Assessing Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury: Animal Models in Preclinical Neuro-Urology Research
Abstract
:1. Background
2. Literature Review
3. Animal Cystometry
4. Cystometric Findings in Animal Models of SCI
4.1. Mice and Rats
| Species | Mechanism | Level | Catheter | Time Post-SCI | Anesthetic Used | Ref. |
|---|---|---|---|---|---|---|
| Mouse | Transection | T8 | Transurethral | 18 weeks | Isoflurane | [35] |
| T8-9 | Suprapubic | 2 weeks | [121] | |||
| T8-9 | Suprapubic | 2 weeks | [119] | |||
| T8-9 | Suprapubic | 2–4 weeks | EMLA | [118] | ||
| T8-9 | Suprapubic | 4 weeks | ELMA | [116] | ||
| T8-9 | Suprapubic | 4 weeks | Isoflurane | [162] | ||
| T8-9 | Suprapubic | 4 weeks | [120] | |||
| T8-9 | Suprapubic | 4 weeks | [122] | |||
| T8-9 | Suprapubic | 4 weeks | [117] | |||
| Rat & Mouse | Transection | T8-9 | Suprapubic | 4 weeks | [36] | |
| Rat | Compression | T8 | No cath | 10 weeks | Isoflurane | [100] |
| T8-T9 | Suprapubic | 2, 4 weeks | [75] | |||
| Contusion | L5-S2 | Suprapubic | 2–8 weeks | [109] | ||
| T10 | corpus spongiosum | 3–21 days | [86] | |||
| T10 | No cath | 2–3 d–2 weeks | Isoflurane | [85] | ||
| T10 | No cath | 4 weeks | [56] | |||
| T10 | Suprapubic | 4 weeks | Urethane | [59] | ||
| T10 | Transurethral | 4 weeks | Not specified | [115] | ||
| T8 | Suprapubic | 2–3 d–2 weeks | [71] | |||
| T8 | Suprapubic | 2–4 months | Urethane | [114] | ||
| T8 | Transurethral | 8 weeks | [69] | |||
| T8 | Transurethral | 8 weeks | [72] | |||
| T8-T9 | Suprapubic | 2 weeks | Urethane | [111] | ||
| T9-T10 | Suprapubic | 2 weeks | [108] | |||
| T9-T10 | Suprapubic | 4 d, 2–8 weeks | Isoflurane | [93] | ||
| T9-T10 | Suprapubic | 5 weeks | Ketoprofen | [112] | ||
| Heat Injury | T12 | Suprapubic | 30 days | Urethane and α-chlor. | [62] | |
| Transection | L4-L5 | Transurethral | 6 weeks | Pentobarbital | [110] | |
| T10 | Suprapubic | 3 weeks | [67] | |||
| T10 | Suprapubic | 4 weeks | [95] | |||
| T10 | Suprapubic | 4,5 weeks | [101] | |||
| T10 | Suprapubic | 6 weeks | [44] | |||
| T10 | Suprapubic | 6–8 weeks | [74] | |||
| T10 | Suprapubic | 8–12 weeks | Urethane | [163] | ||
| T10 | Transurethral | 8 weeks | Ketamine | [164] | ||
| T11 | Suprapubic | 3 weeks | [102] | |||
| T4 | Suprapubic | 3 weeks | Xylazine/Ketamine | [97] | ||
| T6-T7 | Suprapubic | 4 weeks | [79] | |||
| T7-T9 | Suprapubic | 2–3 weeks | [40] | |||
| T7-T9 | Suprapubic | 2–3 weeks | [73] | |||
| T7-T9 | Suprapubic | 6 weeks | Urethane | [66] | ||
| T7-T9 | Suprapubic | 7 weeks | Urethane | [88] | ||
| T8 | Suprapubic | 1–4 weeks | [53] | |||
| T8 | Suprapubic | 4 weeks | [54] | |||
| T8 | Suprapubic | 4 weeks | Urethane | [96] | ||
| T8 | Suprapubic | 6 weeks | [105] | |||
| T8-9,L3-4,L6-S1 | Suprapubic | 4 weeks | Urethane | [90] | ||
| T8-T10 | Suprapubic | 2–3 weeks | Halothane | [64] | ||
| T8-T10 | Suprapubic | 4–6 months | Urethane | [57] | ||
| T8-T9 | Suprapubic | 1,3,4 weeks | [52] | |||
| T8-T9 | Suprapubic | 1–2 days | Urethane | [77] | ||
| T8-T9 | Suprapubic | 1–4 weeks | [165] | |||
| T8-T9 | Suprapubic | 2 weeks | Halothane | [70] | ||
| T8-T9 | Suprapubic | 3 weeks | Halothane | [78] | ||
| T8-T9 | Suprapubic | 3 weeks | [83] | |||
| T8-T9 | Suprapubic | 3 weeks | [98] | |||
| T8-T9 | Suprapubic | 4 weeks | [166] | |||
| T8-T9 | Suprapubic | 4 weeks | Urethane | [113] | ||
| T8-T9 | Suprapubic | 4 weeks | Urethane | [107] | ||
| T8-T9 | Suprapubic | 4 weeks | [65] | |||
| T8-T9 | Suprapubic | 4–5 weeks | Urethane | [61] | ||
| T8-T9 | Suprapubic | 4–5 weeks | Urethane | [60] | ||
| T8-T9 | Suprapubic | 6 weeks | Urethane | [167] | ||
| T8-T9 | Transurethral | 6–8 weeks | Urethane | [58] | ||
| T8-T9 | Transurethral | 7 weeks | Ketamine | [84] | ||
| T9 | Suprapubic | 3–28 days | [89] | |||
| T9-T11 | Suprapubic | 6 weeks | Halothane | [80] | ||
| T9-T10 | Suprapubic | 1–2 weeks | Chloral hydrate | [104] | ||
| T9-T10 | Suprapubic | 1–2 weeks | [91] | |||
| T9-T10 | Suprapubic | 1–8 weeks | Urethane | [99] | ||
| T9-T10 | Suprapubic | 2, 4 weeks | [168] | |||
| T9-T10 | Suprapubic | 4 weeks | [92] | |||
| T9-T10 | Suprapubic | 4 weeks | [103] | |||
| T9-T10 | Suprapubic | 4 weeks | Urethane | [81] | ||
| T9-T10 | Suprapubic | 6 weeks | Urethane | [68] | ||
| T9-T10 | Transurethral | 1 day | Urethane | [87] | ||
| T9-T10 | Transurethral | 1 d, 4 weeks | Urethane | [76] | ||
| T9-T10 | Transurethral | 4 weeks | Urethane | [82] | ||
| T9-T11 | Suprapubic | 4–7 weeks | Urethane | [55] | ||
| T9-T11 | Suprapubic | 6–8 weeks | Urethane | [94] | ||
| Transection/Compression | T8 | Suprapubic | 6, 14 weeks | [106] | ||
| Transection/Contusion | T8 | Suprapubic | 2, 6 weeks | [150] | ||
| T8 | Transurethral | 2 d–2 weeks | Chloral hydrate | [63] | ||
| Rabbit | Cauderization | T12-L2 | Transurethral | 10–12 weeks | Not specified | [169] |
| Transection | T10 | Transurethral | 3 weeks | [170] | ||
| Cat | Compression | T1 | Suprapubic | 1–2 weeks | [48] | |
| T1 | Suprapubic | 8–10 weeks | [123] | |||
| Transection | C6-T1 | Transurethral | 3–10 weeks | [124] | ||
| L1 | Suprapubic | 6 h–38 days | Halothane | [125] | ||
| Transection | T10 | Suprapubic | 3–6 weeks | [126] | ||
| T10 | Suprapubic | immediately | Halothane | [33] | ||
| T10-T11 | Transurethral | 2–6 months | [127] | |||
| T10-T11 | Transurethral | 3–10 months | [128] | |||
| T11-T12 | Suprapubic | 8 weeks | Isoflurane + α-chlor. | [129] | ||
| T11-T12 | Suprapubic | not specified | [130] | |||
| T12 | Suprapubic | hours | Ketamine + α-chlor. | [131] | ||
| T12-T13 | Suprapubic | 8 weeks | Isofluorane + α-chlor. | [29] | ||
| T13 | Transurethral | 2–8 weeks | [132] | |||
| T8-T12 | Suprapubic/Transurethral | 2–12 h, 4–14 w | Urethane or α-chlor. | [32] | ||
| T9-T10 | Suprapubic | 6–8 weeks | α-chloralose | [133] | ||
| T9-T10 | Transurethral | 18–24 weeks | [30] | |||
| T9-T10 | Transurethral | 4–5 weeks | α-chloralose | [134] | ||
| T9-T10 | Transurethral | 4–50 weeks | α-chloralose | [135] | ||
| Transection/Contusion | T8 | Transurethral | 3 weeks | Ketamine/Xylazine. | [136] | |
| Dog | Transection | T10 | Suprapubic/Transurethral | 1–4 weeks | Halothane | [51] |
| T10 | Transurethral | 1–8 months | Not specified | [137] | ||
| T8-T9 | no cath | not specified | Pentobarbital | [138] | ||
| T8-T9 | Transurethral | 1–8 weeks | not specified | [139] | ||
| T8-T9 | Urethral + Ureteral cath | not specified | Pentobarbital | [140] | ||
| T11-T12 | Transurethral | 1–6 weeks | Ketamine | [44] | ||
| Pig | Compression | T11-T12 | Transurethral | 1–16 weeks | Propofol/Xylazine | [141] |
| Contusion | T2/T10 | Transurethral | 4–13 weeks | Dexmedetomidine + atipamezole | [31] |
4.2. Cats
4.3. Dogs
4.4. Pigs
5. Considerations for the Application of Animal Models in NLUTD Research
5.1. Basic Functional Differences
5.2. Technical and Practical Considerations
6. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, K.D. Targeting Recovery: Priorities of the Spinal Cord-Injured Population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef]
- Bloemen-Vrencken, J.H.A.; Post, M.W.M.; Hendriks, J.M.S.; De Reus, E.C.E.; de Witte, L. Health problems of persons with spinal cord injury living in the Netherlands. Disabil. Rehabil. 2005, 27, 1381–1389. [Google Scholar] [CrossRef]
- Huh, S.; Ko, H.-Y. Recovery target priorities of people with spinal cord injuries in Korea compared with other countries: A survey. Spinal Cord 2020, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Ditunno, P.L.; Patrick, M.; Stineman, M.; Ditunno, J.F. Who wants to walk? Preferences for recovery after SCI: A longitudinal and cross-sectional study. Spinal Cord 2008, 46, 500–506. [Google Scholar] [CrossRef]
- Snoek, G.J.; Ijzerman, M.J.; Post, M.W.; Stiggelbout, A.M.; Roach, M.J.; Zilvold, G. Choice-Based Evaluation for the Improvement of Upper-Extremity Function Compared with Other Impairments in Tetraplegia. Arch. Phys. Med. Rehabil. 2005, 86, 1623–1630. [Google Scholar] [CrossRef]
- Snoek, G.J.; Ijzerman, M.J.; Hermens, H.J.; Maxwell, D.; Biering-Sørensen, F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004, 42, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Pavese, C.; Schneider, M.P.; Schubert, M.; Curt, A.; Scivoletto, G.; Finazzi-Agrò, E.; Mehnert, U.; Maier, D.; Abel, R.; Röhrich, F.; et al. Prediction of Bladder Outcomes after Traumatic Spinal Cord Injury: A Longitudinal Cohort Study. PLoS Med. 2016, 13, e1002041. [Google Scholar] [CrossRef]
