Cell-Free Supernatant Derived from a Lactobacillus casei BL23 Culture Modifies the Antiviral and Immunomodulatory Capacity of Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
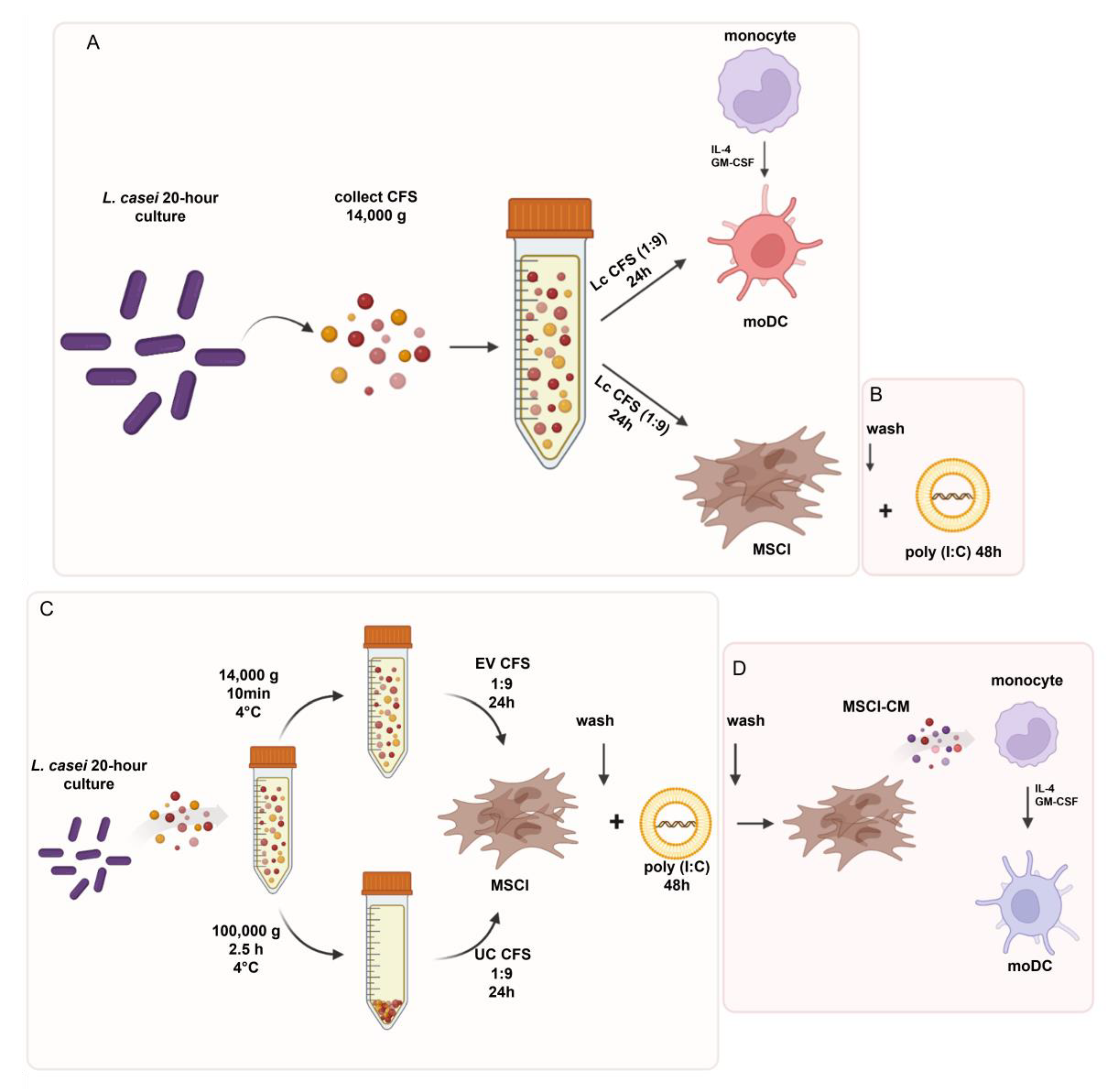
2.1. Preparation of Cell-Free Supernatants (CFS) from Lactobacillus Strains
2.2. Generation and Maintenance of Human moDC Cultures
2.3. Maintenance of the Mesenchymal Stromal Cell-like Cell Line
2.4. Experimental Models
2.5. Flow Cytometry
2.6. Measurement of the Cytokine Concentrations via Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. RNA Isolation, DNase Treatment, Reverse Transcription, and Real-Time Quantitative Polymerase Chain Reaction (PCR)
2.8. Statistical Analysis
3. Results
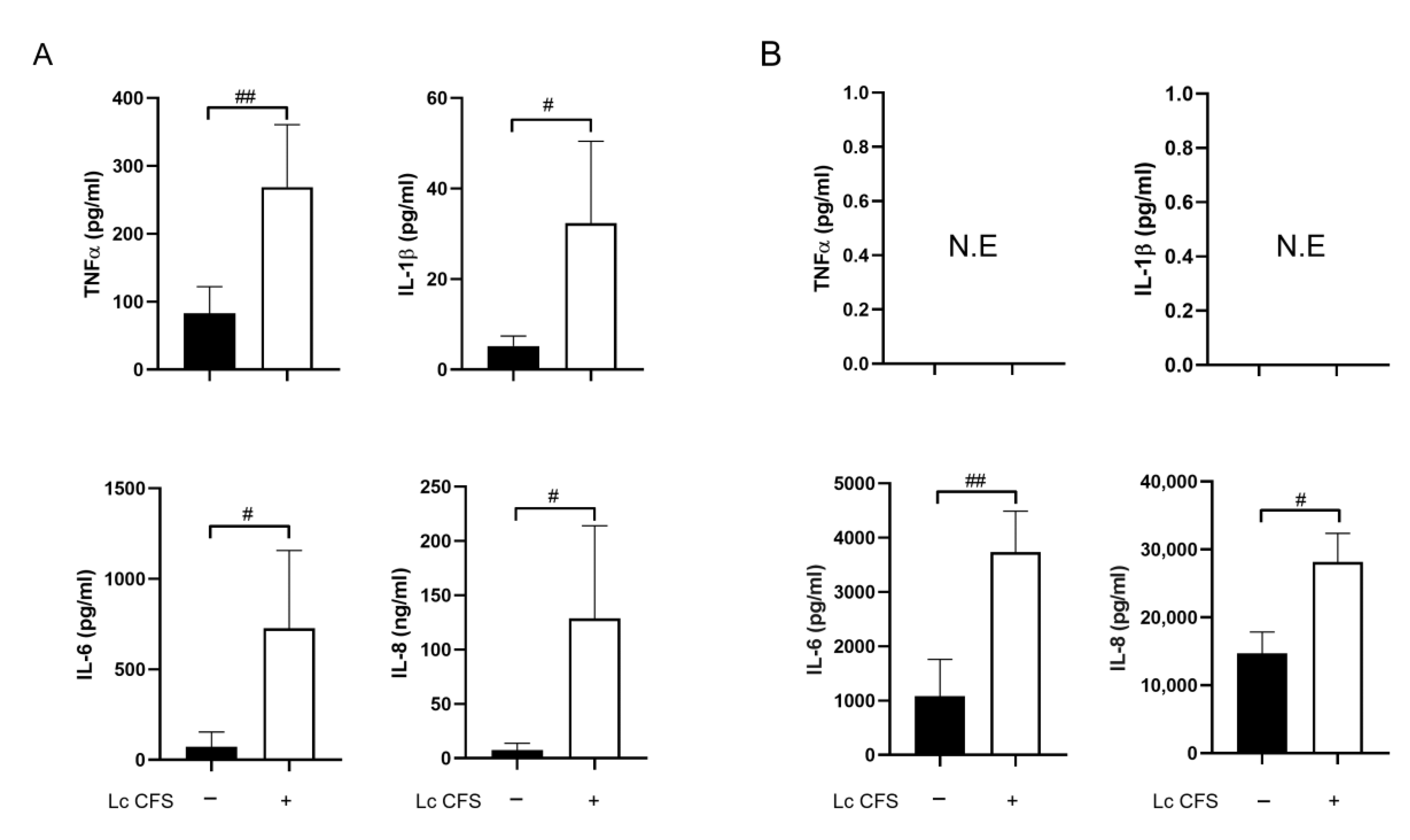
3.1. L. casei CFS Induces Inflammatory Cytokine Production by moDCs while Triggering IL-6 and IL-8 Production by MSCl Cells
3.2. L. casei CFS Enhances the Sensitivity of MSCl Cells to Viral Stimulus and Induces the Increased mRNA Expression of Antiviral IFNβ Cytokine
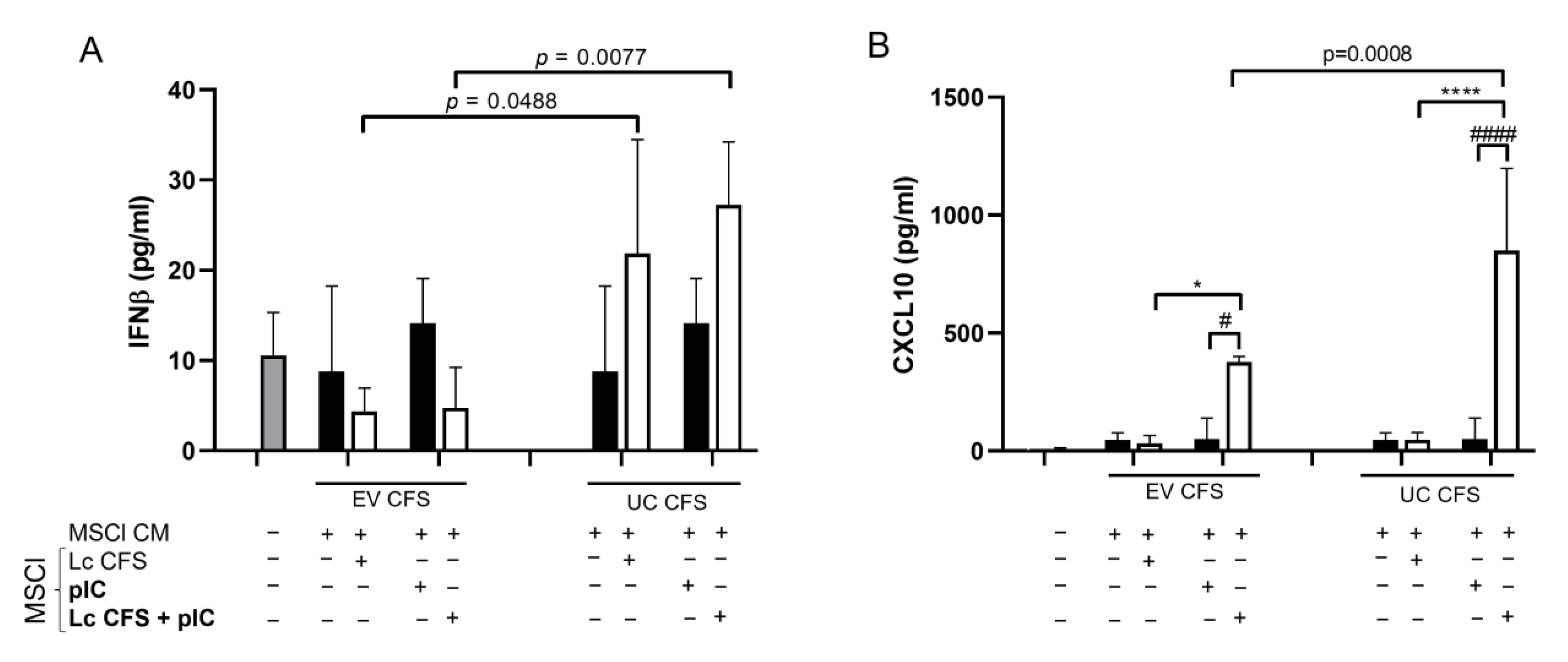
3.3. L. casei-Derived EVs Alter IFNβ and CXCL10 Production by MSCl Cells
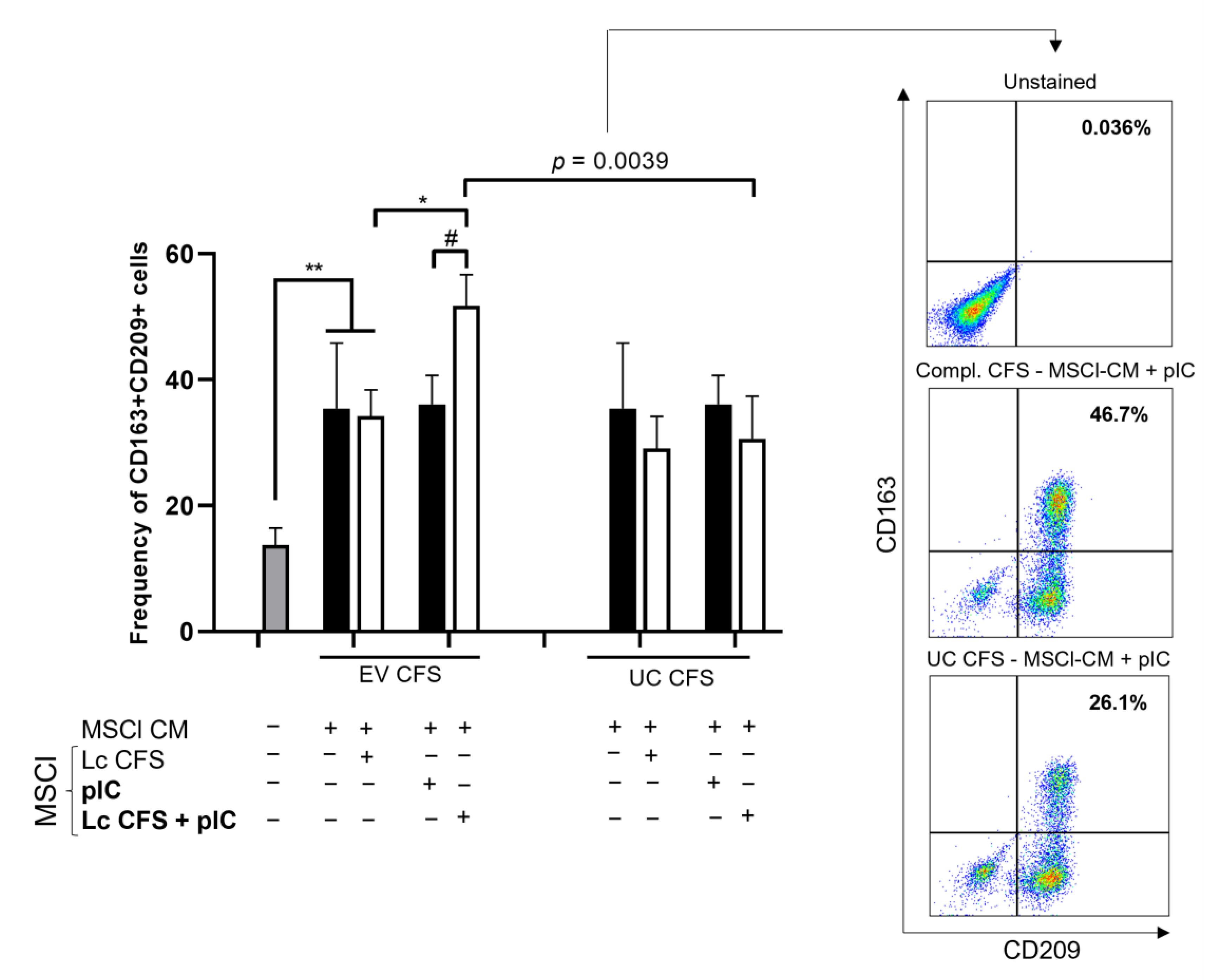
3.4. MSCl-Modulated moDC Differentiation Is Influenced by Lactobacillus-Derived Metabolites
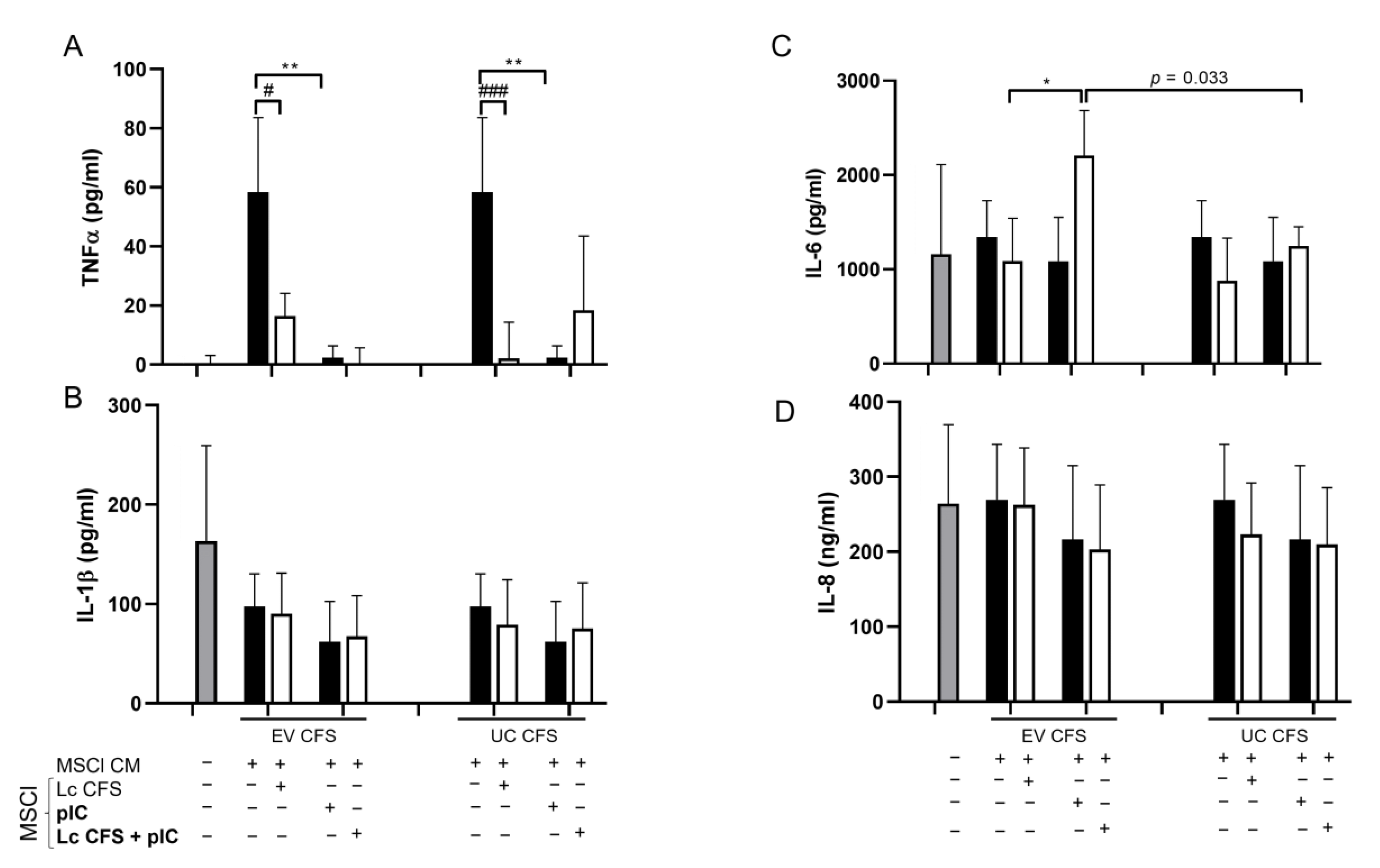
3.5. Cytokine/Chemokine Production by moDCs Is Indirectly Affected by MSCl Cells Exposed to Various Microenvironmental Signals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, N.; Bosco-Levy, P.; Thurin, N.; Blin, P.; Droz-Perroteau, C. NSAIDs and COVID-19: A Systematic Review and Meta-analysis. Drug Saf. 2021, 44, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Capuano, A.; Scavone, C.; Racagni, G.; Scaglione, F. NSAIDs in patients with viral infections, including COVID-19: Victims or perpetrators? Pharmacol. Res. 2020, 157, 104849. