New Synthesized Activating Transcription Factor 3 Inducer SW20.1 Suppresses Resistin-Induced Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Adipocyte Differentiation
2.2. Oil Red O Staining
2.3. Quantitative Real-Time Polymerase Chain Reaction
2.4. Chromatin Immunoprecipitation Assay
2.5. Animal Studies
2.6. Glucose and Insulin Tolerance Tests
2.7. Histology, Adipocyte Size Measurement, and Adipocyte Number Estimation
2.8. Measurement of Biochemical Parameters
2.9. Measurement of the Serum Resistin Level
2.10. Statistical Analysis
3. Results
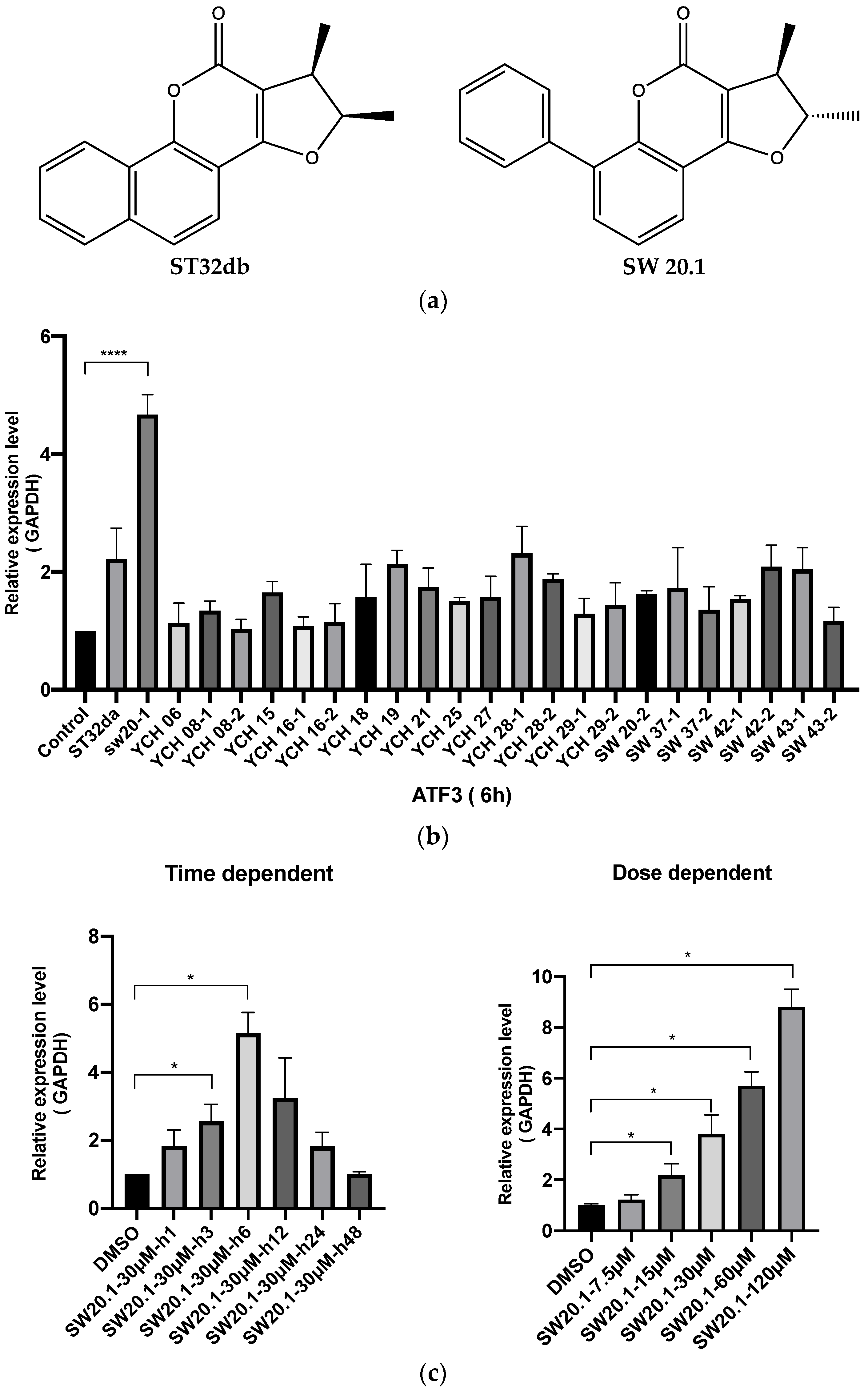
3.1. SW20.1 Induces ATF3 Expression
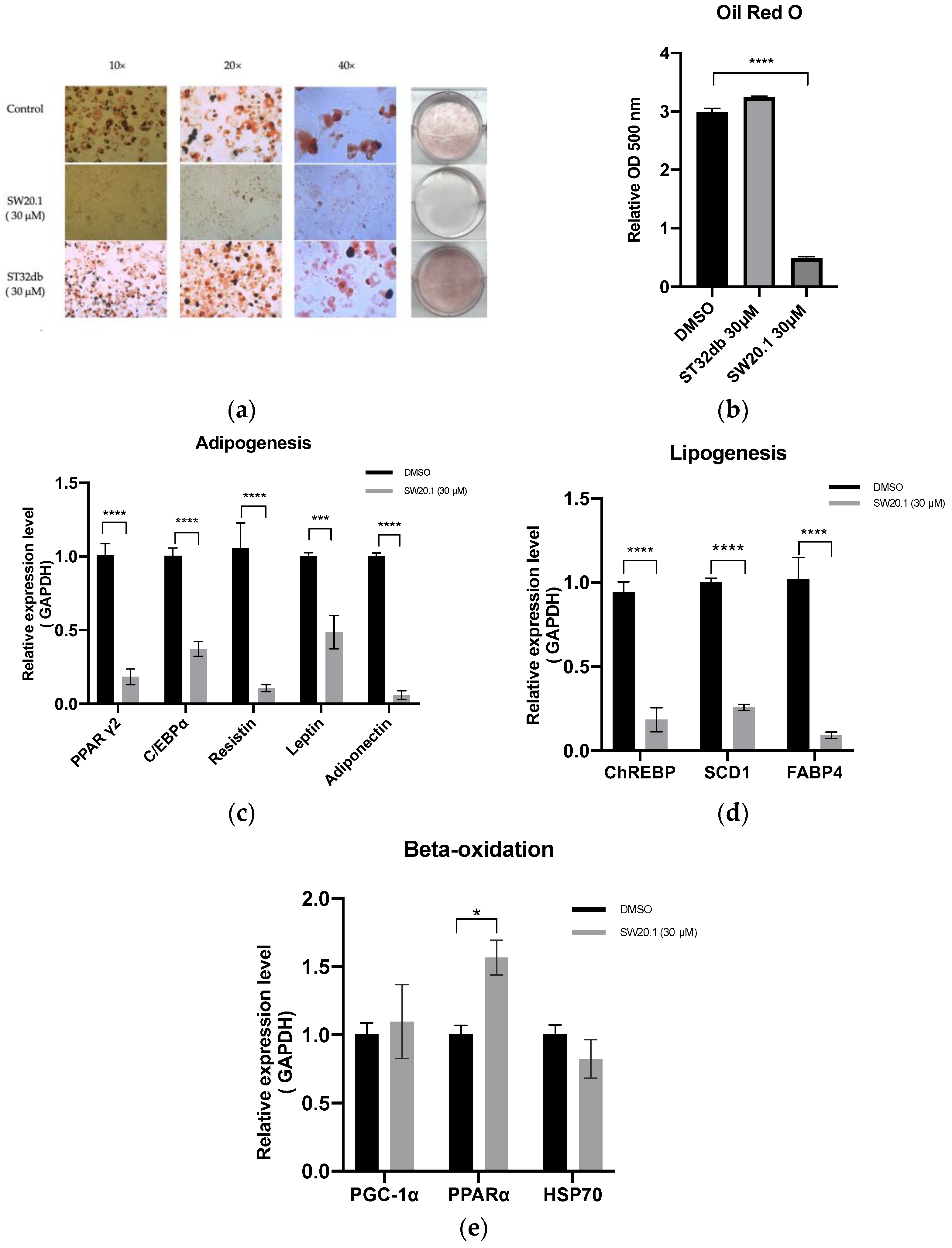
3.2. SW20.1 Suppresses 3T3-L1 Preadipocyte Differentiation through the Resistin Pathway
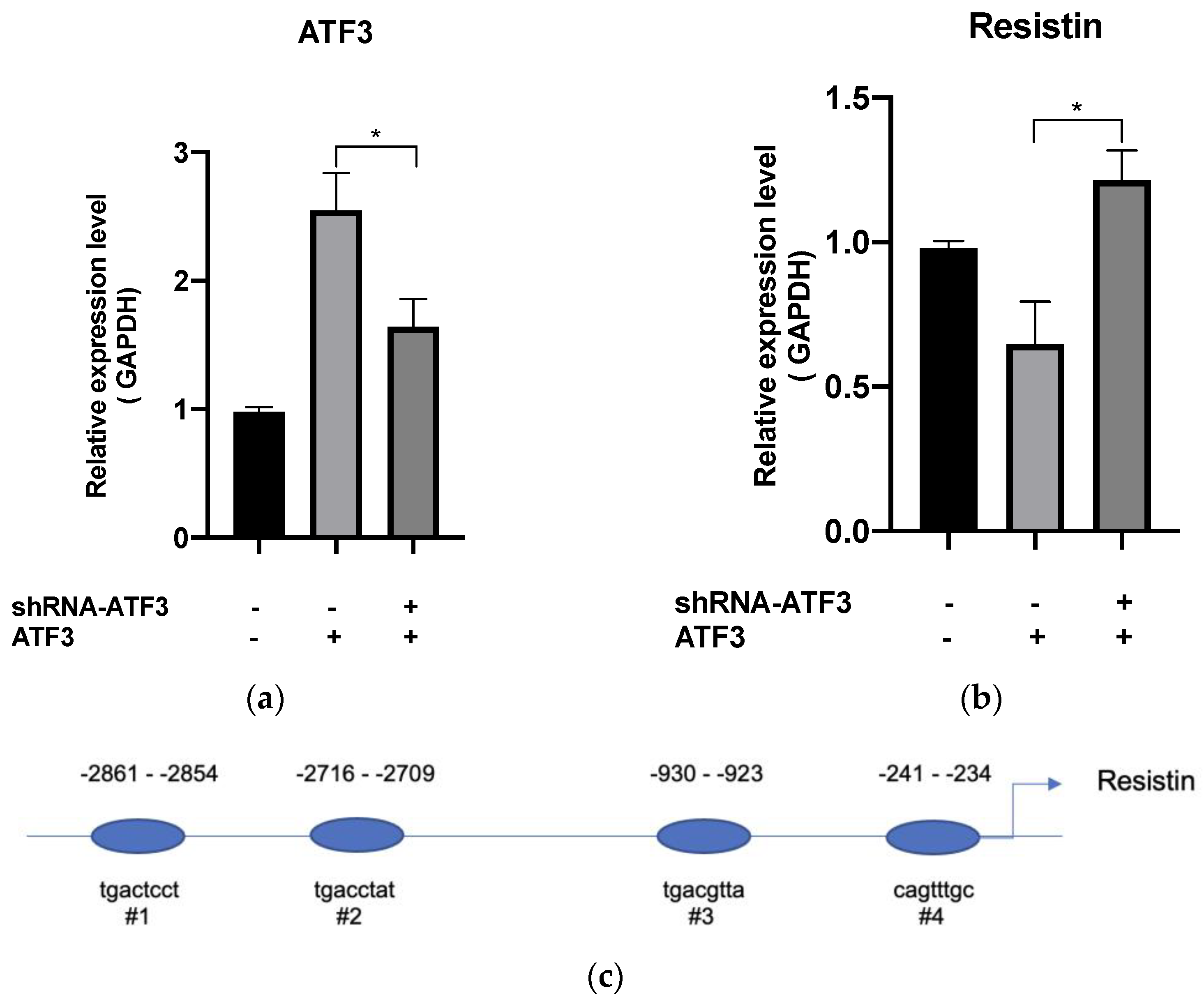
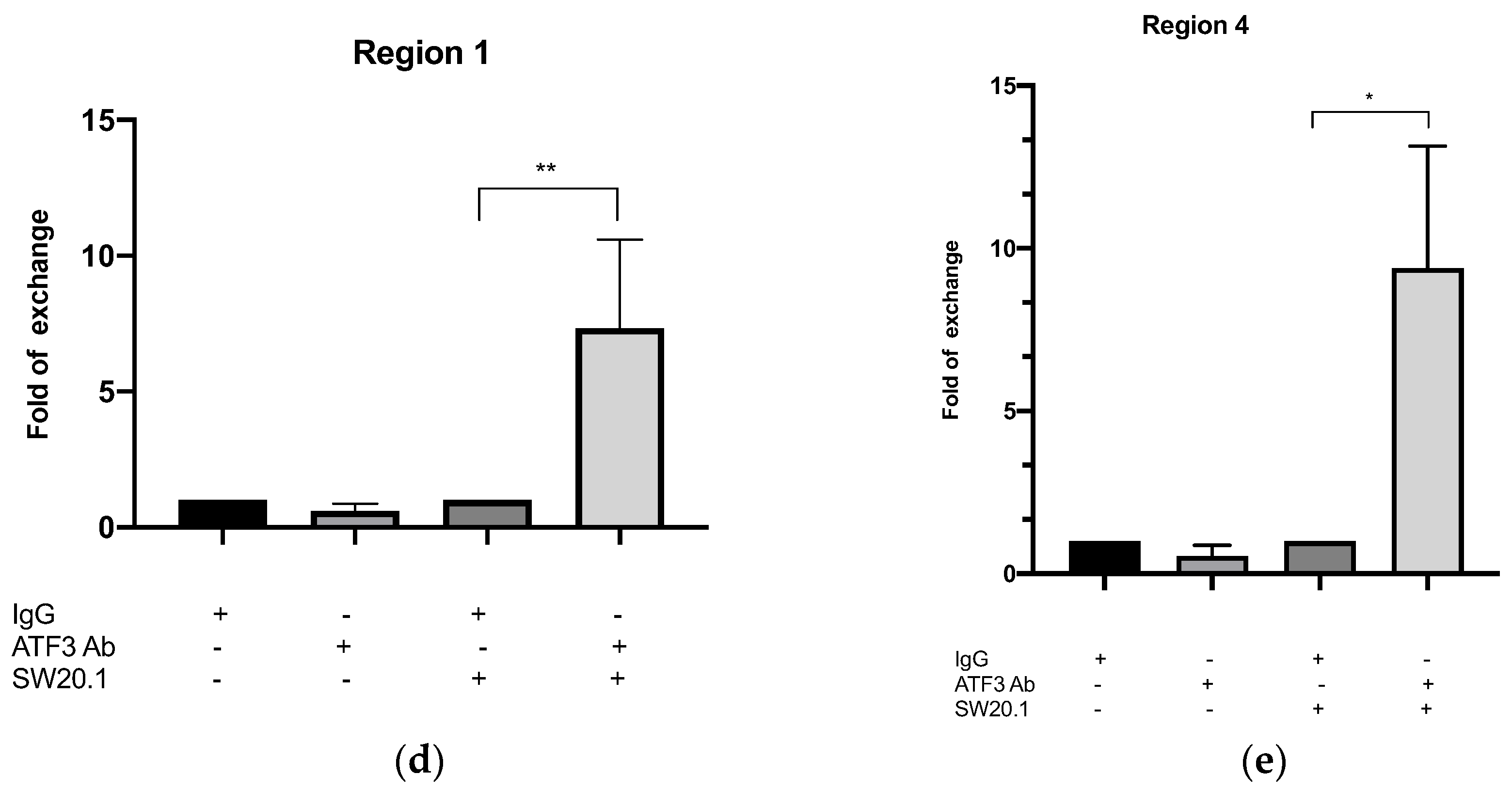
3.3. SW20.1 Inhibits Adipogenesis Directly through the ATF3-Resistin Pathway
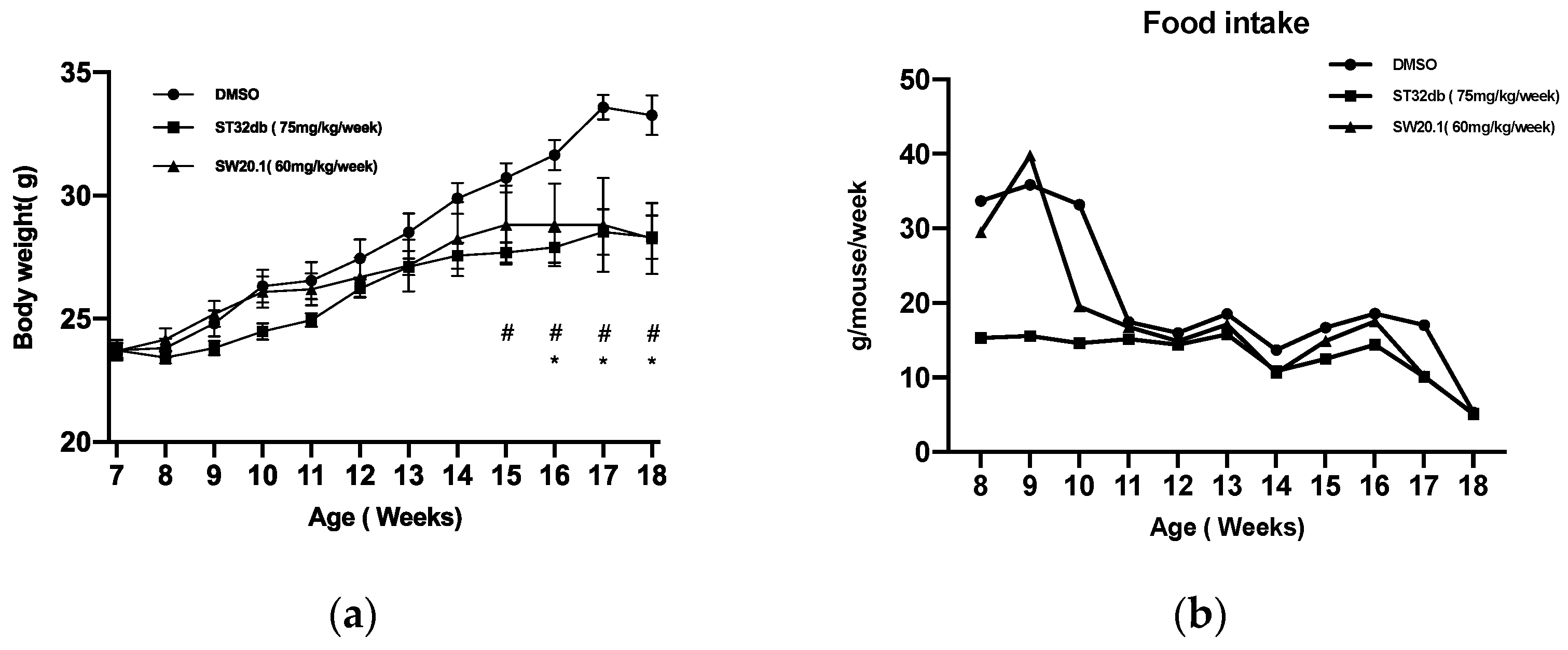
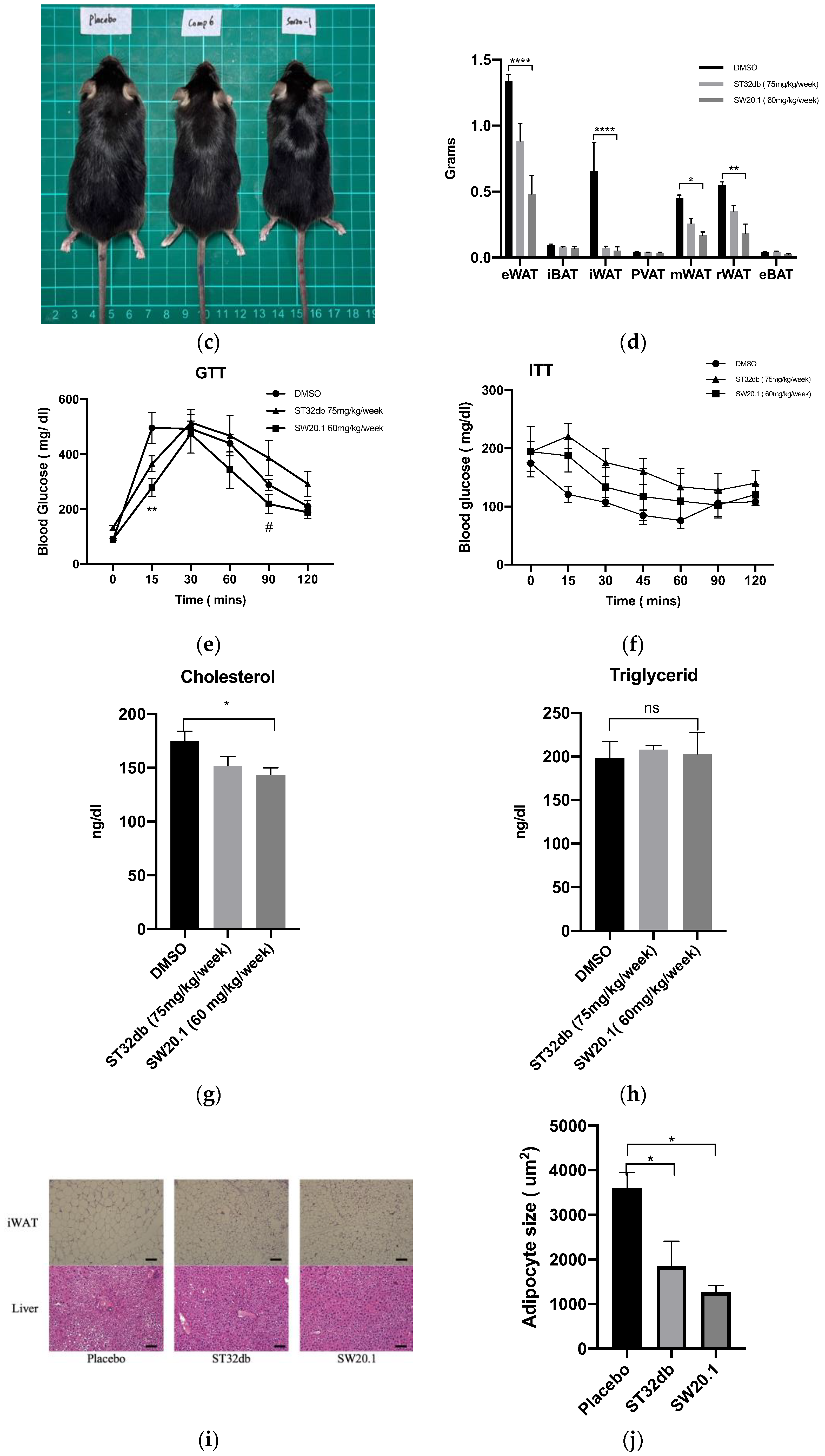
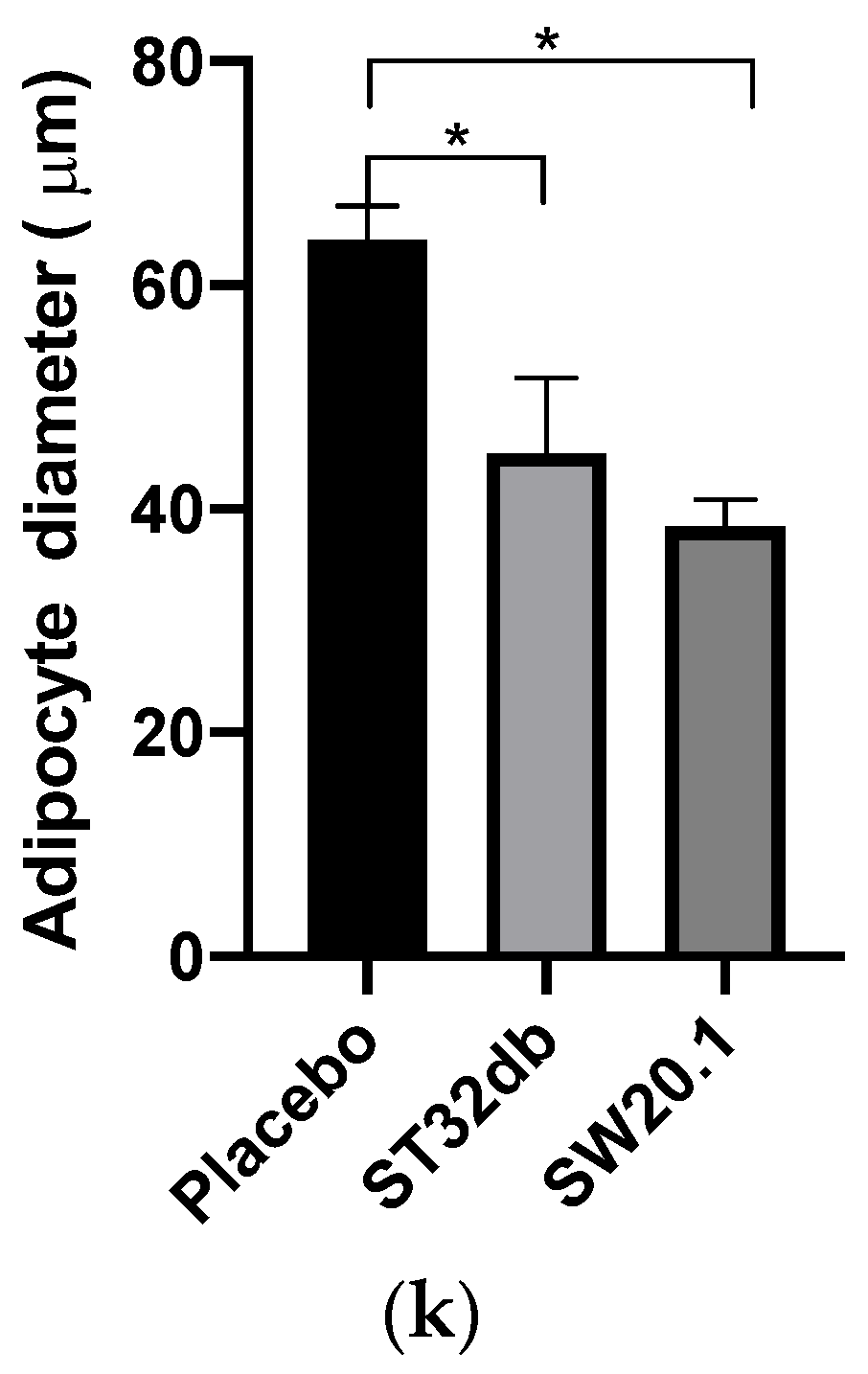
3.4. SW20.1 Ameliorates Obesity and Obesity-Related Metabolic Syndrome in Mice
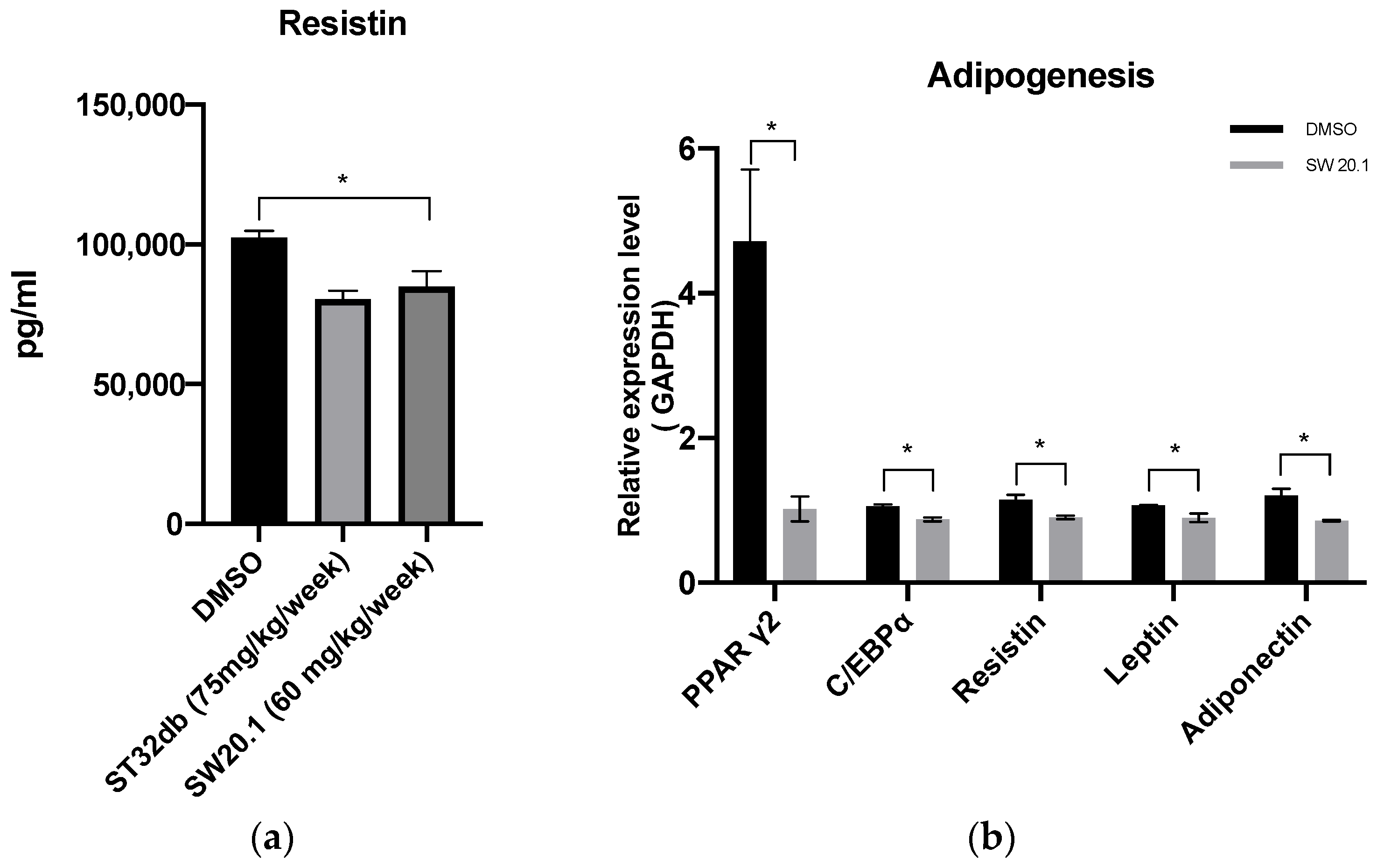
3.5. SW20.1 Treatment Reduces Serum Resistin Levels to Inhibit Adipogenesis in HFD-Fed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Yanovski, S.Z.; Carroll, M.D.; Flegal, K.M. The Epidemiology of Obesity. Gastroenterology 2007, 132, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, M.