Development of a Decision Support Tool for Anticoagulation in Critically Ill Patients Admitted for SARS-CoV-2 Infection: The CALT Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Definitions
2.3. Ethics Statement
2.4. Statistical Analysis
2.4.1. Exploratory Analysis
2.4.2. Data Imputation
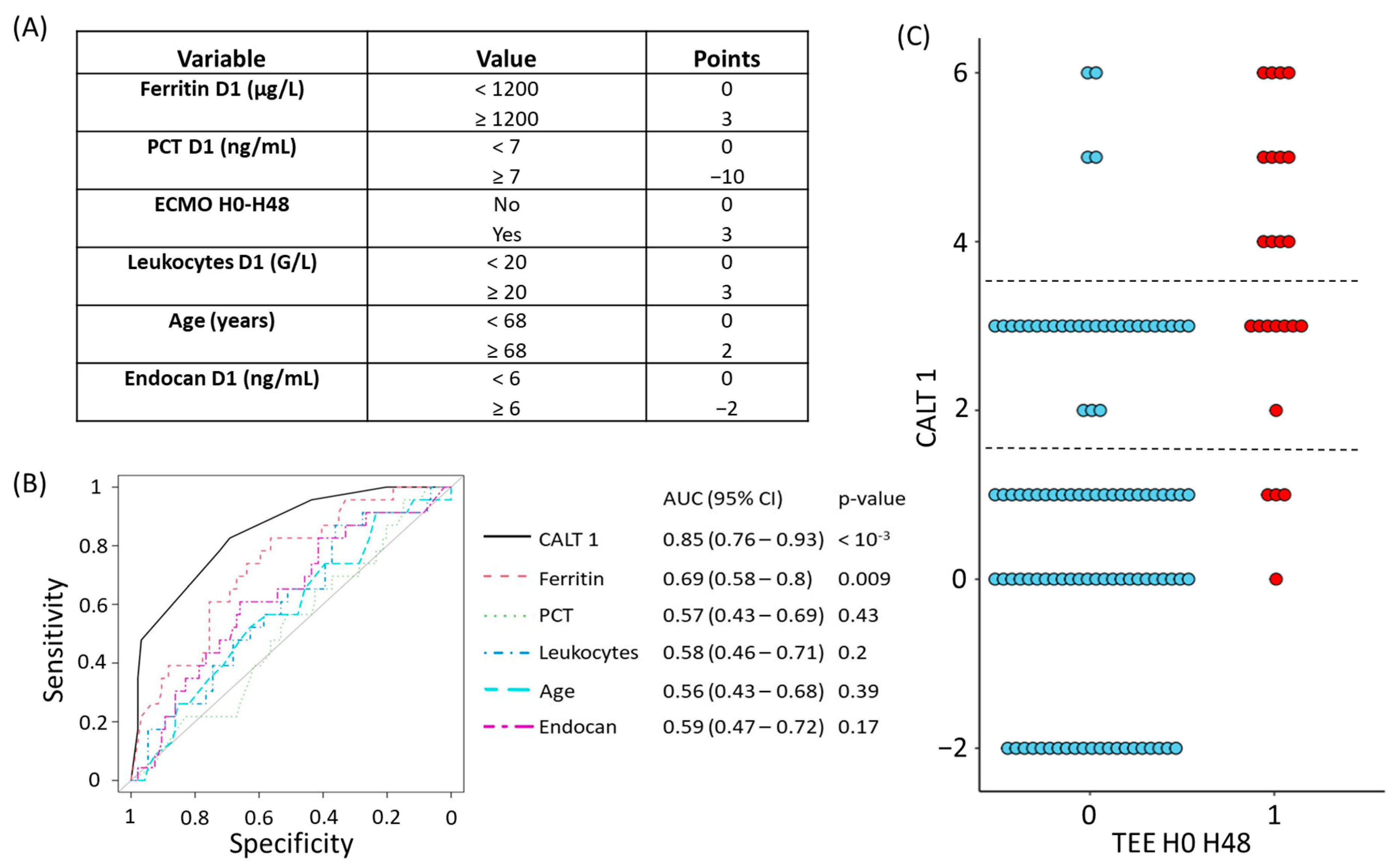
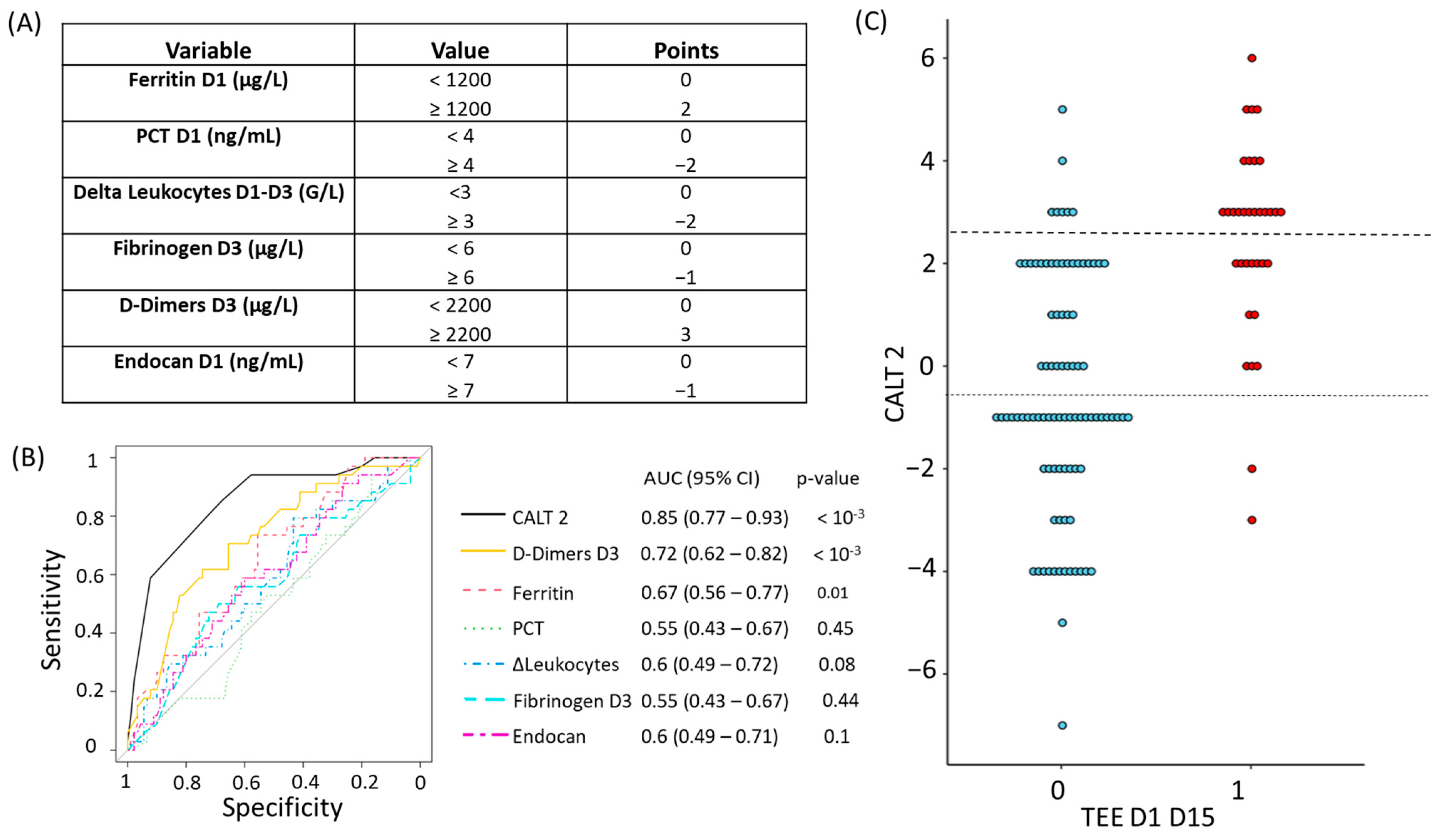
2.4.3. Derivation of CALT 1 and CALT 2
3. Results
3.1. Patients’ Characteristics according to the Presence or Absence of TEEs
3.2. Construction of CALT 1 and CALT 2 Scores
3.2.1. Construction and Evaluation of the CALT 1 Score
3.2.2. Construction and Evaluation of the CALT 2 Score
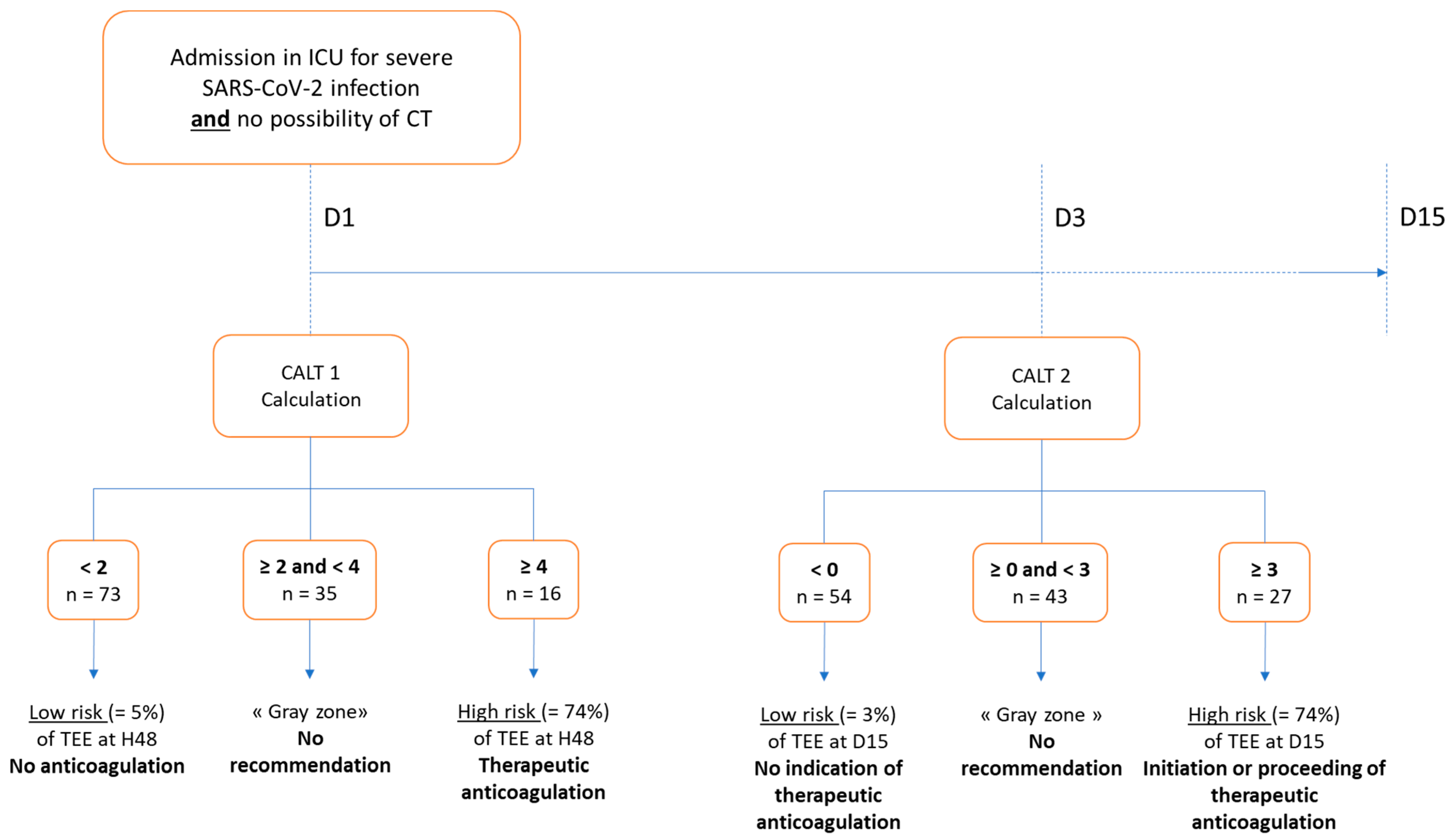
3.2.3. Proposal of a Decision Support Algorithm for Anticoagulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S.; et al. Pulmonary Embolism in Patients With COVID-19. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Remap-Cap, T.; ACTIV-4a; Investigators, A.; Zarychanski, R. Therapeutic Anticoagulation in Critically Ill Patients with COVID-19–Preliminary Report. medRxiv 2021. medRxiv:2021.03.10.21252749. [Google Scholar] [CrossRef]
- Godon, A.; Tacquard, C.A.; Mansour, A.; Garrigue, D.; Nguyen, P.; Lasne, D.; Testa, S.; Levy, J.H.; Albaladejo, P.; Gruel, Y.; et al. Prevention of Venous Thromboembolism and Haemostasis Monitoring in Patients with COVID-19: Updated Proposals (April 2021): From the French Working Group on Perioperative Haemostasis (GIHP) and the French Study Group on Thrombosis and Haemostasis (GFHT), in Collaboration with the French Society of Anaesthesia and Intensive Care (SFAR). Anaesth. Crit. Care Pain. Med. 2021, 40, 100919. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence from a Single-Centre, Cross-Sectional Study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.; Ghozlan, B.; Dupont, A.; Parmentier-Decrucq, E.; Rosa, M.; Jeanpierre, E.; Bayon, C.; Tsicopoulos, A.; Duburcq, T.; Susen, S.; et al. Derivation and Validation of a Predictive Score for Respiratory Failure Worsening Leading to Secondary Intubation in COVID-19: The CERES Score. J. Clin. Med. 2022, 11, 2172. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.E.; Kaatz, S.; Rosovsky, R.P.; Zon, R.L.; Pillai, S.; Robertson, W.E.; Elavalakanar, P.; Patell, R.; Khorana, A. COVID-19 and Venous Thromboembolism: A Narrative Review. Res. Pract. Thromb. Haemost. 2022, 6, e12666. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and Novel Biomarkers of Thrombotic Risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- De Freitas Caires, N.; Gaudet, A.; Portier, L.; Tsicopoulos, A.; Mathieu, D.; Lassalle, P. Endocan, Sepsis, Pneumonia, and Acute Respiratory Distress Syndrome. Crit. Care 2018, 22, 280. [Google Scholar] [CrossRef]
- Güzel, A.; Duran, L.; Köksal, N.; Torun, A.C.; Alaçam, H.; Ekiz, B.C.; Murat, N. Evaluation of Serum Endothelial Cell Specific Molecule-1 (Endocan) Levels as a Biomarker in Patients with Pulmonary Thromboembolism. Blood Coagul. Fibrinolysis 2014, 25, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; Redant, S.; Preseau, T.; Cismas, B.V.; Kaefer, K.; Barreto Gutierrez, L.; Anane, S.; Attou, R.; Gallerani, A.; De Bels, D. Is Endocan Correlated to ARDS Severity or an Epiphenomenon of Thrombo-Embolic Disease in COVID. Crit. Care 2021, 25, 425. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.; Chenevier-Gobeaux, C.; Parmentier, E.; Delobel, J.-E.; Dubucquoi, S.; Mathieu, D.; Lassalle, P.; De Freitas Caires, N. Endocan Is a Stable Circulating Molecule in ICU Patients. Clin. Biochem. 2017, 50, 870–877. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology 2021 Guidelines on the Use of Anticoagulation for Thromboprophylaxis in Patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef] [PubMed]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wang, H.-F.; Yin, P.; Li, D.; Wang, D.-L.; Peng, P.; Wang, W.-H.; Wang, L.; Yuan, X.-W.; Xie, J.-Y.; et al. Clinical Characteristics and Risk Factors for Symptomatic Venous Thromboembolism in Hospitalized COVID-19 Patients: A Multicenter Retrospective Study. J. Thromb. Haemost. 2021, 19, 1038–1048. [Google Scholar] [CrossRef]
- Linkins, L.-A.; Takach Lapner, S. Review of D-Dimer Testing: Good, Bad, and Ugly. Int. J. Lab. Hematol. 2017, 39, 98–103. [Google Scholar] [CrossRef]