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in autoimmune and inflammatory disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Lamas, A.; Mondragón, A.D.C.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic effects against virus infections: New weapons for an old war. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front. Cell. Infect. Microbiol. 2021, 10, 596166. [Google Scholar] [CrossRef] [PubMed]
- Bene, K.P.; Kavanaugh, D.W.; Leclaire, C.; Gunning, A.P.; MacKenzie, D.A.; Wittmann, A.; Young, I.D.; Kawasaki, N.; Rajnavolgyi, E.; Juge, N. Lactobacillus reuteri surface mucus adhesins upregulate inflammatory responses through interactions with innate C-Type lectin receptors. Front. Microbiol. 2017, 8, 321. [Google Scholar] [CrossRef]
- Tóth, M.; Muzsai, S.; Regulski, K.; Szendi-Szatmári, T.; Czimmerer, Z.; Rajnavölgyi, É.; Chapot-Chartier, M.P.; Bácsi, A. The Phagocytosis of Lacticaseibacillus casei and Its Immunomodulatory Properties on Human Monocyte-Derived Dendritic Cells Depend on the Expression of Lc-p75, a Bacterial Peptidoglycan Hydrolase. Int. J. Mol. Sci. 2022, 23, 7620. [Google Scholar] [CrossRef]
- Lasaviciute, G.; Barz, M.; van der Heiden, M.; Arasa, C.; Tariq, K.; Quin, J.; Östlund Farrants, A.K.; Sverremark-Ekström, E. Gut commensal Limosilactobacillus reuteri induces atypical memory-like phenotype in human dendritic cells in vitro. Gut Microbes 2022, 14, 2045046. [Google Scholar] [CrossRef]
- Engevik, M.A.; Ruan, W.; Esparza, M.; Fultz, R.; Shi, Z.; Engevik, K.A.; Engevik, A.C.; Ihekweazu, F.D.; Visuthranukul, C.; Venable, S.; et al. Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites. Physiol. Rep. 2021, 9, e14719. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Lactobacillus reuteri extracts promoted wound healing via PI3K/AKT/β-catenin/TGFβ1 pathway. Stem Cell Res. Ther. 2019, 10, 243. [Google Scholar] [CrossRef]