- Chen, Y.; Lo, S.; Meng, E.; Shen, J.; Chou, E.C.-L.; Chen, S.; Lee, M.; Hsu, C.; Ong, H.; Chen, J.; et al. Clinical Guidelines of Patient-centered Bladder Management of Neurogenic Lower Urinary Tract Dysfunction Due to Chronic Spinal Cord Injury-Part 1: Pathophysiology, Treatment Strategy, and Priority. Urol. Sci. 2023, 34, 3. [Google Scholar]
- Ku, J.H.; Choi, W.J.; Lee, K.Y.; Jung, T.Y.; Lee, J.K.; Park, W.H.; Shim, H.B. Complications of the upper urinary tract in patients with spinal cord injury: A long-term follow-up study. Urol. Res. 2005, 33, 435–439. [Google Scholar] [CrossRef]
- Musco, S.; Padilla-Fernández, B.; Del Popolo, G.; Bonifazi, M.; Blok, B.F.M.; Groen, J.; Hoen, L.; Pannek, J.; Bonzon, J.; Kessler, T.M.; et al. Value of urodynamic findings in predicting upper urinary tract damage in neuro-urological patients: A systematic review. Neurourol. Urodyn. 2018, 37, 1522–1540. [Google Scholar] [CrossRef]
- Shin, J.C.; Lee, Y.; Yang, H.; Kim, D.H. Clinical Significance of Urodynamic Study Parameters in Maintenance of Renal Function in Spinal Cord Injury Patients. Ann. Rehabil. Med. 2014, 38, 353. [Google Scholar] [CrossRef]
- Veenboer, P.W.; Bosch, J.L.H.R.; Rosier, P.; Dik, P.; Van Asbeck, F.W.A.; de Jong, T.P.; De Kort, L.M.O. Cross-Sectional Study of Determinants of Upper and Lower Urinary Tract Outcomes in Adults with Spinal Dysraphism—New Recommendations for Urodynamic Followup Guidelines? J. Urol. 2014, 192, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Sahai, A.; Cortes, E.; Seth, J.; Khan, M.S.; Panicker, J.; Kelleher, C.; Kessler, T.M.; Fowler, C.J.; Dasgupta, P. Neurogenic Detrusor Overactivity in Patients with Spinal Cord Injury: Evaluation and Management. Curr. Urol. Rep. 2011, 12, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, J.T. Detrusor sphincter dyssynergia: A review of physiology, diagnosis, and treatment strategies. Transl. Androl. Urol. 2016, 5, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; Pannek, J.; Diaz, D.C.; Del Popolo, G.; Gross, T.; Hamid, R.; Karsenty, G.; Kessler, T.M.; Schneider, M.; Hoen, L.; et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur. Urol. 2016, 69, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, A.; Baverstock, R.; Campeau, L.; Carlson, K.; Cox, A.; Hickling, D.; Nadeau, G.; Stothers, L.; Welk, B. Canadian Urological Association guideline: Diagnosis, management, and surveillance of neurogenic lower urinary tract dysfunction—Executive summary. Can. Urol. Assoc. J. 2019, 13, 156–165. [Google Scholar] [CrossRef]
- Al Taweel, W.; Seyam, R. Neurogenic bladder in spinal cord injury patients. Res. Rep. Urol. 2015, 7, 85–99. [Google Scholar] [CrossRef]
- Gao, Y.; Danforth, T.; Ginsberg, D.A. Urologic Management and Complications in Spinal Cord Injury Patients: A 40- to 50-year Follow-up Study. Urology 2017, 104, 52–58. [Google Scholar] [CrossRef]
- Khanna, R.; Sandhu, A.; Doddamani, D. Urodynamic Management of Neurogenic Bladder in Spinal Cord Injury. Med. J. Armed Forces India 2009, 65, 300–304. [Google Scholar] [CrossRef]
- Shimizu, N.; Saito, T.; Wada, N.; Hashimoto, M.; Shimizu, T.; Kwon, J.; Cho, K.J.; Saito, M.; Karnup, S.; de Groat, W.C.; et al. Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 7885. [Google Scholar] [CrossRef]
- Shen, J.-D.; Chen, S.-J.; Chen, H.-Y.; Chiu, K.-Y.; Chen, Y.-H.; Chen, W.-C. Review of Animal Models to Study Urinary Bladder Function. Biology 2021, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Weber-Levine, C.; Hersh, A.M.; Jiang, K.; Routkevitch, D.; Tsehay, Y.; Perdomo-Pantoja, A.; Judy, B.F.; Kerensky, M.; Liu, A.; Adams, M.; et al. Porcine Model of Spinal Cord Injury: A Systematic Review. Neurotrauma Rep. 2022, 3, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, Y.; Bian, T.; He, M.; Xu, Y.; Wang, G.; Guo, J.; Wang, H. An optimized transurethral catheterization cystometry in mice and comparison with classic suprapubic catheterization cystometry: Optimized, Minimally Invasive, Repeatable. Neurourol. Urodyn. 2017, 36, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Meerveld, B.G.-V.; Foreman, R.D. Spinal Neuronal Responses to Urinary Bladder Stimulation in Rats with Corticosterone or Aldosterone onto the Amygdala. J. Neurophysiol. 2003, 90, 2180–2189. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Takeda, H.; Akahane, M.; Igawa, Y.; Nishizawa, O.; Ajisawa, Y. Species Differences in the Distribution of B-Adrenoceptor Subtypes in Bladder Smooth Muscle. Br. J. Pharmacol. 1998, 124, 593–599. [Google Scholar] [CrossRef]
- Moody, B.J.; Liberman, C.; Zvara, P.; Smith, P.P.; Freeman, K.; Zvarova, K. Acute lower urinary tract dysfunction (LUTD) following traumatic brain injury (TBI) in rats: Experimental TBI: Hemodynamics and Urodynamics. Neurourol. Urodyn. 2014, 33, 1159–1164. [Google Scholar] [CrossRef]
- Huppertz, N.D.; Kirschner-Hermanns, R.; Tolba, R.H.; Grosse, J.O. Telemetric monitoring of bladder function in female Göttingen minipigs. BJU Int. 2015, 116, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.P.; Kuchel, G.A. Continuous uroflow cystometry in the urethane-anesthetized mouse: Mouse cystometry, urethane, and uroflow. Neurourol. Urodyn. 2010, 29, 1344–1349. [Google Scholar] [CrossRef]
- Gu, B.; Olejar, K.J.; Reiter, J.P.; Thor, K.B.; Dolber, P.C. Inhibition of Bladder Activity by 5-Hydroxytryptamine1 Serotonin Receptor Agonists in Cats with Chronic Spinal Cord Injury. J. Pharmacol. Exp. Ther. 2004, 310, 1266–1272. [Google Scholar] [CrossRef]
- Gu, B.; Thor, K.B.; Reiter, J.P.; Dolber, P.C. Effect of 5-Hydroxytryptamine1 Serotonin Receptor Agonists on Noxiously Stimulated Micturition in Cats with Chronic Spinal Cord Injury. J. Urol. 2007, 177, 2381–2385. [Google Scholar] [CrossRef]
- Keung, M.S.; Streijger, F.; Herrity, A.; Ethridge, J.; Dougherty, S.M.; Aslan, S.; Webster, M.; Fisk, S.; Deegan, E.; Tessier-Cloutier, B.; et al. Characterization of Lower Urinary Tract Dysfunction after Thoracic Spinal Cord Injury in Yucatan Minipigs. J. Neurotrauma 2021, 38, 1306–1326. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.; Wenzel, B.J.; Gustafson, K.J.; Grill, W.M. Spinal Micturition Reflex Mediated by Afferents in the Deep Perineal Nerve. J. Neurophysiol. 2005, 93, 2688–2697. [Google Scholar] [CrossRef]
- Kerns, J.M.; Truong, T.T.; Walter, J.S.; Khan, T. Do Direct Current Electric Fields Enhance Micturition in the Spinal Cat? J. Spinal Cord Med. 1996, 19, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.S.; Youssef, H.A.; Saleh, A.S.; Bollen, P.; Zvara, P. Anesthetic protocols for urodynamic studies of the lower urinary tract in small rodents—A systematic review. PLoS ONE 2021, 16, e0253192. [Google Scholar] [CrossRef] [PubMed]
- DePaul, M.; Lin, C.-Y.; Silver, J.; Lee, Y.-S. Peripheral Nerve Transplantation Combined with Acidic Fibroblast Growth Factor and Chondroitinase Induces Regeneration and Improves Urinary Function in Complete Spinal Cord Transected Adult Mice. PLoS ONE 2015, 10, e0139335. [Google Scholar] [CrossRef]
- Kadekawa, K.; Yoshimura, N.; Majima, T.; Wada, N.; Shimizu, T.; Birder, L.A.; Kanai, A.J.; de Groat, W.C.; Sugaya, K.; Yoshiyama, M. Characterization of bladder and external urethral activity in mice with or without spinal cord injury—A comparison study with rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R752–R758. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, M.; Roppolo, J.R.; de Groat, W.C. Effects of LY215490, a Competitive α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid (AMPA) Receptor Antagonist, on the Micturition Reflex in the Rat. J. Pharmacol. Exp. Ther. 1997, 280, 894–904. [Google Scholar]
- Yoshiyama, M.; Mochizuki, T.; Nakagomi, H.; Miyamoto, T.; Kira, S.; Mizumachi, R.; Sokabe, T.; Takayama, Y.; Tominaga, M.; Takeda, M. Functional roles of TRPV1 and TRPV4 in control of lower urinary tract activity: Dual analysis of behavior and reflex during the micturition cycle. Am. J. Physiol. Physiol. 2015, 308, F1128–F1134. [Google Scholar] [CrossRef]