- Keller, K.; Sagoschen, I.; Schmitt, V.H.; Sivanathan, V.; Espinola-Klein, C.; Lavie, C.J.; Münzel, T.; Hobohm, L. Obesity and Its Impact on Adverse In-Hospital Outcomes in Hospitalized Patients With COVID-19. Front. Endocrinol. 2022, 13, 876028. [Google Scholar] [CrossRef]
- Mf, Y.M.; Ms, M. Epidemiological Characteristics, Clinical Relevance, and Risk Factors of Thromboembolic Complications among Patients with COVID-19 Pneumonia at A Teaching Hospital: Retrospective Observational Study. Ann. Med. Surg. 2022, 77, 103660. [Google Scholar] [CrossRef]
- Lobbes, H.; Mainbourg, S.; Mai, V.; Douplat, M.; Provencher, S.; Lega, J.-C. Risk Factors for Venous Thromboembolism in Severe COVID-19: A Study-Level Meta-Analysis of 21 Studies. Int. J. Env. Res. Public. Health 2021, 18, 12944. [Google Scholar] [CrossRef]
- Bohula, E.A.; Berg, D.D.; Lopes, M.S.; Connors, J.M.; Babar, I.; Barnett, C.F.; Chaudhry, S.-P.; Chopra, A.; Ginete, W.; Ieong, M.H.; et al. Anticoagulation and Antiplatelet Therapy for Prevention of Venous and Arterial Thrombotic Events in Critically Ill Patients With COVID-19: COVID-PACT. Circulation 2022, 146, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Hosoya, T.; Amamiya, M.; Mitsumura, T.; Kawata, D.; Sasaki, H.; Kamiya, M.; Yamamoto, A.; Ando, T.; Shimada, S.; et al. Arterial and Venous Thrombosis Complicated in COVID-19: A Retrospective Single Center Analysis in Japan. Front. Cardiovasc. Med. 2021, 8, 767074. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, W.C.; Ramos, L.E.F.; Silva, R.T.; de Paiva, B.B.M.; Pereira, P.D.; Schwarzbold, A.V.; Garbini, A.F.; Barreira, B.S.M.; de Castro, B.M.; Ramos, C.M.; et al. Predictors of Venous Thromboembolism in COVID-19 Patients: Results of the COVID-19 Brazilian Registry. Intern. Emerg. Med. 2022, 17, 1863–1878. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Kohler, H.-P.; Dornhöfer, T.; Wallmann, D.; Lämmle, B. Accuracy of D-Dimer/Fibrinogen Ratio to Predict Pulmonary Embolism: A Prospective Diagnostic Study. J. Thromb. Haemost. 2003, 1, 708–713. [Google Scholar] [CrossRef]