- Cai, C.; Chen, D.Z.; Ge, L.C.; Chen, W.K.; Ye, S.S.; Ye, W.W.; Tao, Y.; Wang, R.; Li, J.; Lin, Z.; et al. Synergistic effects of Lactobacillus rhamnosus culture supernatant and bone marrow mesenchymal stem cells on the development of alcoholic steatohepatitis in mice. Am. J. Transl. Res. 2019, 11, 5703–5715. [Google Scholar] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Correction to: Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal stromal cells. Curr. Opin. Hematol. 2006, 13, 419–425. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef]
- Gieseke, F.; Böhringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Müller, I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Wang, F.; Li, G.; Zhang, L.; Luan, X. Expression and biological function of programmed death ligands in human placenta mesenchymal stem cells. Cell Biol. Int. 2013, 37, 137–148. [Google Scholar] [CrossRef]
- Canham, M.A.; Campbell, J.D.M.; Mountford, J.C. The use of mesenchymal stromal cells in the treatment of coronavirus disease 2019. J. Transl. Med. 2020, 18, 359. [Google Scholar] [CrossRef]
- da Silva, K.N.; Gobatto, A.L.N.; Costa-Ferro, Z.S.M.; Cavalcante, B.R.R.; Caria, A.C.I.; de Aragão França, L.S.; Nonaka, C.K.V.; de Macêdo Lima, F.; Lopes-Pacheco, M.; Rocco, P.R.M.; et al. Is there a place for mesenchymal stromal cell-based therapies in the therapeutic armamentarium against COVID-19? Stem Cell Res. Ther. 2021, 12, 425. [Google Scholar] [CrossRef]
- Chan, M.C.W.; Kuok, D.I.T.; Leung, C.Y.H.; Hui, K.P.Y.; Valkenburg, S.A.; Lau, E.H.Y.; Nicholls, J.M.; Fang, X.; Guan, Y.; Lee, J.W.; et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 3621–3626. [Google Scholar] [CrossRef]