- Kira, S.; Yoshiyama, M.; Tsuchiya, S.; Shigetomi, E.; Miyamoto, T.; Nakagomi, H.; Shibata, K.; Mochizuki, T.; Takeda, M.; Koizumi, S. P2Y6-deficiency increases micturition frequency and attenuates sustained contractility of the urinary bladder in mice. Sci. Rep. 2017, 7, 771. [Google Scholar] [CrossRef]
- Yokoyama, O.; Yoshiyama, M.; Namiki, M.; de Groat, W.C. Role of the Forebrain in Bladder Overactivity Following Cerebral Infarction in the Rat. Exp. Neurol. 2000, 163, 469–476. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Roppolo, J.R.; Takeda, M.; De Groat, W.C. Effects of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. Am. J. Physiol. Physiol. 2013, 304, F390–F396. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, M.; Roppolo, J.; Thor, K.; de Groat, W. Effects of LY274614, a competitive NMDA receptor antagonist, on the micturition reflex in the urethane-anaesthetized rat. Br. J. Pharmacol. 1993, 110, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, M.; Roppolo, J.R.; De Groat, W.C. Alteration by urethane of glutamatergic control of micturition. Eur. J. Pharmacol. 1994, 264, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, M.; Boyer, S.; Sawan, M.; Elhilali, M. Reduction of bladder outlet resistance by selective stimulation of the ventral sacral root using high frequency blockade: A chronic study in spinal cord transected dogs. J. Urol. 2001, 166, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Giuliani, S.; Santicioli, P.; Meli, A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am. J. Physiol. Integr. Comp. Physiol. 1986, 251, R250–R257. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.O.; Smith, P.P.; Sullivan, M.P.; Bjorling, D.E.; Campeau, L.; Andersson, K.; Yoshiyama, M. Best practices for cystometric evaluation of lower urinary tract function in muriform rodents. Neurourol. Urodyn. 2020, 39, 1868–1884. [Google Scholar] [CrossRef]
- Barrington, F.J.F. The nervous mechanism of micturition. Q. J. Exp. Physiol. 1914, 8, 33–71. [Google Scholar] [CrossRef]
- de Groat, W.C.; Ryall, R.W. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J. Physiol. 1969, 200, 87–108. [Google Scholar] [CrossRef]
- de Groat, W. Inhibition and excitation of sacral parasympathetic neurons by visceral and cutaneous stimuli in the cat. Brain Res. 1971, 33, 499–503. [Google Scholar] [CrossRef]
- De Groat, W. Nervous control of the urinary bladder of the cat. Brain Res. 1975, 87, 201–211. [Google Scholar] [CrossRef]
- Van Gool, J.D.; Schmidt, R.A.; Tanagho, E.A. Development of Reflex Activity of Detrusor and Striated Sphincter Muscles in Experimental Paraplegia. Urol. Int. 1978, 33, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.M.; Salemi, S.; Hofer, A.-S.; Baumgartner, V.; Eberli, D.; Liechti, M.D.; Schwab, M.E.; Kessler, T.M. Early Transcutaneous Tibial Nerve Stimulation Acutely Improves Lower Urinary Tract Function in Spinal Cord Injured Rats. Neurotrauma Rep. 2022, 3, 15–26. [Google Scholar] [CrossRef]
- Schneider, M.P.; Sartori, A.M.; Ineichen, B.V.; Moors, S.; Engmann, A.K.; Hofer, A.-S.; Weinmann, O.; Kessler, T.M.; Schwab, M.E. Anti-Nogo-A Antibodies as a Potential Causal Therapy for Lower Urinary Tract Dysfunction after Spinal Cord Injury. J. Neurosci. 2019, 39, 4066–4076. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Yamauchi, H.; Ito, H.; Akino, H.; Yokoyama, O. α1D-Adrenoceptor blockade increases voiding efficiency by improving external urethral sphincter activity in rats with spinal cord injury. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R971–R978. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.N.; Belton, A.L.; De Groat, W.C. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1993, 264, R1157–R1163. [Google Scholar] [CrossRef]
- Chancellor, M.B.; Rivas, D.A.; Abdill, C.K.; Karasick, S.; Ehrlich, S.M.; Staas, W.E. Prospective comparison of external sphincter balloon dilatation and prosthesis placement with external sphincterotomy in spinal cord injured men. Arch. Phys. Med. Rehabil. 1994, 75, 297–305. [Google Scholar] [CrossRef]
- Kruse, M.; Bennett, B.; de Groat, W. Effect of Urinary Diversion on the Recovery of Micturition Reflexes after Spinal Cord Injury in the Rat. J. Urol. 1994, 151, 1088–1091. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Ma, C.-P.; de Groat, W.C. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995, 678, 40–48. [Google Scholar] [CrossRef]
- Shenot, P.J.; Chancellor, M.B.; Rivas, D.A.; Watanabe, T.; Kumon, H.; Figueroa, T.E. In-Vivo Whole Bladder Response to Anticholinergic and Musculotropic Agents in Spinal Cord Injured Rats. J. Spinal Cord Med. 1997, 20, 31–35. [Google Scholar] [CrossRef]
- Kakizaki, H.; Fraser, M.O.; De Groat, W.C. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 272, R1647–R1656. [Google Scholar] [CrossRef]
- Kakizaki, H.; de Groat, W.C. Reorganization of somato-urethral reflexes following spinal cord injury in the rat. J. Urol. 1997, 158, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Kontani, H.; Hayashi, K. Urinary Bladder Response to Hypogastric Nerve Stimulation After Bilateral Resection of the Pelvic Nerve or Spinal Cord Injury in Rats. Int. J. Urol. 1997, 4, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Pikov, V.; Gillis, R.A.; Jasmin, L.; Wrathall, J.R. Assessment of Lower Urinary Tract Functional Deficit in Rats with Contusive Spinal Cord Injury. J. Neurotrauma 1998, 15, 375–386. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Nezu, F.M.; Yokoyama, O.; de Groat, W.C.; Chancellor, M.B. Changes in micturition after spinal cord injury in conscious rats. Urology 1999, 54, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, I.; Igawa, Y.; Ishizuka, O.; Andersson, K.-E. Effects of intravesical capsaicin and resiniferatoxin on distension-induced bladder contraction in conscious rats with and without chronic spinal cord injury. J. Urol. 1999, 161, 314–319. [Google Scholar] [CrossRef]
- Callsen-Cencic, P.; Mense, S. Control of the unstable urinary bladder by graded thermoelectric cooling of the spinal cord: Unstable urinary bladder control. BJU Int. 2001, 84, 1084–1092. [Google Scholar] [CrossRef]
- Shaker, H.; Wang, Y.; Loung, D.; Balbaa, L.; Fehlings, M.; Hassouna, M. Role of C-afferent fibres in the mechanism of action of sacral nerve root neuromodulation in chronic spinal cord injury: Sacral nerve root neuromodulation. BJU Int. 2001, 85, 905–910. [Google Scholar] [CrossRef]
- Chang, S.; Mao, S.-T.; Hu, S.-J.; Lin, W.-C.; Cheng, C.-L. Studies of detrusor-sphincter synergia and dyssynergia during micturition in rats via fractional Brownian motion. IEEE Trans. Biomed. Eng. 2000, 47, 1066–1073. [Google Scholar] [CrossRef]
- Pikov, V.; Wrathall, J.R. Coordination of the Bladder Detrusor and the External Urethral Sphincter in a Rat Model of Spinal Cord Injury: Effect of Injury Severity. J. Neurosci. 2001, 21, 559–569. [Google Scholar] [CrossRef]
- Seki, S.; Sasaki, K.; Fraser, M.O.; Igawa, Y.; Nishizawa, O.; Chancellor, M.B.; Groat, W.C.D.; Yoshimura, N. Immunoneutralization of nerve growth factor in the lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J. Urol. 2002, 168, 2269–2274. [Google Scholar] [CrossRef]
- Smith, C.P.; Somogyi, G.T.; Bird, E.T.; Chancellor, M.B.; Boone, T.B. Neurogenic bladder model for spinal cord injury: Spinal cord microdialysis and chronic urodynamics. Brain Res. Protoc. 2002, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pikov, V.; Wrathall, J.R. Altered glutamate receptor function during recovery of bladder detrusor-external urethral sphincter coordination in a rat model of spinal cord injury. Experiment 2002, 300, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, M.; de Groat, W.C. Effect of Bilateral Hypogastric Nerve Transection on Voiding Dysfunction in Rats with Spinal Cord Injury. Exp. Neurol. 2002, 175, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Karim, A.M.; Abdel-Gawad, M.; Huynh, H.; Elhilali, M.M. Modulation of insulin-like growth factor-i system of the bladder using a somatostatin analogue in chronic spinalized rats. J. Urol. 2002, 168, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Kakizaki, H.; Tanaka, H.; Shibata, T.; Matsuoka, I.; Koyanagi, T. Immortalized Neural Stem Cells Transplanted into the Injured Spinal Cord Promote Recovery of Voiding Function in the Rat. J. Urol. 2003, 170, 1421–1425. [Google Scholar] [CrossRef]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Ashitomi, K.; Hatano, T.; Ogawa, Y. Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp. Neurol. 2003, 183, 232–240. [Google Scholar] [CrossRef]
