosnard, G.; Thonnard, J.-L.; Olivier, E. Dissociating the Role of Ventral and Dorsal Premotor Cortex in Precision Grasping. J. Neurosci. 2006, 26, 2260–2268. [Google Scholar] [CrossRef]
- Jacquet, P.O.; Avenanti, A. Perturbing the Action Observation Network During Perception and Categorization of Actions’ Goals and Grips: State-Dependency and Virtual Lesion TMS Effects. Cereb. Cortex 2015, 25, 598–608. [Google Scholar] [CrossRef]
- Avenanti, A.; Urgesi, C. Understanding “what” Others Do: Mirror Mechanisms Play a Crucial Role in Action Perception. Soc. Cogn. Affect. Neurosci. 2011, 6, 257–259. [Google Scholar] [CrossRef]
- Avenanti, A.; Candidi, M.; Urgesi, C. Vicarious Motor Activation during Action Perception: Beyond Correlational Evidence. Front. Hum. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef]
- Bäumer, T.; Schippling, S.; Kroeger, J.; Zittel, S.; Koch, G.; Thomalla, G.; Rothwell, J.C.; Siebner, H.R.; Orth, M.; Münchau, A. Inhibitory and Facilitatory Connectivity from Ventral Premotor to Primary Motor Cortex in Healthy Humans at Rest—A Bifocal TMS Study. Clin. Neurophysiol. 2009, 120, 1724–1731. [Google Scholar] [CrossRef]
- Kammer, T.; Beck, S.; Thielscher, A.; Laubis-Herrmann, U.; Topka, H. Motor Thresholds in Humans: A Transcranial Magnetic Stimulation Study Comparing Different Pulse Waveforms, Current Directions and Stimulator Types. Clin. Neurophysiol. 2001, 112, 250–258. [Google Scholar] [CrossRef]
- Mora, F.; Segovia, G.; del Arco, A. Aging, Plasticity and Environmental Enrichment: Structural Changes and Neurotransmitter Dynamics in Several Areas of the Brain. Brain Res. Rev. 2007, 55, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Farzan, F.; Pascual-Leone, A. Assessing Brain Plasticity across the Lifespan with Transcranial Magnetic Stimulation: Why, How, and What Is the Ultimate Goal? Front. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Iezzi, E.; Gilio, L.; Centonze, D.; Buttari, F. Synaptic Plasticity Shapes Brain Connectivity: Implications for Network Topology. Int. J. Mol. Sci. 2019, 20, 6193. [Google Scholar] [CrossRef]
- Davare, M.; Kraskov, A.; Rothwell, J.C.; Lemon, R.N. Interactions between Areas of the Cortical Grasping Network. Curr. Opin. Neurobiol. 2011, 21, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Cattaneo, L.; Fabbri-Destro, M.; Rozzi, S. Cortical Mechanisms Underlying the Organization of Goal-Directed Actions and Mirror Neuron-Based Action Understanding. Physiol. Rev. 2014, 94, 655–706. [Google Scholar] [CrossRef] [PubMed]
- Davare, M.; Rothwell, J.C.; Lemon, R.N. Causal Connectivity between the Human Anterior Intraparietal Area and Premotor Cortex during Grasp. Curr. Biol. 2010, 20, 176–181. [Google Scholar] [CrossRef]
- Grol, M.J.; Majdandžić, J.; Stephan, K.E.; Verhagen, L.; Dijkerman, H.C.; Bekkering, H.; Verstraten, F.A.J.; Toni, I. Parieto-Frontal Connectivity during Visually Guided Grasping. J. Neurosci. 2007, 27, 11877–11887. [Google Scholar] [CrossRef]
- Sala-Llonch, R.; Bartrés-Faz, D.; Junqué, C. Reorganization of Brain Networks in Aging: A Review of Functional Connectivity Studies. Front. Psychol. 2015, 6, 633. [Google Scholar] [CrossRef]
- Carment, L.; Abdellatif, A.; Lafuente-Lafuente, C.; Pariel, S.; Maier, M.A.; Belmin, J.; Lindberg, P.G. Manual Dexterity and Aging: A Pilot Study Disentangling Sensorimotor From Cognitive Decline. Front. Neurol. 2018, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Inghilleri, M.; Conte, A.; Currà, A.; Frasca, V.; Lorenzano, C.; Berardelli, A. Ovarian Hormones and Cortical Excitability. An RTMS Study in Humans. Clin. Neurophysiol. 2004, 115, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Minkova, L.; Peter, J.; Abdulkadir, A.; Schumacher, L.V.; Kaller, C.P.; Nissen, C.; Klöppel, S.; Lahr, J. Determinants of Inter-Individual Variability in Corticomotor Excitability Induced by Paired Associative Stimulation. Front. Neurosci. 2019, 13, 841. [Google Scholar] [CrossRef]
- Davare, M.; Montague, K.; Olivier, E.; Rothwell, J.C.; Lemon, R.N. Ventral Premotor to Primary Motor Cortical Interactions during Object-Driven Grasp in Humans. Cortex 2009, 45, 1050–1057. [Google Scholar] [CrossRef] [PubMed]

| Group | Age | Gender |
|---|---|---|
| Elderly | 71.21 years ± 6.95 | Males = 11, Females = 3 |
| Young | 23.08 years ± 2.91 | Males = 6, Females = 8 |
| Statistical analyses | t26 = 23.13, p < 0.0001 | Yates’s χ2 = 2.40, p = 0.12 |
| Group | M1 | PMv | ||||
|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |
| Older | −33.6 ± 6.3 | −18.6 ± 7.7 | 59.7 ± 4.2 | −53.6 ± 2.0 | 9.6 ± 1.5 | 23.7 ± 1.1 |
| Young | −30.5 ± 5.7 | −16.5 ± 6.1 | 59.0 ± 4.8 | −51.6 ± 2.2 | 9.1 ± 1.8 | 23.2 ± 2.6 |
| Statistical analyses | No effect of group. All t ≤ 1.43, all p ≥ 0.14 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrini, S.; Bevacqua, N.; Cataneo, A.; Chiappini, E.; Fiori, F.; Battaglia, S.; Romei, V.; Avenanti, A. Neurophysiological Markers of Premotor–Motor Network Plasticity Predict Motor Performance in Young and Older Adults. Biomedicines 2023, 11, 1464. https://doi.org/10.3390/biomedicines11051464
Turrini S, Bevacqua N, Cataneo A, Chiappini E, Fiori F, Battaglia S, Romei V, Avenanti A. Neurophysiological Markers of Premotor–Motor Network Plasticity Predict Motor Performance in Young and Older Adults. Biomedicines. 2023; 11(5):1464. https://doi.org/10.3390/biomedicines11051464
Chicago/Turabian StyleTurrini, Sonia, Naomi Bevacqua, Antonio Cataneo, Emilio Chiappini, Francesca Fiori, Simone Battaglia, Vincenzo Romei, and Alessio Avenanti. 2023. "Neurophysiological Markers of Premotor–Motor Network Plasticity Predict Motor Performance in Young and Older Adults" Biomedicines 11, no. 5: 1464. https://doi.org/10.3390/biomedicines11051464
APA StyleTurrini, S., Bevacqua, N., Cataneo, A., Chiappini, E., Fiori, F., Battaglia, S., Romei, V., & Avenanti, A. (2023). Neurophysiological Markers of Premotor–Motor Network Plasticity Predict Motor Performance in Young and Older Adults. Biomedicines, 11(5), 1464. https://doi.org/10.3390/biomedicines11051464