- Harrell, C.R.; Popovska Jovicic, B.; Djonov, V.; Volarevic, V. Molecular mechanisms responsible for mesenchymal stem cell-based treatment of viral diseases. Pathogens 2021, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, Z.; Yan, Z.; Ji, N.; Yang, X.; Gao, D.; Hu, L.; Lv, H.; Zhang, J.; Li, M. Mesenchymal Stem Cell Immunomodulation: A Novel Intervention Mechanism in Cardiovascular Disease. Front. Cell Dev. Biol. 2022, 9, 3781. [Google Scholar] [CrossRef] [PubMed]
- Mázló, A.; Kovács, R.; Miltner, N.; Tóth, M.; Veréb, Z.; Szabó, K.; Bacskai, I.; Pázmándi, K.; Apáti, Á.; Bíró, T.; et al. MSC-like cells increase ability of monocyte-derived dendritic cells to polarize IL-17-/IL-10-producing T cells via CTLA-4. iScience 2021, 24, 102312. [Google Scholar] [CrossRef] [PubMed]
- Bacskai, I.; Mázló, A.; Kis-Tóth, K.; Szabó, A.; Panyi, G.; Sarkadi, B.; Apáti, Á.; Rajnavölgyi, É. Mesencyhmal stromal cell-like cells set the balance of stimulatory and inhibitory signals in monocyte-derived dendritic cells. Stem Cells Dev. 2015, 24, 1805–1816. [Google Scholar] [CrossRef]
- Oth, T.; Schnijderberg, M.C.A.; Senden-Gijsbers, B.L.M.G.; Germeraad, W.T.V.; Bos, G.M.J.; Vanderlocht, J. Monitoring the initiation and kinetics of human dendritic cell-induced polarization of autologous naive CD4+ T cells. PLoS ONE 2014, 9, e103725. [Google Scholar] [CrossRef]
- Raicevic, G.; Najar, M.; Busser, H.; Crompot, E.; Bron, D.; Toungouz, M.; Lagneaux, L. Comparison and immunobiological characterization of retinoic acid inducible gene-I-like receptor expression in mesenchymal stromal cells. Sci. Rep. 2017, 7, 2896. [Google Scholar] [CrossRef]
- Chen, H.; Min, X.H.; Wang, Q.Y.; Leung, F.W.; Shi, L.; Zhou, Y.; Yu, T.; Wang, C.M.; An, G.; Sha, W.H.; et al. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β 2 and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci. Rep. 2015, 5, 8718. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Vanpouille, C.; Laghi, L.; Parolin, C.; Melikov, K.; Backlund, P.; Vitali, B.; Margolis, L. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat. Commun. 2019, 10, 5656. [Google Scholar] [CrossRef]