-Y.; Yoon, M. The anti-angiogenic herbal extracts Ob-X from Morus alba, Melissa officinalis, and Artemisia capillaris suppresses adipogenesis in 3T3-L1 adipocytes. Pharm. Biol. 2011, 49, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J.; Muruzábal, F.J.; Burrell, M.A. The adipocyte: A model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E827–E847. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green Tea Polyphenol Epigallocatechin Gallate Inhibits Adipogenesis and Induces Apoptosis in 3T3-L1 Adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Taouis, M.; Benomar, Y. Is resistin the master link between inflammation and inflammation-related chronic diseases? Mol. Cell. Endocrinol. 2021, 533, 111341. [Google Scholar] [CrossRef]
- Aboudeya, H.M.; Shaker, S.A.; Salama, M. Effect of short-term high fat diet on resistin levels and expression of autophagy-related genes in the cartilage of male rats. Sci. Rep. 2022, 12, 15313. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.; Wang, S.; Gan, Y.; Chen, X.; Tao, Y.; Gao, J. Alteration of Peripheral Resistin and the Severity of Acute Pancreatitis: A Meta-Analysis. Front. Med. 2022, 9, 915152. [Google Scholar] [CrossRef]
- Kukla, A.; Piotrowska, K.; Misiek, M.; Chudecka-Glaz, A.M. Role of adipokines in ovarian cancer epidemiology and prognosis. Ginekol. Pol. 2022, 93, 496–500. [Google Scholar] [CrossRef]
- Nieva-Vazquez, A.; Pérez-Fuentes, R.; Torres-Rasgado, E.; López-López, J.G.; Romero, J.R. Serum resistin levels are associated with adiposity and insulin sensitivity in obese Hispanic subjects. Metab. Syndr. Relat. Disord. 2014, 12, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.Y.; Kim, J.