- Cheng, C. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp. Neurol. 2004, 187, 445–454. [Google Scholar] [CrossRef]
- Seki, S.; Sasaki, K.; Igawa, Y.; Nishizawa, O.; Chancellor, M.B.; De Groat, W.C.; Yoshimura, N. Suppression of Detrusor-Sphincter Dyssynergia by Immunoneutralization of Nerve Growth Factor in Lumbosacral Spinal Cord in Spinal Cord Injured Rats. J. Urol. 2004, 171, 478–482. [Google Scholar] [CrossRef]
- Yokoyama, O.; Mita, E.; Akino, H.; Tanase, K.; Ishida, H.; Namiki, M. Roles of Opiate in Lower Urinary Tract Dysfunction Associated with Spinal Cord Injury in Rats. J. Urol. 2004, 171, 963–967. [Google Scholar] [CrossRef]
- Chang, S.; Hu, S.-J.; Lin, W.-C. Fractal dynamics and synchronization of rhythms in urodynamics of female Wistar rats. J. Neurosci. Methods 2004, 139, 271–279. [Google Scholar] [CrossRef]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Ashitomi, K.; Morozumi, M.; Ogawa, Y. Dietary glycine inhibits bladder activity in normal rats and rats with spinal cord injury. J. Urol. 2005, 173, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Kadekawa, K.; Ashimine, S.; Ogawa, Y. Intrathecal or dietary glycine inhibits bladder and urethral activity in rats with spinal cord injury. J. Urol. 2005, 174, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- Khera, M.; Somogyi, G.T.; Salas, N.A.; Kiss, S.; Boone, T.B.; Smith, C.P. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology 2005, 66, 208–212. [Google Scholar] [CrossRef]
- Temeltas, G.; Tikiz, C.; Dagci, T.; Tuglu, I.; Yavasoglu, A. The effects of botulinum-a toxin on bladder function and histology in spinal cord injured rats: Is there any difference between early and late application? J. Urol. 2005, 174, 2393–2396. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, H.S.; Fedulov, V.; Cloutier, F.; Steward, O.; Duel, B.P. A noninvasive ultrasonographic method to evaluate bladder function recovery in spinal cord injured rats. Exp. Neurol. 2005, 194, 120–127. [Google Scholar] [CrossRef]
- Nout, Y.S.; Schmidt, M.H.; Tovar, C.A.; Culp, E.; Beattie, M.S.; Bresnahan, J.C. Telemetric Monitoring of Corpus Spongiosum Penis Pressure in Conscious Rats for Assessment of Micturition and Sexual Function following Spinal Cord Contusion Injury. J. Neurotrauma 2005, 22, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ashitomi, K.; Sugaya, K.; Miyazato, M.; Nishijima, S.; Ogawa, Y. Intrathecal glutamate promotes glycinergic neuronal activity and inhibits the micturition reflex in urethane-anesthetized rats: Glutamate promotes glycinergic neurons. Int. J. Urol. 2006, 13, 1519–1524. [Google Scholar] [CrossRef]
- Cruz, C.D.; McMahon, S.B.; Cruz, F. Spinal ERK activation contributes to the regulation of bladder function in spinal cord injured rats. Exp. Neurol. 2006, 200, 66–73. [Google Scholar] [CrossRef]
- Takahara, Y.; Maeda, M.; Nakatani, T.; Kiyama, H. Transient suppression of the vesicular acetylcholine transporter in urinary bladder pathways following spinal cord injury. Brain Res. 2007, 1137, 20–28. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Cheng, C.-L.; Chen, J.-J.J.; De Groat, W.C. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1044–F1053. [Google Scholar] [CrossRef]
- Zinck, N.; Downie, J. IB4 afferent sprouting contributes to bladder dysfunction in spinal rats. Exp. Neurol. 2008, 213, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Sasatomi, K.; Hiragata, S.; Sugaya, K.; Chancellor, M.B.; de Groat, W.C.; Yoshimura, N. GABA Receptor Activation in the Lumbosacral Spinal Cord Decreases Detrusor Overactivity in Spinal Cord Injured Rats. J. Urol. 2008, 179, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.K.; Lee, Y.I.; Son, Y.-J.; Park, J.-S. Serial Changes in Bladder, Locomotion, and Levels of Neurotrophic Factors in Rats with Spinal Cord Contusion. J. Neurotrauma 2009, 26, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Li, S.J.; Hu, S.J.; Cheng, H.Y.; Hsieh, S.H.; Cheng, C.L. Dynamic Performance Evaluation on the Synergy of Micturition in Spinal Cord-Injured Female Rats under Pharmacological Effects. Chin. J. Physiol. 2009, 52, 23–34. [Google Scholar] [CrossRef]
- Zhang, F.; Liao, L.; Ju, Y.; Song, A.; Liu, Y. Neurochemical Plasticity of Nitric Oxide Synthase Isoforms in Neurogenic Detrusor Overactivity after Spinal Cord Injury. Neurochem. Res. 2011, 36, 1903–1909. [Google Scholar] [CrossRef]
- Munoz, A.; Somogyi, G.T.; Boone, T.B.; Smith, C.P. Central inhibitory effect of intravesically applied botulinum toxin A in chronic spinal cord injury. Neurourol. Urodyn. 2011, 30, 1376–1381. [Google Scholar] [CrossRef]
- Elkelini, M.S.; Bagli, D.J.; Fehlings, M.; Hassouna, M. Effects of intravesical onabotulinumtoxinA on bladder dysfunction and autonomic dysreflexia after spinal cord injury: Role of nerve growth factor: Onabotulinumtoxin a controls bladder dysfunction and autonomic dysreflexia after spinal cord injury. BJU Int. 2012, 109, 402–407. [Google Scholar] [CrossRef]
- Elkelini, M.S.; Pravdivyi, I.; Hassouna, M.M. Mechanism of action of sacral nerve stimulation using a transdermal amplitude-modulated signal in a spinal cord injury rodent model. Can. Urol. Assoc. J. 2012, 6, 227–230. [Google Scholar] [CrossRef]
- D’Amico, S.C.; Schuster, I.P.; Collins, W.F. Quantification of external urethral sphincter and bladder activity during micturition in the intact and spinally transected adult rat. Exp. Neurol. 2011, 228, 59–68. [Google Scholar] [CrossRef]
- Ozsoy, O.; Ozsoy, U.; Stein, G.; Semler, O.; Skouras, E.; Schempf, G.; Wellmann, K.; Wirth, F.; Angelova, S.; Ankerne, J.; et al. Functional deficits and morphological changes in the neurogenic bladder match the severity of spinal cord compression. Restor. Neurol. Neurosci. 2012, 30, 363–381. [Google Scholar] [CrossRef]
- Loutochin, O.; Al Afraa, T.; Campeau, L.; Mahfouz, W.; Elzayat, E.; Corcos, J. Effect of the anticonvulsant medications Pregabalin and Lamotrigine on urodynamic parameters in an animal model of neurogenic detrusor overactivity. Neurourol. Urodyn. 2012, 31, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.-M.; Mohammadinejad, P.; Hojjat, A.; Nezami, B.G.; Talab, S.S.; Emami, H.; Esfahani, S.A. The timing of established detrusor hyperreflexia in a rat model of neuropathic bladder. J. Surg. Res. 2012, 178, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kadekawa, K.; Sugaya, K.; Nishijima, S.; Ashitomi, K.; Miyazato, M.; Ueda, T.; Yamamoto, H. Effect of naftopidil, an alpha1D/A-adrenoceptor antagonist, on the urinary bladder in rats with spinal cord injury. Life Sci. 2013, 92, 1024–1028. [Google Scholar] [CrossRef]
- Shi, P.; Zhao, X.; Wang, J.; Lan, N. Effects of Acute Sacral Neuromodulation on Bladder Reflex in Complete Spinal Cord Injury Rats. Neuromodul. Technol. Neural Interface 2013, 16, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.G.; Algarrahi, K.; Franck, D.; Tu, D.D.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R.; Mauney, J.R. The use of bi-layer silk fibroin scaffolds and small intestinal submucosa matrices to support bladder tissue regeneration in a rat model of spinal cord injury. Biomaterials 2014, 35, 7452–7459. [Google Scholar] [CrossRef]
- Chung, Y.G.; Seth, A.; Doyle, C.; Franck, D.; Kim, D.; Cristofaro, V.; Benowitz, L.I.; Tu, D.D.; Estrada, C.R.; Mauney, J.R.; et al. Inosine Improves Neurogenic Detrusor Overactivity following Spinal Cord Injury. PLoS ONE 2015, 10, e0141492. [Google Scholar] [CrossRef]
- Wong, K.; Boone, T.B.; Wong, S.T.; Munoz, A. Functional brain interactions during reflexive micturition are absent from spinal cord injured rats with neurogenic bladder: FMRI in Normal and SCI Rats. Neurourol. Urodyn. 2015, 34, 469–474. [Google Scholar] [CrossRef]
- Shunmugavel, A.; Khan, M.; Hughes, F.M.; Purves, J.T.; Singh, A.; Singh, I. S-Nitrosoglutathione protects the spinal bladder: Novel therapeutic approach to post-spinal cord injury bladder remodeling: GSNO Protects Spinal Bladder. Neurourol. Urodyn. 2015, 34, 519–526. [Google Scholar] [CrossRef]