- Gul, M.H.; Htun, Z.M.; de Jesus Perez, V.; Suleman, M.; Arshad, S.; Imran, M.; Vyasabattu, M.; Wood, J.P.; Anstead, M.; Morris, P.E. Predictors and Outcomes of Acute Pulmonary Embolism in COVID-19; Insights from US National COVID Cohort Collaborative. Respir. Res. 2023, 24, 59. [Google Scholar] [CrossRef]
- Carbonell, R.; Urgelés, S.; Salgado, M.; Rodríguez, A.; Reyes, L.F.; Fuentes, Y.V.; Serrano, C.C.; Caceres, E.L.; Bodí, M.; Martín-Loeches, I.; et al. Negative Predictive Value of Procalcitonin to Rule out Bacterial Respiratory Co-Infection in Critical COVID-19 Patients. J. Infect. 2022, 85, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS): The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Decavele, M.; Trosini-Désert, V.; Boussouar, S.; Duceau, B.; Dres, M.; Demoule, A. Endobronchial Ultrasound Is Feasible and Safe to Diagnose Pulmonary Embolism in Non-Transportable SARS-CoV-2 ARDS Patients Requiring Extracorporeal Lung Support. Crit. Care 2020, 24, 564. [Google Scholar] [CrossRef]
- Battistoni, I.; Francioni, M.; Morici, N.; Rubboli, A.; Podda, G.M.; Pappalardo, A.; Abdelrahim, M.E.A.; Elgendy, M.O.; Elgendy, S.O.; Khalaf, A.M.; et al. Pre- and in-Hospital Anticoagulation Therapy in Coronavirus Disease 2019 Patients: A Propensity-Matched Analysis of in-Hospital Outcomes. J. Cardiovasc. Med. 2022, 23, 264–271. [Google Scholar] [CrossRef]
- Novelli, C.; Borotto, E.; Beverina, I.; Punzi, V.; Radrizzani, D.; Brando, B. Heparin Dosage, Level, and Resistance in SARS-CoV2 Infected Patients in Intensive Care Unit. Int. J. Lab. Hematol. 2021, 43, 1284–1290. [Google Scholar] [CrossRef]
- Singson, J.R.C. Factors Associated with Severe Outcomes Among Immunocompromised Adults Hospitalized for COVID-19— COVID-NET, 10 States, March 2020–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 878–884. [Google Scholar] [CrossRef] [PubMed]
| TEE H0–H48 | p | TEE D1–D15 | p | |||
|---|---|---|---|---|---|---|
| No (n = 100) | Yes (n = 24) | No (n = 90) | Yes (n = 34) | |||
| Demographics | ||||||
| Gender (male), n (%), n′ = 124 | 68 (68) | 17 (71) | 0.98 | 60 (67) | 25 (74) | 0.60 |
| Age (years), mean (SD), n′ = 124 | 61 (11) | 62 (11) | 0.49 | 61 (11) | 61 (11) | 1 |
| Comorbidities, | ||||||