-H.; Jung, M.H. Anti-Obesity Effects of Tanshinone I from Salvia miltiorrhiza Bunge in Mice Fed a High-Fat Diet through Inhibition of Early Adipogenesis. Nutrients 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-M.; Xu, S.-W.; Liu, P.-Q. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Jung, I.; Kim, H.; Moon, S.; Lee, H.; Kim, B. Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action. Antioxidants 2020, 9, 857. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, C.W.T.; Woo, K.-S.; Fung, K.-P. Chemistry and Biological Activities of Caffeic Acid Derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological activity and mechanism of Tanshinone IIA in related diseases. Drug Des. Dev. Ther. 2020, 14, 4735. [Google Scholar] [CrossRef]
- Park, Y.-K.; Obiang-Obounou, B.W.; Lee, J.; Lee, T.-Y.; Bae, M.-A.; Hwang, K.-S.; Lee, K.-B.; Choi, J.-S.; Jang, B.-C. Anti-Adipogenic Effects on 3T3-L1 Cells and Zebrafish by Tanshinone IIA. Int. J. Mol. Sci. 2017, 18, 2065. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, C.; Sheng, X.; Zhang, Y.; Li, Q.; Wang, M.-W.; Peng, L.; Zang, Y.Q. The Role of Tanshinone IIA in the Treatment of Obesity through Peroxisome Proliferator-Activated Receptor γ Antagonism. Endocrinology 2009, 150, 104–113. [Google Scholar] [CrossRef]
- Montminy, M.R.; Bilezikjian, L.M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 1987, 328, 175–178. [Google Scholar] [CrossRef]
- Deutsch, P.J.; Hoeffler, J.; Jameson, J.L.; Lin, J.; Habener, J.F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J. Biol. Chem. 1988, 263, 18466–18472. [Google Scholar] [CrossRef]