- Afrashteh, B.; Roider1, K.; Bauer, S.; Lusuardi, L.; Heimel, P.; Hercher, D.; Aigner, L.; Keller, E. A Rodent Lumbosacral Spinal Cord Injury Model Reflecting Neurological and Urological Deficits of Humans. J. Exp. Neurol. 2022, 3, 24–34. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, M.; Chen, S.; Ji, Z.; Zheng, X. TGF-β1 and connexin-43 expression in neurogenic bladder from rats with sacral spinal cord injury. Neurourol. Urodyn. 2018, 37, 2502–2509. [Google Scholar] [CrossRef]
- Salazar, B.H.; Hoffman, K.A.; Zhang, C.; Zhang, Y.; Cruz, Y.; Boone, T.B.; Munoz, A. Modulatory effects of intravesical P2X2/3 purinergic receptor inhibition on lower urinary tract electromyographic properties and voiding function of female rats with moderate or severe spinal cord injury. BJU Int. 2019, 123, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Yoon, C.Y.; Lee, M.S.; Song, B.D.; Lee, S.W.; Jeong, S.J. Effect of Early Sacral Neuromodulation on Bladder Function in a Rat Model of Incomplete Spinal Cord Injury Due to Focal Contusion. Neuromodul. Technol. Neural Interface 2019, 22, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Yazdi, I.K.; Tang, X.; Rivera, C.; Taghipour, N.; Grossman, R.G.; Boone, T.B.; Tasciotti, E. Localized inhibition of P2X7R at the spinal cord injury site improves neurogenic bladder dysfunction by decreasing urothelial P2X3R expression in rats. Life Sci. 2017, 171, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Sparks, A.; Lee, Y.-S. Improvement of lower urinary tract function by a selective serotonin 5-HT1A receptor agonist, NLX-112, after chronic spinal cord injury. Exp. Neurol. 2020, 332, 113395. [Google Scholar] [CrossRef]
- Song, J.; Cao, X.; Zhang, A.; Fang, Z.; Xu, J.; Gao, X. Posterior tibial nerve stimulation improves neurogenic bladder in rats with spinal cord injury through transient receptor potential/P2X signaling pathway. Neurourol. Urodyn. 2022, 41, 756–764. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, E.; Cho, H.; Jang, J.; Han, M.; Kwak, E.; Kim, H.; An, J.; Park, D.; Han, S.; et al. Antifibrosis treatment by inhibition of VEGF, FGF, and PDGF receptors improves bladder wall remodeling and detrusor overactivity in association with modulation of C-fiber afferent activity in mice with spinal cord injury. Neurourol. Urodyn. 2021, 40, 1460–1469. [Google Scholar] [CrossRef]
- Shimizu, N.; Gotoh, D.; Nishimoto, M.; Hashimoto, M.; Saito, T.; Fujita, K.; Hirayama, A.; Yoshimura, N.; Uemura, H. Efficacy of vibegron, a novel β3-adrenoreceptor agonist, for lower urinary tract dysfunction in mice with spinal cord injury. Int. J. Urol. 2021, 28, 1068–1072. [Google Scholar] [CrossRef]
- Saito, T.; Gotoh, D.; Wada, N.; Tyagi, P.; Minagawa, T.; Ogawa, T.; Ishizuka, O.; Yoshimura, N. Time-dependent progression of neurogenic lower urinary tract dysfunction after spinal cord injury in the mouse model. Am. J. Physiol. Ren. Physiol. 2021, 321, F26–F32. [Google Scholar] [CrossRef]
- Shimizu, T.; Majima, T.; Suzuki, T.; Shimizu, N.; Wada, N.; Kadekawa, K.; Takai, S.; Takaoka, E.; Kwon, J.; Kanai, A.J.; et al. Nerve growth factor-dependent hyperexcitability of capsaicin-sensitive bladder afferent neurones in mice with spinal cord injury. Exp. Physiol. 2018, 103, 896–904. [Google Scholar] [CrossRef]
- Wada, N.; Shimizu, T.; Takai, S.; Shimizu, N.; Kanai, A.J.; Tyagi, P.; Kakizaki, H.; Yoshimura, N. Post-injury bladder management strategy influences lower urinary tract dysfunction in the mouse model of spinal cord injury. Neurourol. Urodyn. 2017, 36, 1301–1305. [Google Scholar] [CrossRef]
- Zabbarova, I.V.; Ikeda, Y.; Carder, E.; Wipf, P.; Wolf-Johnston, A.S.; Birder, L.A.; Yoshimura, N.; Getchell, S.E.; Almansoori, K.; Tyagi, P.; et al. Targeting p75 neurotrophin receptors ameliorates spinal cord injury-induced detrusor sphincter dyssynergia in mice. Neurourol. Urodyn. 2018, 37, 2452–2461. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Shimizu, T.; Shimizu, N.; Kurobe, M.; de Groat, W.C.; Tyagi, P.; Kakizaki, H.; Yoshimura, N. Therapeutic effects of inhibition of brain-derived neurotrophic factor on voiding dysfunction in mice with spinal cord injury. Am. J. Physiol.-Ren. Physiol. 2019, 317, F1305–F1310. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.; Kawatani, M.; Hisamitsu, T.; Cheng, C.-L.; Ma, C.-P.; Thor, K.; Steers, W.; Roppolo, J. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 1990, 30, S71–S77. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.S.; Wheeler, J.S.; Robinson, C.J.; Wurster, R.D. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol. Urodyn. 1993, 12, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.S.; Zaszczurynski, P.; Cai, W.; Wheeler, J.S.; Riedy, L.; Scarpine, V.E. Direct Bladder Stimulation with Percutaneous Electrodes and Impedance Monitoring of Volume in an SCI Animal Model. J. Spinal Cord Med. 1995, 18, 98–102. [Google Scholar] [CrossRef]
- Espey, M.J.; Downie, J.W. Serotonergic modulation of cat bladder function before and after spinal transection. Eur. J. Pharmacol. 1995, 287, 173–177. [Google Scholar] [CrossRef]
- Walter, J.S.; Wheeler, J.S.; Cai, W.; Wurster, R.D. Direct bladder stimulation with suture electrodes promotes voiding in a spinal animal model: A technical report. J. Rehabil. Res. Dev. 1997, 34, 72–81. [Google Scholar]
- Sugaya, K.; Ogawa, Y.; Hatano, T.; Koyama, Y.; Miyazato, T.; Oda, M. Effect of Injury to the Dorsal Funiculus of the Thoracic Spinal Cord on Micturition in Decerebrate and Freely-Moving Cats. Urol. Int. 1999, 63, 179–184. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Liu, J.-C.; Chang, S.-Y.; Ma, C.-P.; De Groat, W.C. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol. Integr. Comp. Physiol. 1999, 277, R786–R794. [Google Scholar] [CrossRef]
- Sugaya, K.; Ogawa, Y.; Hatano, T.; Nishizawa, O. Micturition in thoracic spinal cord injured cats with autografting of the adrenal medulla to the sacral spinal cord. J. Urol. 2001, 166, 2525–2529. [Google Scholar] [CrossRef]
- Tai, C.; Booth, A.M.; de Groat, W.C.; Roppolo, J.R. Bladder and urethral sphincter responses evoked by microstimulation of S2 sacral spinal cord in spinal cord intact and chronic spinal cord injured cats. Exp. Neurol. 2004, 190, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.S.; Wheeler, J.S.; Fitzgerald, M.P.; McDonnell, A.; Wurster, R.D. A Spinal Cord Injured Animal Model of Lower Urinary Tract Function: Observations Using Direct Bladder and Pelvic Plexus Stimulation with Model Microstimulators. J. Spinal Cord Med. 2005, 28, 73–81. [Google Scholar] [CrossRef]
- Tai, C.; Miscik, C.L.; Ungerer, T.D.; Roppolo, J.R.; de Groat, W.C. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp. Neurol. 2006, 199, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, T.D.; Kim, K.A.; Daugherty, S.L.; Roppolo, J.R.; Tai, C.; de Groat, W.C. Influence of urothelial or suburothelial cholinergic receptors on bladder reflexes in chronic spinal cord injured cats. Exp. Neurol. 2016, 285, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Van Ba, O.L.; Barbe, M.; Caremel, R.; Aharony, S.; Loutochin, O.; Jacques, L.; Wood, M.W.; Tiwari, E.; Tuite, G.F.; Campeau, L.; et al. Lumbar to sacral root rerouting to restore bladder function in a feline spinal cord injury model: Urodynamic and retrograde nerve tracing results from a pilot study. Neurourol. Urodyn. 2018, 37, 153–162. [Google Scholar] [CrossRef]
- Guo, W.; Shapiro, K.; Wang, Z.; Armann, K.; Shen, B.; Wang, J.; Roppolo, J.R.; de Groat, W.C.; Tai, C. Restoring both continence and micturition after chronic spinal cord injury by pudendal neuromodulation. Exp. Neurol. 2021, 340, 113658. [Google Scholar] [CrossRef]
- Tang, P.C.; Walter, J.S. Voiding in anesthetized spinal dogs induced by stimulating sacral and coccygeal roots with the “volume conduction” method. Neurourol. Urodynamics 1984, 3, 51–61. [Google Scholar] [CrossRef]
- Tang, P.C.; Walter, J.S. Voiding in conscious spinal dogs induced by stimulating sacral and coccygeal roots with the “volume conduction” method. Neurourol. Urodynamics 1984, 3, 43–50. [Google Scholar] [CrossRef]
- Walter, J.S.; Wheeler, J.S.; Robinson, C.J.; Bolam, J.; Wurster, R.D. Urodynamic responses to sacral stimulation in the chronic spinal dog. Neurourol. Urodynamics 1988, 7, 13–25. [Google Scholar] [CrossRef]
- Hassouna, M.; Li, J.; Sawan, M.; Duval, F.; Latt, R.; Elhilali, M. Effect of early bladder stimulation on spinal shock: Experimental approach. Urology 1992, 40, 563–573. [Google Scholar] [CrossRef]