| BMI (kg/m2), mean (SD), n′ = 116 | 31 (7) | 31 (7) | 0.61 | 32 (7) | 30 (6) | 0.22 |
| BMI > 30 kg/m2, n (%), n′ = 116 | 57 (61) | 7 (32) | 0.027 | 52 (62) | 12 (38) | 0.031 |
| Diabetes, n (%), n′ = 124 | 42 (42) | 4 (17) | 0.038 | 39 (43) | 7 (21) | 0.033 |
| Chronic respiratory failure, n (%), n′ = 124 | 7 (7) | 0 (0) | 0.4 | 7 (8) | 0 (0) | 0.22 |
| COPD, n (%), n′ = 124 | 4 (4) | 1 (4) | 1 | 4 (4) | 1 (3) | 1 |
| Chronic heart failure, n (%), n′ = 124 | 7 (7) | 0 (0) | 0.4 | 7 (8) | 0 (0) | 0.22 |
| Cirrhosis (Child B or C), n (%), n′ = 124 | 1 (1) | 0 (0) | 1 | 1 (1) | 0 (0) | 1 |
| End-stage kidney disease, n (%), n′ = 124 | 4 (4) | 0 (0) | 0.72 | 4 (4) | 1 (3) | 0.5 |
| Immunocompromised, n (%), n′ = 124 | 14 (14) | 2 (8) | 0.69 | 14 (16) | 2 (6) | 0.26 |
| Antiplatelet drug prior to hospitalization, n (%), n′ = 100 | 22 (27) | 0 (0) | 0.01 | 22 (29) | 0 (0) | 0.001 |
| SAPS 2, mean (SD), n′ = 123 | 41 (14) | 43 (17) | 0.45 | 41 (14) | 42 (15) | 0.58 |
| SOFA, mean (SD), n′ = 123 | 5 (4) | 6 (4) | 0.36 | 5 (4) | 6 (4) | 0.28 |
| SARS-CoV-2 infection | ||||||
| Variant, n′ = 103 | 0.36 | 0.83 | ||||
| Wild, n (%) | 53 (63) | 13 (68) | 48 (65) | 18 (62) | ||
| Alpha, n (%) | 16 (19) | 5 (26) | 14 (19) | 7 (24) | ||
| Delta, n (%) | 15 (18) | 1 (5) | 12 (16) | 4 (14) | ||
| Vaccination status, n′ = 100 | 0.56 | 0.67 | ||||
| Not vaccinated, n (%) | 87 (91) | 21 (88) | 77 (90) | 31 (91) | ||
| Complete scheme, n (%) | 2 (2) | 0 (0) | 2 (2) | 0 (0) | ||
| Incomplete scheme, n (%) | 7 (7) | 3 (13) | 7 (8) | 3 (9) | ||
| Symptoms onset—ICU admission (days), mean (SD), n′ = 121 | 9 (6) | 9 (5) | 0.97 | 9 (5) | 9 (5) | 0.76 |
| Extension of lung injury on CT scan (%), mean (SD), n′ = 105 | 52 (21) | 57 (20) | 0.41 | 51 (21) | 58 (10) | 0.16 |
| Predominant findings in CT, n′ = 98 | 1 | 0.71 | ||||
| Consolidation, n (%) | 58 (73) | 14 (74) | 55 (71) | 20 (77) | ||
| Ground-glass opacities, n (%) | 42 (27) | 10 (26) | 35 (29) | 14 (23) | ||
| Treatments during ICU hospitalization | ||||||
| Corticosteroids, n (%), n′ = 124 | 83 (83) | 19 (80) | 0.89 | 77 (86) | 25 (74) | 0.19 |
| Remdesivir, n (%), n′ = 124 | 9 (9) | 1 (4) | 0.72 | 2 (2) | 1 (3) | 1 |