- Chen, B.; Liang, G.; Whelan, J.; Hai, T. ATF3 and ATF3 delta Zip. Transcriptional repression versus activation by alternatively spliced isoforms. J. Biol. Chem. 1994, 269, 15819–15826. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.; Curran, T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA 1991, 88, 3720–3724. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Wolfgang, C.D.; Chen, B.P.; Chen, T.-H.; Hai, T. ATF3 Gene: GENOMIC ORGANIZATION, PROMOTER, AND REGULATION (∗). J. Biol. Chem. 1996, 271, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Roesler, W.; Vandenbark, G.; Hanson, R. Cyclic AMP and the induction of eukaryotic gene transcription. J. Biol. Chem. 1988, 263, 9063–9066. [Google Scholar] [CrossRef]
- Jang, M.K.; Jung, M.H. ATF3 represses PPARγ expression and inhibits adipocyte differentiation. Biochem. Biophys. Res. Commun. 2014, 454, 58–64. [Google Scholar] [CrossRef]
- Jang, M.K.; Kim, C.H.; Seong, J.K.; Jung, M.H. ATF3 inhibits adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2012, 421, 38–43. [Google Scholar] [CrossRef]
- Cheng, C.F.; Ku, H.C.; Cheng, J.J.; Chao, S.W.; Li, H.F.; Lai, P.F.; Chang, C.C.; Don, M.J.; Chen, H.H.; Lin, H. Adipocyte browning and resistance to obesity in mice is induced by expression of ATF3. Commun. Biol. 2019, 2, 389. [Google Scholar] [CrossRef]