- Keller, E.E.; Patras, I.; Hutu, I.; Roider, K.; Sievert, K.; Aigner, L.; Janetschek, G.; Lusuardi, L.; Zimmermann, R.; Bauer, S. Early sacral neuromodulation ameliorates urinary bladder function and structure in complete spinal cord injury minipigs. Neurourol. Urodyn. 2020, 39, 586–593. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C.; Yoshimura, N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp. Neurol. 2012, 235, 123–132. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C.; Yoshimura, N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 2006, 152, 59–84. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Spinal Cord 1995, 33, 493–505. [Google Scholar] [CrossRef]
- Wada, N.; Karnup, S.; Kadekawa, K.; Shimizu, N.; Kwon, J.; Shimizu, T.; Gotoh, D.; Kakizaki, H.; de Groat, W.C.; Yoshimura, N. Current knowledge and novel frontiers in lower urinary tract dysfunction after spinal cord injury: Basic research perspectives. Urol. Sci. 2022, 33, 101. [Google Scholar] [CrossRef]
- Pandita, R.K.; Fujiwara, M.; Alm, P.; Andersson, K.-E. Cystometric evaluation of bladder function in non- anesthetized mice with and without bladder outlet obstruction. J. Urol. 2000, 164, 1385–1389. [Google Scholar] [CrossRef]
- Schnegelsberg, B.; Sun, T.-T.; Cain, G.; Bhattacharya, A.; Nunn, P.A.; Ford, A.P.D.W.; Vizzard, M.A.; Cockayne, D.A. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R534–R547. [Google Scholar] [CrossRef]
- Petrosyan, H.A.; Alessi, V.; Lasek, K.; Gumudavelli, S.; Muffaletto, R.; Liang, L.; Collins, W.F.; Levine, J.; Arvanian, V.L. AAV Vector Mediated Delivery of NG2 Function Neutralizing Antibody and Neurotrophin NT-3 Improves Synaptic Transmission, Locomotion, and Urinary Tract Function after Spinal Cord Contusion Injury in Adult Rats. J. Neurosci. 2023, 43, 1492–1508. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lin, C.-Y.; Jiang, H.-H.; DePaul, M.; Lin, V.W.; Silver, J. Nerve Regeneration Restores Supraspinal Control of Bladder Function after Complete Spinal Cord Injury. J. Neurosci. 2013, 33, 10591–10606. [Google Scholar] [CrossRef]
- Leung, P.Y.; Johnson, C.S.; Wrathall, J.R. Comparison of the effects of complete and incomplete spinal cord injury on lower urinary tract function as evaluated in unanesthetized rats. Exp. Neurol. 2007, 208, 80–91. [Google Scholar] [CrossRef]
- Mitsui, T.; Murray, M.; Nonomura, K. Lower urinary tract function in spinal cord-injured rats: Midthoracic contusion versus transection. Spinal Cord 2014, 52, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Hubscher, C.H.; Montgomery, L.R.; Fell, J.D.; Armstrong, J.E.; Poudyal, P.; Herrity, A.N.; Harkema, S.J. Effects of exercise training on urinary tract function after spinal cord injury. Am. J. Physiol.-Ren. Physiol. 2016, 310, F1258–F1268. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Hughes, F.M.; Engmann, A.K.; Purves, J.T.; Kasper, H.; Tedaldi, M.; Spruill, L.S.; Gullo, M.; Schwab, M.E.; Kessler, T.M. A novel urodynamic model for lower urinary tract assessment in awake rats: Lower Urinary Tract Assessment in Awake Rats. BJU Int. 2015, 115, 8–15. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Soler, R.; Füllhase, C. Rodent models for urodynamic investigation. Neurourol. Urodyn. 2011, 30, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Downie, J. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol. Urodyn. 1999, 19, 87–99. [Google Scholar] [CrossRef]
- Mann-Gow, T.K.; Larson, T.R.; Wøien, C.T.; Andersen, T.M.; Andersson, K.-E.; Zvara, P. Evaluating the Procedure for Performing Awake Cystometry in a Mouse Model. J. Vis. Exp. 2017, 123, e55588. [Google Scholar] [CrossRef]
- Yokoyama, O.; Ishiura, Y.; Komatsu, K.; Mita, E.; Nakamura, Y.; Kunimi, K.; Morikawa, K.; Namiki, M. Effects of mk-801 on bladder overactm’iy in rats with cerebral infarction. J. Urol. 1998, 159, 571–576. [Google Scholar] [CrossRef]
- Morikawa, K.; Ichihashi, M.; Kakiuchi, M.; Yamauchi, T.; Kato, H.; Ito, Y.; Gomi, Y. Effects of Various Drugs on Bladder Function in Conscious Rats. Jpn. J. Pharmacol. 1989, 50, 369–376. [Google Scholar] [CrossRef]
- Streng, T.; Santti, R.; Andersson, K.-E.; Talo, A. The role of the rhabdosphincter in female rat voiding. BJU Int. 2004, 94, 138–142. [Google Scholar] [CrossRef]
- Streng, T.; Santti, R.; Talo, A. Possible action of the proximal rhabdosphincter muscle in micturition of the adult male rat. Neurourol. Urodyn. 2001, 20, 197–213. [Google Scholar] [CrossRef]
- Holstege, G.; Griffiths, D.; De Wall, H.; Dalm, E. Anatomical and physiological observations on suprapinal control of bladder and urethral sphincter muscles in the cat. J. Comp. Neurol. 1986, 250, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, D.; Shimizu, N.; Wada, N.; Kadekawa, K.; Saito, T.; Mizoguchi, S.; Morizawa, Y.; Hori, S.; Miyake, M.; Torimoto, K.; et al. Effects of a new β3-adrenoceptor agonist, vibegron, on neurogenic bladder dysfunction and remodeling in mice with spinal cord injury. Neurourol. Urodyn. 2020, 39, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Dolber, P.C.; Gu, B.; Zhang, X.; Fraser, M.O.; Thor, K.B.; Reiter, J.P. Activation of the external urethral sphincter central pattern generator by a 5-HT1A receptor agonist in rats with chronic spinal cord injury. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R1699–R1706. [Google Scholar] [CrossRef] [PubMed]
- Urakami, S.; Shiina, H.; Enokida, H.; Kawamoto, K.; Kikuno, N.; Fandel, T.; Vejdani, K.; Nunes, L.; Igawa, M.; Tanagho, E.A.; et al. Functional improvement in spinal cord injury-induced neurogenic bladder by bladder augmentation using bladder acellular matrix graft in the rat. World J. Urol. 2007, 25, 207–213. [Google Scholar] [CrossRef]
- Sartori, A.M.; Hofer, A.-S.; Scheuber, M.I.; Rust, R.; Kessler, T.M.; Schwab, M.E. Slow development of bladder malfunction parallels spinal cord fiber sprouting and interneurons’ loss after spinal cord transection. Exp. Neurol. 2022, 348, 113937. [Google Scholar] [CrossRef]
- Aizawa, N.; Ogawa, S.; Sugiyama, R.; Homma, Y.; Igawa, Y. Influence of urethane-anesthesia on the effect of resiniferatoxin treatment on bladder function in rats with spinal cord injury: Influence of Urethane on the Effect of RTX. Neurourol. Urodyn. 2015, 34, 274–279. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Hsieh, T.-H.; Chen, S.-C.; Lai, C.-H.; Kuo, T.-S.; Chen, C.-P.; Lin, C.-W.; Young, S.-T.; Peng, C.-W. Effects of pudendal neuromodulation on bladder function in chronic spinal cord-injured rats. J. Formos. Med. Assoc. 2016, 115, 703–713. [Google Scholar] [CrossRef]
- Zinck, N.; Rafuse, V.; Downie, J. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp. Neurol. 2007, 204, 777–790. [Google Scholar] [CrossRef]
- Sadeghmousavi, S.; Khaboushan, A.S.; Jafarnezhad-Ansariha, F.; Nejad-Gashti, R.; Farsi, M.; Esmaeil-Pour, R.; Alijani, M.; Zolbin, M.M.; Niknejad, H.; Kajbafzadeh, A. The role of spinal cord tractography in detecting lesions following selective bladder afferent and efferent fibers injury: A novel method for induction of neurogenic lower urinary tract dysfunction in rabbit. Neurourol. Urodyn. 2022, 41, 1539–1552. [Google Scholar] [CrossRef]
- Kameoka, H.; Shiraiwa, Y.; Fukaya, Y.; Yokota, T.; Shishido, K.; Yamaguchi, O. Effect of naloxone on the bladder activity of rabbits with acute spinal injury. Int. J. Urol. 1998, 5, 588–594. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Durant, P.A.; Brent, C.R. Micturition in rats: A chronic model for study of bladder function and effect of anesthetics. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1986, 251, R1177–R1185. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Havton, L.A. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am. J. Physiol. Physiol. 2008, 295, F1248–F1253. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.W.; Damaser, M.S. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001, 69, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Pickering, A.E.; Igawa, Y.; Kanai, A.J.; Fry, C.H.; Drake, M.J. Muro-Neuro-Urodynamics; a Review of the Functional Assessment of Mouse Lower Urinary Tract Function. Front. Physiol. 2017, 8, 49. [Google Scholar] [CrossRef]