| Tocilizumab, (n%), n′ = 123 | 2 (2) | 1 (4) | 1 | 7 (8) | 3 (9) | 1 |
| HFNO H0–H48, n (%), n′ = 124 | 57 (57) | 14 (58) | 1 | 52 (58) | 19 (56) | 1 |
| CPAP H0–H48, n (%), n′ = 124 | 28 (28) | 7 (29) | 1 | 27 (30) | 8 (24) | 0.62 |
| NIV H0–H48, n (%), n′ = 124 | 25 (25) | 4 (17) | 0.55 | 22 (24) | 7 (21) | 0.83 |
| IV H0–H48, n (%), n′ = 124 | 50 (50) | 12 (50) | 1 | 45 (50) | 17 (50) | 1 |
| ECMO H0–H48, n (%), n′ = 124 | 12 (12) | 6 (25) | 0.19 | 10 (11) | 8 (24) | 0.14 |
| Prone positioning H0–H48, n (%), n′ = 100 | 19 (19) | 4 (17) | 1 | 16 (18) | 7 (21) | 0.92 |
| Antibiotherapy H0–H48, n (%), n′ = 124 | 66 (66) | 17 (71) | 0.83 | 59 (65) | 24 (71) | 0.75 |
| Anticoagulation H0–H48, n′ = 121 | <0.001 | <0.001 | ||||
| Prophylactic dose, n (%) | 60 (62) | 0 (0) | 56 (64) | 4 (12) | ||
| Intermediate dose, n (%) | 20 (21) | 0 (0) | 16 (18) | 4 (12) | ||
| Therapeutic dose, n (%) | 17 (18) | 24 (100) | 15 (17) | 26 (76) | ||
| Outcomes | ||||||
| Duration of IV (days), mean (SD), n′ = 120 | 14 (15) | 17 (20) | 0.43 | 13 (15) | 19 (18) | 0.063 |
| Duration of antibiotherapy (days), mean (SD), n′ = 123 | 11 (11) | 12 (13) | 0.76 | 10 (11) | 13 (12) | 0.27 |
| ICU length of stay (days), mean (SD), n′ = 123 | 19 (18) | 21 (22) | 0.58 | 18 (19) | 22 (20) | 0.3 |
| Mortality at D28, n (%), n′ = 124 | 34 (34) | 4 (17) | 0.16 | 31 (34) | 7 (21) | 0.2 |
| Mortality at ICU discharge, n (%, n′ = 124) | 38 (38) | 6 (25) | 0.34 | 33 (37) | 11 (32) | 0.81 |
| TEE H0–H48 | p | TEE D1–D15 | p | |||
|---|---|---|---|---|---|---|
| No (n = 100) | Yes (n = 24) | No (n = 90) | Yes (n = 34) | |||
| CRP (mg/L), median (IQR) | ||||||
| D1, n′ = 123 | 133 [68; 186] | 156 [81; 211] | 0.30 | 130 [70; 196] | 150 [83; 182] | 0.48 |
| D3, n′ = 101 | 67 [37;135] | 45 [20; 124] | 0.47 | 66 [34; 120] | 58 [36; 153] | 0.94 |
| Delta, n′ = 101 | −50 [−125; 21] | −53 [−128; 39] | 0.86 | −57 [−125; 8] | −48 [−126; 45] | 0.52 |
| Leukocytes (G/L), median (IQR) | ||||||
| D1, n′ = 124 | 9 [5.7; 12] | 10.2 [7;14] | 0.2 | 9 [6; 12] | 9 [6; 12] | 0.22 |
| D3, n′ = 107 | 10 [8; 13] | 13 [9; 15] | 0.14 | 11 [8; 13] | 11 [8; 13] | 0.49 |