- Kim, S.; Song, N.J.; Bahn, G.; Chang, S.H.; Yun, U.J.; Ku, J.M.; Jo, D.G.; Park, K.W. Atf3 induction is a therapeutic target for obesity and metabolic diseases. Biochem. Biophys. Res. Commun. 2018, 504, 903–908. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Lin, H.; Li, H.-F.; Chen, H.-H.; Hung, H.-Y. Exploration and biological evaluation of 7-methoxy-3-methyl-1H-chromeno[4,3-c]pyrazol-4-one as an activating transcription factor 3 inducer for managing metabolic syndrome. Eur. J. Med. Chem. 2023, 246, 114951. [Google Scholar] [CrossRef]
- Galarraga, M.; Campión, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-de-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tsuchiya, H.; Hama, S.; Kajimoto, K.; Kogure, K. Resistin affects lipid metabolism during adipocyte maturation of 3T3-L1 cells. FEBS J. 2013, 280, 5884–5895. [Google Scholar] [CrossRef]
- van Raalte, D.H.; Li, M.; Pritchard, P.H.; Wasan, K.M. Peroxisome proliferator-activated receptor (PPAR)-alpha: A pharmacological target with a promising future. Pharm. Res. 2004, 21, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, X.; Li, Y.; Zhang, S.; Cai, X.; Zhang, R.; Gong, S.; Han, X.; Ji, L. Serum leptin, resistin, and adiponectin levels in obese and non-obese patients with newly diagnosed type 2 diabetes mellitus: A population-based study. Medicine 2020, 99, e19052. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Thomas, T.C.; Storlien, L.H.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. 2000, 24, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lin, H.; Li, H.-F.; Don, M.-J.; King, P.-C.; Chen, H.