- Salehi-Pourmehr, H.; Mahmoudi, J.; Vahdat, A.S.; Hajebrahimi, S.; Abolhasanpour, N. A comprehensive visual report of urodynamic study in rats with spinal cord injury. Curr. Urol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Kihara, K.; de Groat, W.C. Sympathetic efferent pathways projecting to the bladder neck and proximal urethra in the rat. J. Auton. Nerv. Syst. 1997, 62, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Birder, L.; Nakamura, Y.; Kiss, S.; Nealen, M.; Barrick, S.; Kanai, A.; Wang, E.; Ruiz, G.; de Groat, W.; Apodaca, G.; et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002, 5, 856–860. [Google Scholar] [CrossRef]
- Cornelissen, L.L.; Misajet, B.; Brooks, D.P.; Hicks, A. Influence of genetic background and gender on bladder function in the mouse. Auton. Neurosci. 2008, 140, 53–58. [Google Scholar] [CrossRef]
- Comiter, C.; Phull, H.S. Angiotensin II type 1 (AT-1) receptor inhibition partially prevents the urodynamic and detrusor changes associated with bladder outlet obstruction: A mouse model: Effects of an at-1 antagonist in boo. BJU Int. 2011, 109, 1841–1846. [Google Scholar] [CrossRef]
- Mingin, G.C.; Heppner, T.J.; Tykocki, N.R.; Erickson, C.S.; Vizzard, M.A.; Nelson, M.T. Social stress in mice induces urinary bladder overactivity and increases TRPV1 channel-dependent afferent nerve activity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R629–R638. [Google Scholar] [CrossRef]
- Smith, P.P.; DeAngelis, A.; Kuchel, G.A. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012, 302, R577–R586. [Google Scholar] [CrossRef]
- Chancellor, M.B.; Rivas, D.A.; Acosta, R.; Erhard, M.J.; Moore, J.; Salzman, S.K. Detrusor-Myoplasty, Innervated Rectus Muscle Transposition Study, and Functional Effect on the Spinal Cord Zyzxywxvwzuvytsxurw Injury Rat Model. Neurourol. Urodyn. 1994, 13, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.F.; Holstege, G. The central nervous system control of micturition in cats and humans. Behav. Brain Res. 1998, 92, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Gernone, F.; Uva, A.; Cavalera, M.A.; Zatelli, A. Neurogenic Bladder in Dogs, Cats and Humans: A Comparative Review of Neurological Diseases. Animals 2022, 12, 3233. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.; Wang, J.; Wang, X.; Roppolo, J.R.; de Groat, W.C. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol. Urodyn. 2007, 26, 879–886. [Google Scholar] [CrossRef]
- Yoo, P.B.; Woock, J.P.; Grill, W.M. Somatic innervation of the feline lower urinary tract. Brain Res. 2008, 1246, 80–87. [Google Scholar] [CrossRef]
- Nishizawa, O.; Satoh, S.; Harada, T.; Nakamura, H.; Fukuda, T.; Tsukada, T.; Tsuchida, S. Role of the Pudendal Nerves on the Dynamics of Micturition in the Dog Evaluated by Pressure Flow Emg and Pressure Flow Plot Studies. J. Urol. 1984, 132, 1036–1039. [Google Scholar] [CrossRef]
- Tulloch, A.G.S.; Rossier, A.B. The autonomic nervous system and the bladder during spinal shock—An experimental study. Spinal Cord 1975, 13, 42–48. [Google Scholar] [CrossRef]
- Jonas, U.; Jonestr, L.; Tanagho, E. Recovery of Bladder Function after Spinal Cord Transection. J. Urol. 1975, 113, 626–628. [Google Scholar] [CrossRef]
- Levine, J.M.; Levine, G.J.; Porter, B.F.; Topp, K.; Noble-Haeusslein, L.J. Naturally Occurring Disk Herniation in Dogs: An Opportunity for Pre-Clinical Spinal Cord Injury Research. J. Neurotrauma 2011, 28, 675–688. [Google Scholar] [CrossRef]
- Li, P.; Liao, L.; Chen, G.; Zhang, F.; Tian, Y. Early low-frequency stimulation of the pudendal nerve can inhibit detrusor overactivity and delay progress of bladder fibrosis in dogs with spinal cord injuries. Spinal Cord 2013, 51, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Sawan, M.; Duval, F.; Hassouna, M.; Li, J.S.; Elhilali, M.M. A transcutaneous implantable bladder controller. Neurourol. Urodyn. 1993, 12, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Holmquist, B.; Olin, T. Electromicturition in Male Dogs at Pelvic Nerve Stimulation: An Urethrocystographic Study. Scand. J. Urol. Nephrol. 1968, 2, 115–127. [Google Scholar] [CrossRef]
- Liao, L.-M.; Ju, Y.-H. Electrical stimulation of dog pudendal nerve regulates the excitatory pudendal-to-bladder reflex. Neural Regen. Res. 2016, 11, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Cohen, N.D.; Fandel, T.M.; Levine, G.J.; Mankin, J.; Griffin, J.F.; Kerwin, S.C.; Boudreau, C.E.; Trivedi, A.; Noble-Haeusslein, L.J. Early Blockade of Matrix Metalloproteinases in Spinal-Cord–Injured Dogs Results in a Long-Term Increase in Bladder Compliance. J. Neurotrauma 2017, 34, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Dalmose, A.; Bjarkam, C.; Sørensen, J.; Djurhuus, J.; Jørgensen, T. Effects of high frequency deep brain stimulation on urine storage and voiding function in conscious minipigs: Deep Brain Stimulation in Minipigs. Neurourol. Urodyn. 2004, 23, 265–272. [Google Scholar] [CrossRef]
- Mills, I.W.; Noble, J.G.; Brading, A.F. Radiotelemetered cystometry in pigs: Validation and comparison of natural filling versus diuresis cystometry. J. Urol. 2000, 164, 1745–1750. [Google Scholar] [CrossRef]
- Dass, N.; Mcmurray, G.; Greenland, J.E.; Brading, A.F. Morphological aspects of the female pig bladder neck and urethra: Quantitative analysis using computer assisted 3-dimensional reconstructions. J. Urol. 2001, 165, 1294–1299. [Google Scholar] [CrossRef]
- Guan, Z.; Kiruluta, G.; Coolsaet, B.; Elhilali, M. Conscious minipig model for evaluating the lower urinary tract. Neurourol. Urodyn. 1994, 13, 147–158. [Google Scholar] [CrossRef]
- Mitterberger, M.; Pinggera, G.M.; Marksteiner, R.; Margreiter, E.; Plattner, R.; Klima, G.; Bartsch, G.; Strasser, H. Functional and Histological Changes after Myoblast Injections in the Porcine Rhabdosphincter. Eur. Urol. 2007, 52, 1736–1743. [Google Scholar] [CrossRef]
- Herrera-Imbroda, B.; Lara, M.F.; Izeta, A.; Sievert, K.-D.; Hart, M.L. Stress urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter. Adv. Drug Deliv. Rev. 2015, 82–83, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Gabella, G. Intramural Ganglia. In Structure of the Autonomic Nervous System; Springer: Dordrecht, The Netherlands, 1976. [Google Scholar] [CrossRef]
- Brading, A.; Williams, J. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: Effects of atropine and α,β-methylene ATP. Br. J. Pharmacol. 1990, 99, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Streng, T.; Talo, A.; Andersson, K.-E. Transmitters contributing to the voiding contraction in female rats. BJU Int. 2004, 94, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.-E.; Arner, A. Urinary Bladder Contraction and Relaxation: Physiology and Pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef]
- Havton, L.A.; Christe, K.L.; Edgerton, V.R.; Gad, P.N. Noninvasive spinal neuromodulation to map and augment lower urinary tract function in rhesus macaques. Exp. Neurol. 2019, 322, 113033. [Google Scholar] [CrossRef]
- Havton, L.A.; Biscola, N.P.; Christe, K.L.; Colman, R.J. Ketamine-induced neuromuscular reactivity is associated with aging in female rhesus macaques. PLoS ONE 2020, 15, e0236430. [Google Scholar] [CrossRef]
- Fedirchuk, B.; Shefchyk, S.J. Membrane potential changes in sphincter motoneurons during micturition in the decerebrate cat. J. Neurosci. 1993, 13, 3090–3094. [Google Scholar] [CrossRef]
- Langdale, C.L.; Grill, W.M. Phasic activation of the external urethral sphincter increases voiding efficiency in the rat and the cat. Exp. Neurol. 2016, 285, 173–181. [Google Scholar] [CrossRef]
- McGuire, E.J.; Morrissey, S.G. The development of reflex bladder activity following spinal cord injury in cats and a method to control it. Neurourol. Urodyn. 1982, 1, 211–220. [Google Scholar] [CrossRef]
- Streng, T.; Santti, R.; Talo, A. Similarities and differences in female and male rat voiding. Neurourol. Urodyn. 2002, 21, 136–141. [Google Scholar] [CrossRef]
- Janssen, K.; Deng, K.; Majerus, S.J.A.; Lin, D.L.; Hanzlicek, B.; Butler, R.S.; van der Vaart, C.H.; Damaser, M.S. Transurethral versus suprapubic catheterization to test urethral function in rats. Sci. Rep. 2021, 11, 14369. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.; Kung, P.; Hou, W.; Collins, W.F.; Sisto, S.A. Serial transurethral cystometry: A novel method for longitudinal evaluation of reflex lower urinary tract function in adult female rats. Physiol. Rep. 2022, 10, e15131. [Google Scholar] [CrossRef]