- Forero, A.; Ozarkar, S.; Li, H.; Lee, C.H.; Hemann, E.A.; Nadjsombati, M.S.; Hendricks, M.R.; So, L.; Green, R.; Roy, C.N.; et al. Differential Activation of the Transcription Factor IRF1 Underlies the Distinct Immune Responses Elicited by Type I and Type III Interferons. Immunity 2019, 51, 451–464. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchi, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus iuterleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef]
- Weiss, G.; Rasmussen, S.; Zeuthen, L.H.; Nielsen, B.N.; Jarmer, H.; Jespersen, L.; Frøkiær, H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology 2010, 131, 268–281. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Z.; Li, L.; Wang, H.; Zhu, J.; Zhang, P.; Lee, Y.K.; Zhao, J.; Zhang, H.; Lu, W.; et al. Lactobacillus mucosae exerted different antiviral effects on respiratory syncytial virus infection in mice. Front. Microbiol. 2022, 13, 1001313. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.S.; Rault, L.; Seyffert, N.; Azevedo, V.; Le Loir, Y.; Even, S. Lactobacillus casei BL23 modulates the innate immune response in Staphylococcus aureus-stimulated bovine mammary epithelial cells. Benef. Microbes 2018, 9, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Torres-Fuentes, C.; Heeney, D.D.; Marco, M.L. Synergy between Probiotic Lactobacillus casei and Milk to Maintain Barrier Integrity of Intestinal Epithelial Cells. J. Agric. Food Chem. 2019, 67, 1955–1962. [Google Scholar] [CrossRef]
- Bäuerl, C.; Coll-Marqués, J.M.; Tarazona-González, C.; Pérez-Martínez, G. Lactobacillus casei extracellular vesicles stimulate EGFR pathway likely due to the presence of proteins P40 and P75 bound to their surface. Sci. Rep. 2020, 10, 19237. [Google Scholar] [CrossRef]