-H. Salvia miltiorrhiza Extract and Individual Synthesized Component Derivatives Induce Activating-Transcription-Factor-3-Mediated Anti-Obesity Effects and Attenuate Obesity-Induced Metabolic Disorder by Suppressing C/EBPα in High-Fat-Induced Obese Mice. Cells 2022, 11, 1022. [Google Scholar]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid. Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef]
- Wen, F.; Shi, Z.; Liu, X.; Tan, Y.; Wei, L.; Zhu, X.; Zhang, H.; Zhu, X.; Meng, X.; Ji, W.; et al. Acute Elevated Resistin Exacerbates Mitochondrial Damage and Aggravates Liver Steatosis Through AMPK/PGC-1α Signaling Pathway in Male NAFLD Mice. Horm. Metab. Res. 2021, 53, 132–144. [Google Scholar] [CrossRef]
- Bae, Y.A.; Cheon, H.G. Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages. Korean J. Physiol. Pharmacol. 2016, 20, 415–424. [Google Scholar] [CrossRef]
- Martín-Fernández, M.; Valencia, K.; Zandueta, C.; Ormazábal, C.; Martínez-Canarias, S.; Lecanda, F.; de la Piedra, C. The Usefulness of Bone Biomarkers for Monitoring Treatment Disease: A Comparative Study in Osteolytic and Osteosclerotic Bone Metastasis Models. Transl. Oncol. 2017, 10, 255–261. [Google Scholar] [CrossRef] [PubMed]
- How Glucose Gets into Cells. Nutr. Rev. 1990, 48, 357–358. [CrossRef]
- Gallou-Kabani, C.; Vigé, A.; Gross, M.S.; Rabès, J.P.; Boileau, C.; Larue-Achagiotis, C.; Tomé, D.; Jais, J.P.; Junien, C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity 2007, 15, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.; Wolfgang, C.D.; Marsee, D.K.; Allen, A.E.; Sivaprasad, U. ATF3 and stress responses. Gene Expr. 1999, 7, 321–335. [Google Scholar]