- Abelson, B.; Majerus, S.; Sun, D.; Gill, B.C.; Versi, E.; Damaser, M.S. Ambulatory urodynamic monitoring: State of the art and future directions. Nat. Rev. Urol. 2019, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mickle, A.D.; Won, S.M.; Noh, K.N.; Yoon, J.; Meacham, K.W.; Xue, Y.; McIlvried, L.A.; Copits, B.A.; Samineni, V.K.; Crawford, K.E.; et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 2019, 565, 361–365. [Google Scholar] [CrossRef]
- Frainey, B.T.; Majerus, S.J.A.; Derisavifard, S.; Lewis, K.C.; Williams, A.R.; Balog, R.S.; Goldman, H.B.; Damaser, M.S. First in Human Subjects Testing of the UroMonitor: A Catheter-Free Wireless Ambulatory Bladder Pressure Monitor. J. Urol. 2023, in press. [Google Scholar]
- Zhong, Y.; Qian, B.; Zhu, Y.; Ren, Z.; Deng, J.; Liu, J.; Bai, Q.; Zhang, X. Development of an Implantable Wireless and Batteryless Bladder Pressure Monitor System for Lower Urinary Tract Dysfunction. IEEE J. Transl. Eng. Health Med. 2020, 8, 2500107. [Google Scholar] [CrossRef] [PubMed]
- Majerus, S.J.; Hanzlicek, B.; Hacohen, Y.; Cabal, D.; Bourbeau, D.; Damaser, M.S. Wireless and Catheter-Free Bladder Pressure and Volume Sensor. IEEE Sens. J. 2023, in press. [Google Scholar] [CrossRef]
- Medina-Aguinaga, D.; Hoey, R.F.; Munoz, A.; Altamira-Camacho, M.; Quintanar, J.L.; Hubscher, C.H. Choice of cystometric technique impacts detrusor contractile dynamics in wistar rats. Physiol. Rep. 2021, 9, e14724. [Google Scholar] [CrossRef] [PubMed]
- Foditsch, E.E.; Roider, K.; Sartori, A.M.; Kessler, T.M.; Kayastha, S.R.; Aigner, L.; Schneider, M.P. Cystometric and External Urethral Sphincter Measurements in Awake Rats with Implanted Catheter and Electrodes Allowing for Repeated Measurements. J. Vis. Exp. 2018, 131, e56506. [Google Scholar] [CrossRef]
- Monjotin, N.; Farrié, M.; Vergnolle, N.; Le Grand, B.; Gillespie, J.; Junquero, D. Bladder telemetry: A new approach to evaluate micturition behavior under physiological and inflammatory conditions: Bladder Telemetry: A New Approach to Evaluate Micturition Behavior. Neurourol. Urodyn. 2017, 36, 308–315. [Google Scholar] [CrossRef]
- Noël, S.; Massart, L.; Hamaide, A. Urodynamic investigation by telemetry in Beagle dogs: Validation and effects of oral administration of current urological drugs: A pilot study. BMC Vet. Res. 2013, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Praxis Spinal Cord Institute. Rick Hansen Spinal Cord Injury Registry—A Look at Spinal Cord Injury in Canada in 2020; Praxis Spinal Cord Institute: Vancouver, BC, Canada, 2022; p. 20. [Google Scholar]
- Rodríguez-Romero, V.; Cruz-Antonio, L.; Franco-Bourland, R.E.; Guízar-Sahagún, G.; Castañeda-Hernández, G. Changes in renal function during acute spinal cord injury: Implications for pharmacotherapy. Spinal Cord 2013, 51, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Williams, C.R.; Jarrett, S.A.; Garraway, S.M. Spinal Cord Injury Increases Pro-inflammatory Cytokine Expression in Kidney at Acute and Sub-chronic Stages. Inflammation 2021, 44, 2346–2361. [Google Scholar] [CrossRef] [PubMed]
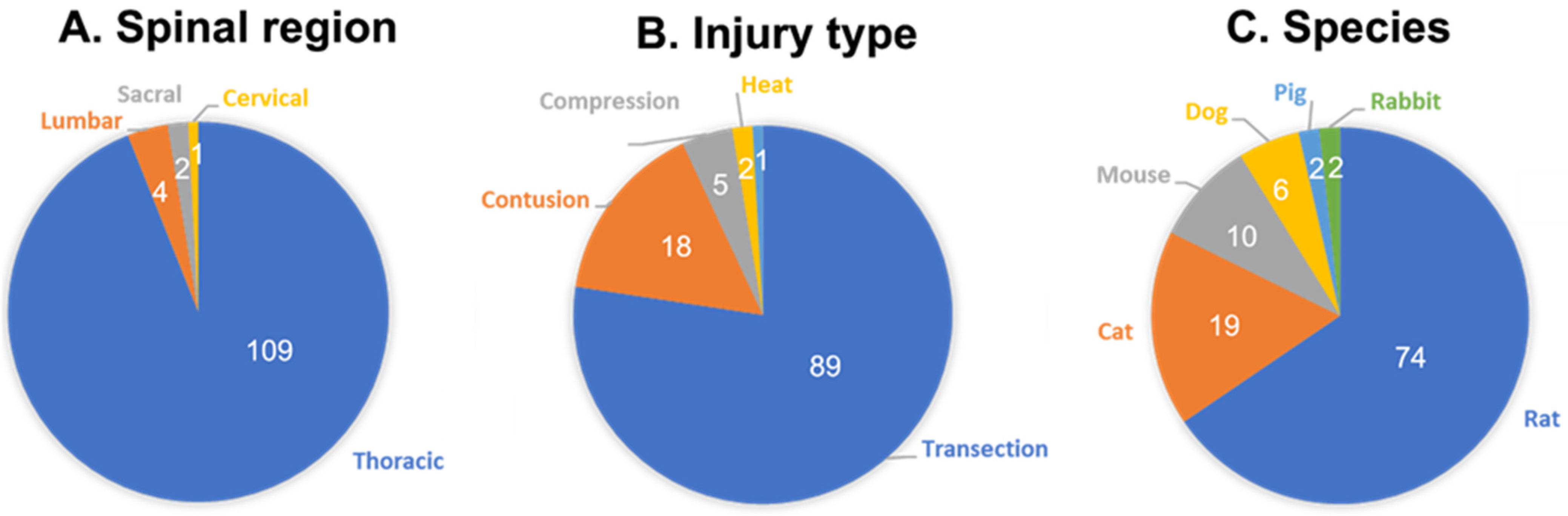

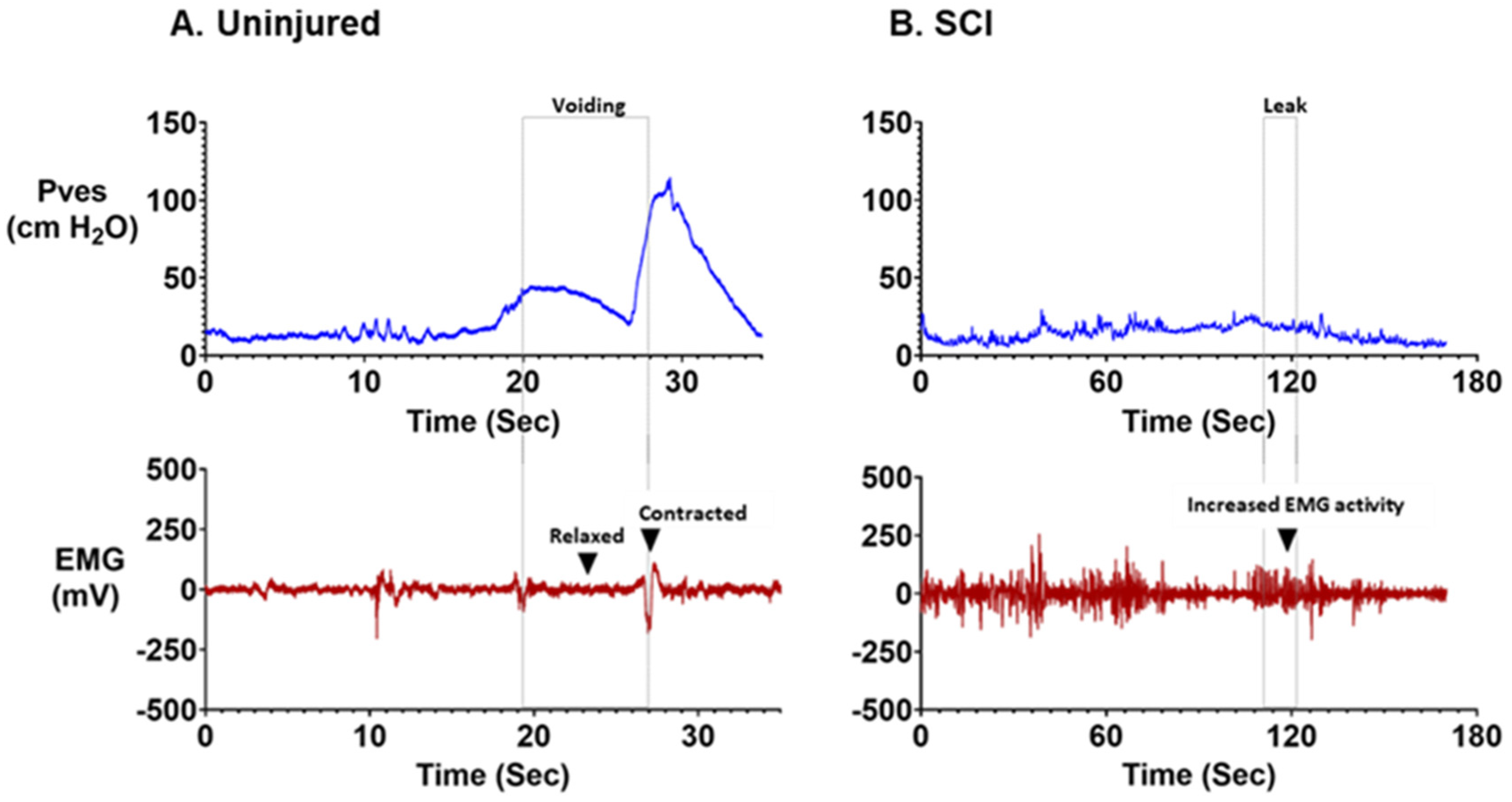
| Animal | What Is the Normal (i.e., Uninjured) Cystometry Pattern Established? | What Has Been Shown to Change after SCI? | What Kinds of Therapies Have Been Tested? | What Are the Disadvantages? | What Are the Advantages? | Refs. |
|---|---|---|---|---|---|---|
| Rats/Mice | EUS EMG silent and active periods (“bursting”) during voiding. Capacity: 0.3–3.8 mL Max detrusor contraction pressure (ΔPdet): 25–50 cm H2O Voided volume: 0.18–1.59 mL Voiding efficiency: 69–94% Contraction duration: 15–85 s | Tonic dyssynergic EUS EMG activation (DSD) Increased capacity: 0.3–26.1 Increased max detrusor ontraction pressure (ΔPdet): 25–77 cm H2O Increased residual volume: 0.13–4 mL Decreased voiding efficiency: 5–78% Neurogenic detrusor overactivity (NDO) present | Drugs: 5′-HT receptor agonists, adrenoreceptor agonists, inosine, n-nitrosoglutathione Cell: stem cells, Anti-Nogo-A antibodies, botulinum toxin-A Tissue: Peripheral nerve transplantation, bladder augmentation, detrusor myoplasty, hypogastric nerve resection, pelvic nerve resection. Device: Tibial, sacral and pudendal neuromodulation | Very small in comparison to human bladder. Challenges with catheterization and EMG recording EUS bursting during normal voiding complicates DSD assessment. | Low cost Widely available Easy access to reagents for imaging studies Well characterized Transgenic approaches Short lifespan | [36,40,44,53,55,56,59,67,69,73,74,75,76,77,78,79,80,81,82,84,85,86,87,88,89,90,92,93,95,97,98,101,102,103,104,105,107,108,109,110,112,115,117,118,118,119,122,150,163,164,165,168] |
| Cats | Silent EUS EMG during voiding Capacity: 5–30 mL Max bladder contraction pressure (ΔPves): 20–60 cm H2O Voided volume: 70 mL | Increased bladder capacity: 30–45 mL Max bladder contraction pressure (ΔPves): 20–40 cm H2O Decreased voided volume: 1–40 mL Reduced voiding efficiency: ~13% Detrusor areflexia immediately after SCI NDO (time-dependent; most prominent at 4 weeks post-SCI) | Stimulation: Pudendal nerve stimulation, sacral neuromodulation, deep perineal nerve stimulation, direct bladder stimulation, pelvic plexus stimulation. 5′-HT1A receptor agonists Autografting adrenal medulla | Less well characterized Challenges obtaining ethical approval More prominent health-risks during anesthesia. | Larger size | [29,30,128,129,130,131] |
| Dogs | EUS EMG bursting activity during voiding. Bladder capacity: 100–200 mL | Acute urinary retention and detrusor areflexia for 2–6 weeks post-SCI Increased capacity: 100–300 mL Decreased max bladder contraction pressure (ΔPves): 20–80 cm H2O NDO at 1 and 3 months post-SCI | Stimulation: Transcutaneous bladder stimulation, Pelvic nerve stimulation, Pudendal nerve stimulation, Sacral root stimulation, Cell: Matrix metalloproteinase inhibitor | Less well characterized Challenges obtaining ethical approval Increased housing cost/requirements | Larger size | [44,137,140] |
| Pigs | Silent EUS EMG during voiding. Capacity: 350–500 mL Max bladder contraction pressure (ΔPves): 15–50 cm H2O Voided vol: 150–800 mL Flow rate: 10–65 mL/s Efficiency: 95–100% Compliance: 40 mL/cm H2O | Increased capacity: 300–600 mL Decreased efficiency: 1–20% Decreased max bladder contraction pressure (ΔPves): ~27 cm H2O Decreased voided volume: 5–50 mL Increased residual volume: 500–700 mL Decreased compliance: 10–40 cm H2O Neurogenic detrusor overactivity noted in most animals at 12 weeks post-SCI Detrusor sphincter dyssynergia present. | Sacral neuromodulation Cell-based therapies including myoblast injections. | Housing costs high. Limited labs performing research. High-level care needed during recovery. Ethical challenges. Less well characterized | Clinical relevance regarding bladder size, LUT physiology, anatomy Ability to test human-sized devices Test same recording equipment as clinical cystometry | [31,141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doelman, A.W.; Streijger, F.; Majerus, S.J.A.; Damaser, M.S.; Kwon, B.K. Assessing Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury: Animal Models in Preclinical Neuro-Urology Research. Biomedicines 2023, 11, 1539. https://doi.org/10.3390/biomedicines11061539
Doelman AW, Streijger F, Majerus SJA, Damaser MS, Kwon BK. Assessing Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury: Animal Models in Preclinical Neuro-Urology Research. Biomedicines. 2023; 11(6):1539. https://doi.org/10.3390/biomedicines11061539
Chicago/Turabian StyleDoelman, Adam W., Femke Streijger, Steve J. A. Majerus, Margot S. Damaser, and Brian K. Kwon. 2023. "Assessing Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury: Animal Models in Preclinical Neuro-Urology Research" Biomedicines 11, no. 6: 1539. https://doi.org/10.3390/biomedicines11061539
APA StyleDoelman, A. W., Streijger, F., Majerus, S. J. A., Damaser, M. S., & Kwon, B. K. (2023). Assessing Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury: Animal Models in Preclinical Neuro-Urology Research. Biomedicines, 11(6), 1539. https://doi.org/10.3390/biomedicines11061539