- Rubio, A.P.D.; Martínez, J.H.; Casillas, D.C.M.; Leskow, F.C.; Piuri, M.; Pérez, O.E. Lactobacillus casei BL23 produces microvesicles carrying proteins that have been associated with its probiotic effect. Front. Microbiol. 2017, 8, 1783. [Google Scholar] [CrossRef]
- Jacouton, E.; Chain, F.; Sokol, H.; Langella, P.; Bermúdez-Humarán, L.G. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front. Immunol. 2017, 8, 1553. [Google Scholar] [CrossRef]
- Lee, B.; Yin, X.; Griffey, S.M.; Marco, M.L. Attenuation of colitis by Lactobacillus casei BL23 is dependent on the dairy delivery matrix. Appl. Environ. Microbiol. 2015, 81, 6425–6435. [Google Scholar] [CrossRef]
- Fathi, E.; Valipour, B.; Sanaat, Z.; Charoudeh, H.N.; Farahzadi, R. Interleukin-6, -8, and TGF-β Secreted from Mesenchymal Stem Cells Show Functional Role in Reduction of Telomerase Activity of Leukemia Cell Via Wnt5a/β-Catenin and P53 Pathways. Adv. Pharm. Bull. 2020, 10, 307–314. [Google Scholar] [CrossRef]
- Putra, A.; Alif, I.; Nazar, M.A.; Prasetio, A.; Satria Irawan, R.C.; Amalina, D.; Sari, E.P.; Kustiyah, A.R.; Adeputra Nasution, I.P. IL-6 and IL-8 Suppression by Bacteria-adhered Mesenchymal Stem Cells Co-cultured with PBMCs under TNF-α Exposure. J. Iran. Med. Counc. 2021, 1, 311–317. [Google Scholar] [CrossRef]
- Merimi, M.; Buyl, K.; Daassi, D.; Rodrigues, R.M.; Melki, R.; Lewalle, P.; Vanhaecke, T.; Fahmi, H.; Rogiers, V.; Lagneaux, L.; et al. Transcriptional profile of cytokines, regulatory mediators and TLR in mesenchymal stromal cells after inflammatory signaling and cell-passaging. Int. J. Mol. Sci. 2021, 22, 7309. [Google Scholar] [CrossRef] [PubMed]