- Takeda, M.; Kato, H.; Takamiya, A.; Yoshida, A.; Kiyama, H. Injury-Specific Expression of Activating Transcription Factor-3 in Retinal Ganglion Cells and Its Colocalized Expression with Phosphorylated c-Jun. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2412–2421. [Google Scholar]
- Li, H.-F.; Cheng, C.-F.; Liao, W.-J.; Lin, H.; Yang, R.-B. ATF3-mediated epigenetic regulation protects against acute kidney injury. J. Am. Soc. Nephrol. 2010, 21, 1003–1013. [Google Scholar] [CrossRef]
- Chung, S.; Choi, H.; Kim, K.; Cho, Y.; Lee, H.; Park, K. Regulation of human resistin gene expression in cell systems: An important role of stimulatory protein 1 interaction with a common promoter polymorphic site. Diabetologia 2005, 48, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Kiryu-Seo, S.; Kato, R.; Ogawa, T.; Nakagomi, S.; Nagata, K.; Kiyama, H. Neuronal injury-inducible gene is synergistically regulated by ATF3, c-Jun, and STAT3 through the interaction with Sp1 in damaged neurons. J. Biol. Chem. 2008, 283, 6988–6996. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strainsof mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Lutz, T.A.; Woods, S.C. Overview of Animal Models of Obesity. Curr. Protoc. Pharmacol. 2012, 58, 5–61. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ATF3 | CTCCTGGGTCACTGGTATTTG | CCGATGGCAGAGGTGTTTAT |
| C/EBPα | GTAACCTTGTGCCTTGGATACT | GGAAGCAGGAATCCTCCAAATA |
| PPARγ2 | CTGGCCTCCCTGATGAATAAA | AGGCTCCATAAAGTCACCAAAG |
| Adiponectin | AAGGGCTCAGGATGCTACTGTT | AGTAACGTCATCTTCGGCATGA |