| Delta, n′ = 107 | 1 [−1; 5] | 2 [−3; 3] | 0.71 | 1 [−1; 5] | 1 [−1; 5] | 0.27 |
| Lymphocytes (G/L), median (IQR) | ||||||
| D1, n′ = 98 | 0.6 [0.4; 0.9] | 0.6 [0.5; 0.9] | 0.66 | 0.6 [0.4; 0.9] | 0.6 [0.5;0.9] | 0.49 |
| D3, n′ = 64 | 0.8 [0.5; 1.1] | 0.7 [0.4; 1.2] | 0.64 | 0.8 [0.5; 1.1] | 0.6 [0.4; 0.9] | 0.071 |
| Delta, n′ = 64 | 0 [−0.2; 0.5] | 0.2 [0; 0.3] | 0.76 | 0.2 [−0.1; 0.5] | −0.1 [−0.3; 0.2] | 0.058 |
| Platelets (G/L), median (IQR) | ||||||
| D1, n′ = 97 | 237 [193; 308] | 254 [201; 319] | 0.4 | 239 [193; 306] | 251 [193; 341] | 0.45 |
| D3, n′ = 92 | 276 [222; 344] | 303 [206; 400] | 0.51 | 278 [218; 350] | 270 [213; 378] | 0.84 |
| Delta, n′ = 92 | 60 [−9; 103] | 31 [−40; 69] | 0.26 | 60 [−9; 105] | 22 [−51; 66] | 0.07 |
| Fibrinogen (g/L), median (IQR) | ||||||
| D1, n′ = 116 | 6.8 [5.9; 7.6] | 6.3 [5.3; 7.4] | 0.32 | 6.8 [5.8; 7.6] | 6.4 [5.7; 7.4] | 0.7 |
| D3, n′ = 87 | 5.8 [5.1; 7] | 5 [3.7; 5.9] | 0.006 | 5.7 [5; 7] | 5.4 [3.9; 6.2] | 0.11 |
| Delta, n′ = 87 | −0.6 [−1.4. −0.1] | −1.7 [−2.3; −0.5] | 0.006 | −0.6 [−1.4; 0.1] | −1.6 [−2.2; −0.5] | 0.02 |
| D-Dimers (µg/L), median (IQR) | ||||||
| D1, n′ = 103 | 1100 [710; 2450] | 2830 [1121; 4054] | 0.033 | 1075 [715; 2293] | 1991 [1048; 4000] | 0.025 |
| D3, n′ = 81 | 1215 [710;1957] | 3590 [1549; 4000] | 0.002 | 1176 [710; 1740] | 3795 [1537; 4000] | <0.001 |
| Delta, n′ = 81 | 0 [−575; 392] | −75 [−3089; 1348] | 0.77 | 0 [−566; 360] | −75 [−3089; 1348] | 0.81 |
| Ferritin (µg/L), median (IQR) | ||||||
| D1, n′ = 100 | 1207 [684; 2149] | 2304 [1373; 3497] | 0.006 | 1175 [675; 2175] | 1692 [1264; 3123] | 0.007 |
| D3, n′ = 74 | 1241 [882; 2161] | 2434 [1305; 3384] | 0.038 | 1223 [853; 2096] | 1468 [1115; 3210] | 0.062 |
| Delta, n′ = 74 | −197 [−787; −24] | 284 [−557; 543] | 0.12 | −227 [−801; −35] | 122 [−430; 543] | 0.057 |
| LDH (IU/L), median (IQR) | ||||||
| D1, n′ = 76 | 458 [357; 588] | 616 [490; 777] | 0.007 | 458 [356; 587] | 616 [470; 769] | 0.004 |
| D3, n′ = 59 | 443 [358; 591] | 564 [465; 676] | 0.084 | 443 [349; 575] | 564 [454; 679] | 0.045 |
| Delta, n′ = 59 | −10 [−64; 66] | −40 [−155; 23] | 0.6 | −11 [−63; 59] | −21 [−113; 34] | 0.67 |
| PCT (ng/mL), median (IQR) | ||||||