- Pricola, K.L.; Kuhn, N.Z.; Haleem-Smith, H.; Song, Y.; Tuan, R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell. Biochem. 2009, 108, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, S.; Cai, J.; Shi, J.; Sui, X.; Cao, Y.; Huang, W.; Chen, X.; Cai, Z.; et al. Bone marrow-derived mesenchymal stem cell-secreted IL-8 promotes the angiogenesis and growth of colorectal cancer. Oncotarget 2015, 6, 42825–42837. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.W.; Matthay, M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kang, S.I.; Shin, D.H.; Oh, S.Y.; Lee, C.W.; Yang, Y.; Son, Y.K.; Yang, H.S.; Lee, B.H.; An, H.J.; et al. Potential of cell-free supernatant from Lactobacillus plantarum nibr97, including novel bacteriocins, as a natural alternative to chemical disinfectants. Pharmaceuticals 2020, 13, 266. [Google Scholar] [CrossRef]
- Suissa, R.; Oved, R.; Jankelowitz, G.; Turjeman, S.; Koren, O.; Kolodkin-Gal, I. Molecular genetics for probiotic engineering: Dissecting lactic acid bacteria. Trends Microbiol. 2022, 30, 293–306. [Google Scholar] [CrossRef]
- Niu, J.; Cui, M.; Yang, X.; Li, J.; Yao, Y.; Guo, Q.; Lu, A.; Qi, X.; Zhou, D.; Zhang, C.; et al. Microbiota-derived acetate enhances host antiviral response via NLRP3. Nat. Commun. 2023, 14, 642. [Google Scholar] [CrossRef]
- Wang, Y.; Moon, A.; Huang, J.; Sun, Y.; Qiu, H.J. Antiviral Effects and Underlying Mechanisms of Probiotics as Promising Antivirals. Front. Cell. Infect. Microbiol. 2022, 12, 724. [Google Scholar] [CrossRef]
- Domínguez Rubio, A.P.; D’Antoni, C.L.; Piuri, M.; Pérez, O.E. Probiotics, Their Extracellular Vesicles and Infectious Diseases. Front. Microbiol. 2022, 13, 1030. [Google Scholar] [CrossRef]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Comi, M.; Avancini, D.; Santoni de Sio, F.; Villa, M.; Uyeda, M.J.; Floris, M.; Tomasoni, D.; Bulfone, A.; Roncarolo, M.G.; Gregori, S. Coexpression of CD163 and CD141 identifies human circulating IL-10-producing dendritic cells (DC-10). Cell. Mol. Immunol. 2020, 17, 95–107. [Google Scholar] [CrossRef]