| Resistin | CTAAGCTGAGGGTCTGGAAATG | CACACACCCTTCTCCACTAAAG |
| Leptin | GGTTGATCTCACAATGCGTTTC | TGGGAGACAGGGTTCTACTT |
| ChREBP | TGTTCAGCATCCTCATCCGACCTT | TGAGTTGGCGAAGGGAATTCAGGA |
| SCD1 | TGGGTTGGCTGCTTGTG | GCGTGGGCAGGATGAAG |
| FABP4 | GCTCCTCCTCGAAGGTTTAC | CCCACTCCCACTTCTTTCAT |
| GAPDH | GGAGCCAAACGGGTCATCATCTC | GAGGGGCCATCCACAGTCTTCT |
| HSP70 | TGGTGCTGACGAAGATGAAG | CGCTGAGAGTCGTTGAAGTAG |
| PGC1α | CTAGCCATGGATGGCCTATTT | GTCTCGACACGGAGAGTTAAAG |
| PPARα | ACCACTACGGAGTTCACGCATG | GAATCTTGCAGCTCCGATCACAC |
| Primer 1 | ATCTGTTTATCTGCTGGTTCCAT | ACATGCACATGTGCACGTGTGT |
| Primer 2 | TGCTTTAATTCCTTTGCTGTGT | AGAGCTAGCAAGATGGTTTGCTG |
| Primer 3 | TAATCTCAATTTTGTCCTATC | AAACATTATTTAGTCATTATTGC |
| Primer 4 | GCAAGGGAGCAGTTGACTAGAT | GGACGTTGTCTGGAGATAACACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.; Huang, W.-J.; Lin, H.; Chen, H.-H. New Synthesized Activating Transcription Factor 3 Inducer SW20.1 Suppresses Resistin-Induced Metabolic Syndrome. Biomedicines 2023, 11, 1509. https://doi.org/10.3390/biomedicines11061509
Tran TT, Huang W-J, Lin H, Chen H-H. New Synthesized Activating Transcription Factor 3 Inducer SW20.1 Suppresses Resistin-Induced Metabolic Syndrome. Biomedicines. 2023; 11(6):1509. https://doi.org/10.3390/biomedicines11061509
Chicago/Turabian StyleTran, Tu T., Wei-Jan Huang, Heng Lin, and Hsi-Hsien Chen. 2023. "New Synthesized Activating Transcription Factor 3 Inducer SW20.1 Suppresses Resistin-Induced Metabolic Syndrome" Biomedicines 11, no. 6: 1509. https://doi.org/10.3390/biomedicines11061509