| D1, n′ = 100 | 0.3 [0.1; 1.2] | 0.2 [0.1; 0.8] | 0.71 | 0.3 [0.1; 1.2] | 0.2 [0.2; 0.7] | 0.73 |
| D3, n′ = 79 | 0.2 [0.1; 0.6] | 0.2 [0.1; 0.3] | 0.48 | 0.2 [0.1; 0.6] | 0.2 [0.1; 0.4] | 0.87 |
| Delta, n′ = 79 | −0.1 [−0.5; −0] | −0.1 [−0.3; 0] | 0.74 | −0.1 [−0.5; 0] | −0.2 [−0.5; 0] | 0.69 |
| Endocan (ng/mL), median (IQR) | ||||||
| D1, n′ = 124 | 6.7 [4; 13] | 4.8 [3.6; 10.2] | 0.17 | 6.7 [4; 14.6] | 6 [3. 11] | 0.1 |
| D3, n′ = 60 | 7.3 [4; 14] | 9.2 [4; 21] | 0.28 | 7 [4.8; 14] | 11 [5.4.;20] | 0.19 |
| Delta, n′ = 60 | 0.4 [−2; 3] | 0.3 [−1.3; 11] | 0.59 | 0 [−3.2; 2.8] | 1.8 [−0.2;7.8] | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubar, V.; Pascreau, T.; Dupont, A.; Dubucquoi, S.; Dautigny, A.-L.; Ghozlan, B.; Zuber, B.; Mellot, F.; Vasse, M.; Susen, S.; et al. Development of a Decision Support Tool for Anticoagulation in Critically Ill Patients Admitted for SARS-CoV-2 Infection: The CALT Protocol. Biomedicines 2023, 11, 1504. https://doi.org/10.3390/biomedicines11061504
Dubar V, Pascreau T, Dupont A, Dubucquoi S, Dautigny A-L, Ghozlan B, Zuber B, Mellot F, Vasse M, Susen S, et al. Development of a Decision Support Tool for Anticoagulation in Critically Ill Patients Admitted for SARS-CoV-2 Infection: The CALT Protocol. Biomedicines. 2023; 11(6):1504. https://doi.org/10.3390/biomedicines11061504
Chicago/Turabian StyleDubar, Victoria, Tiffany Pascreau, Annabelle Dupont, Sylvain Dubucquoi, Anne-Laure Dautigny, Benoit Ghozlan, Benjamin Zuber, François Mellot, Marc Vasse, Sophie Susen, and et al. 2023. "Development of a Decision Support Tool for Anticoagulation in Critically Ill Patients Admitted for SARS-CoV-2 Infection: The CALT Protocol" Biomedicines 11, no. 6: 1504. https://doi.org/10.3390/biomedicines11061504
APA StyleDubar, V., Pascreau, T., Dupont, A., Dubucquoi, S., Dautigny, A.-L., Ghozlan, B., Zuber, B., Mellot, F., Vasse, M., Susen, S., Poissy, J., & Gaudet, A. (2023). Development of a Decision Support Tool for Anticoagulation in Critically Ill Patients Admitted for SARS-CoV-2 Infection: The CALT Protocol. Biomedicines, 11(6), 1504. https://doi.org/10.3390/biomedicines11061504