- Bourdely, P.; Anselmi, G.; Vaivode, K.; Ramos, R.N.; Missolo-Koussou, Y.; Hidalgo, S.; Tosselo, J.; Nuñez, N.; Richer, W.; Vincent-Salomon, A.; et al. Transcriptional and Functional Analysis of CD1c+ Human Dendritic Cells Identifies a CD163+ Subset Priming CD8+CD103+ T Cells. Immunity 2020, 53, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.H.; Farber, D.L. Anti-viral protective capacity of tissue resident memory T cells. Curr. Opin. Virol. 2021, 46, 20–26. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. npj Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzsai, S.; Maryanovsky, O.-M.; Ander, R.; Koncz, G.; Mázló, A.; Bácsi, A.; Tóth, M. Cell-Free Supernatant Derived from a Lactobacillus casei BL23 Culture Modifies the Antiviral and Immunomodulatory Capacity of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 1521. https://doi.org/10.3390/biomedicines11061521
Muzsai S, Maryanovsky O-M, Ander R, Koncz G, Mázló A, Bácsi A, Tóth M. Cell-Free Supernatant Derived from a Lactobacillus casei BL23 Culture Modifies the Antiviral and Immunomodulatory Capacity of Mesenchymal Stromal Cells. Biomedicines. 2023; 11(6):1521. https://doi.org/10.3390/biomedicines11061521
Chicago/Turabian StyleMuzsai, Szabolcs, Ore-Matan Maryanovsky, Roland Ander, Gábor Koncz, Anett Mázló, Attila Bácsi, and Márta Tóth. 2023. "Cell-Free Supernatant Derived from a Lactobacillus casei BL23 Culture Modifies the Antiviral and Immunomodulatory Capacity of Mesenchymal Stromal Cells" Biomedicines 11, no. 6: 1521. https://doi.org/10.3390/biomedicines11061521
APA StyleMuzsai, S., Maryanovsky, O. -M., Ander, R., Koncz, G., Mázló, A., Bácsi, A., & Tóth, M. (2023). Cell-Free Supernatant Derived from a Lactobacillus casei BL23 Culture Modifies the Antiviral and Immunomodulatory Capacity of Mesenchymal Stromal Cells. Biomedicines, 11(6), 1521. https://doi.org/10.3390/biomedicines11061521





