Assessment of the In Vitro and In Vivo Antitumor Activity of Hemocyanins from Helix aspersa, Helix lucorum, and Rapana venosa in a Graffi Myeloid Tumor Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of Hemocyanins from H. lucorum, H. aspersa, and R. venosa and Mucus from H. aspersa
2.1.1. Isolation of Native Hemocyanins and Their Structural Subunits
2.1.2. Isolation of H. aspersa Mucus
2.2. In Vitro Experiments
2.2.1. Cell Cultures and Cultivation
2.2.2. MTT Assay
2.2.3. Acridine Orange/Ethidium Bromide (AO/EB) Dual Staining
2.2.4. DAPI Staining
2.2.5. Transmission Electron Microscopy (TEM)
2.3. In Vivo Experiments
2.3.1. Animals and Animal Care
2.3.2. In Vivo Antitumor Activity Studies
2.3.3. Hematological Analysis
2.3.4. Serological Analysis
2.3.5. Assessment of Biometric Parameters of Tumor Growth
2.3.6. Histopathological Analysis
2.4. Statistical Analysis
3. Results
3.1. Isolation of Hemocyanins and Their Isoforms
3.2. Effects of the Hemocyanins on the Viability and Proliferative Activity of Graffi Tumor Cells In Vitro
3.3. Dual Staining of Graffi Tumor Cells with Acridine Orange/Ethidium Bromide
3.4. DAPI Staining of Graffi Tumor Cells
3.5. Transmission Electron Microscopy
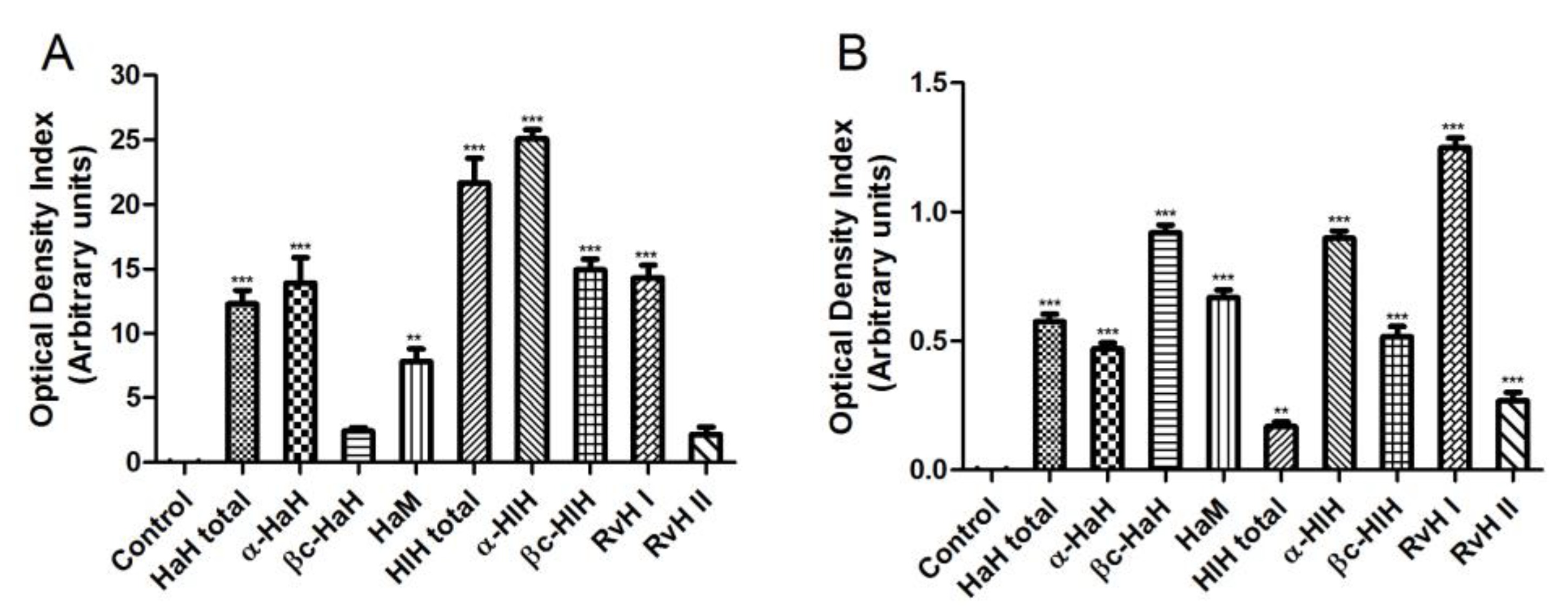
3.6. Hematological Studies
3.7. Serological Studies
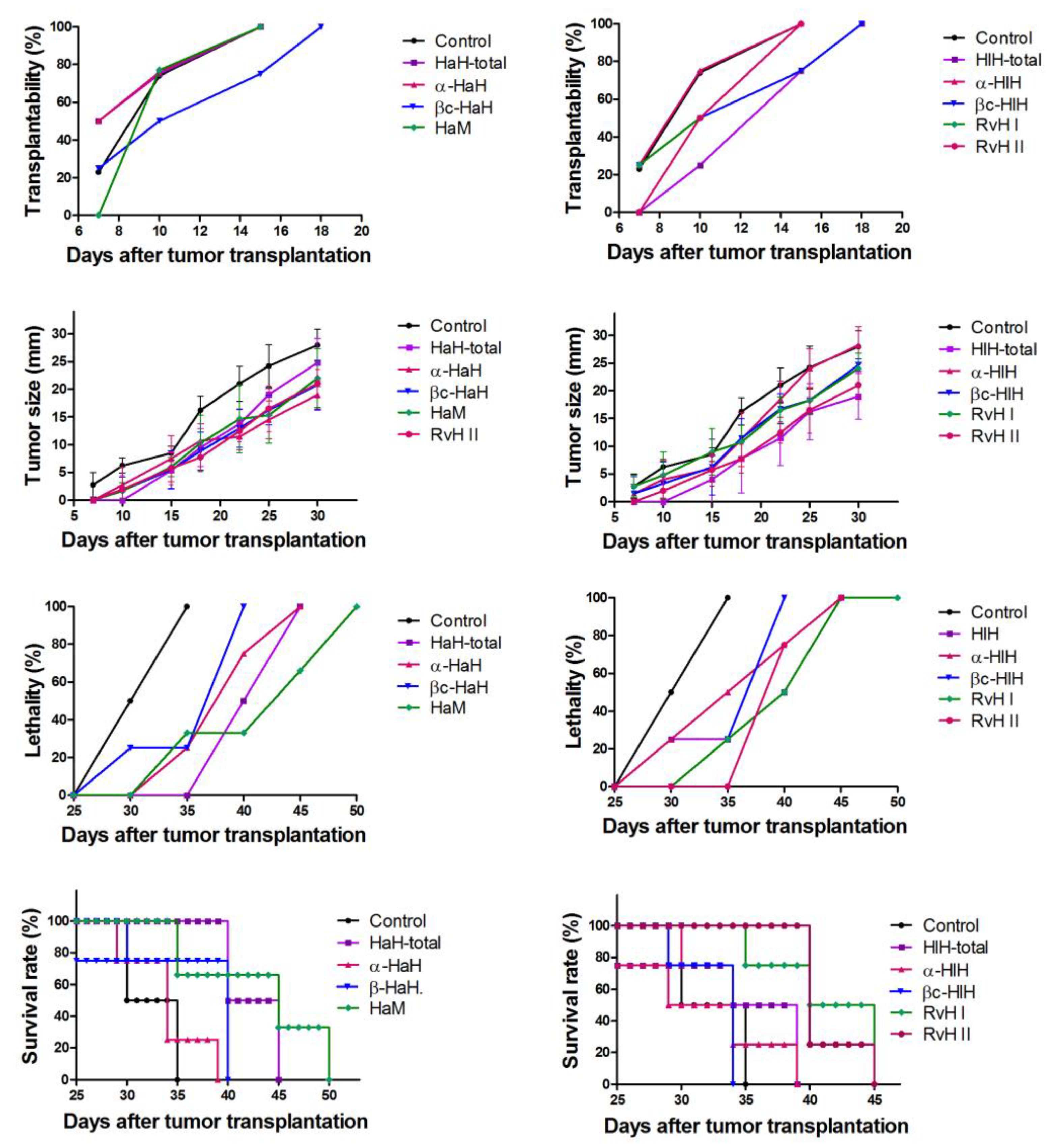
3.8. Assessment of Biometric Parameters of Tumor Growth
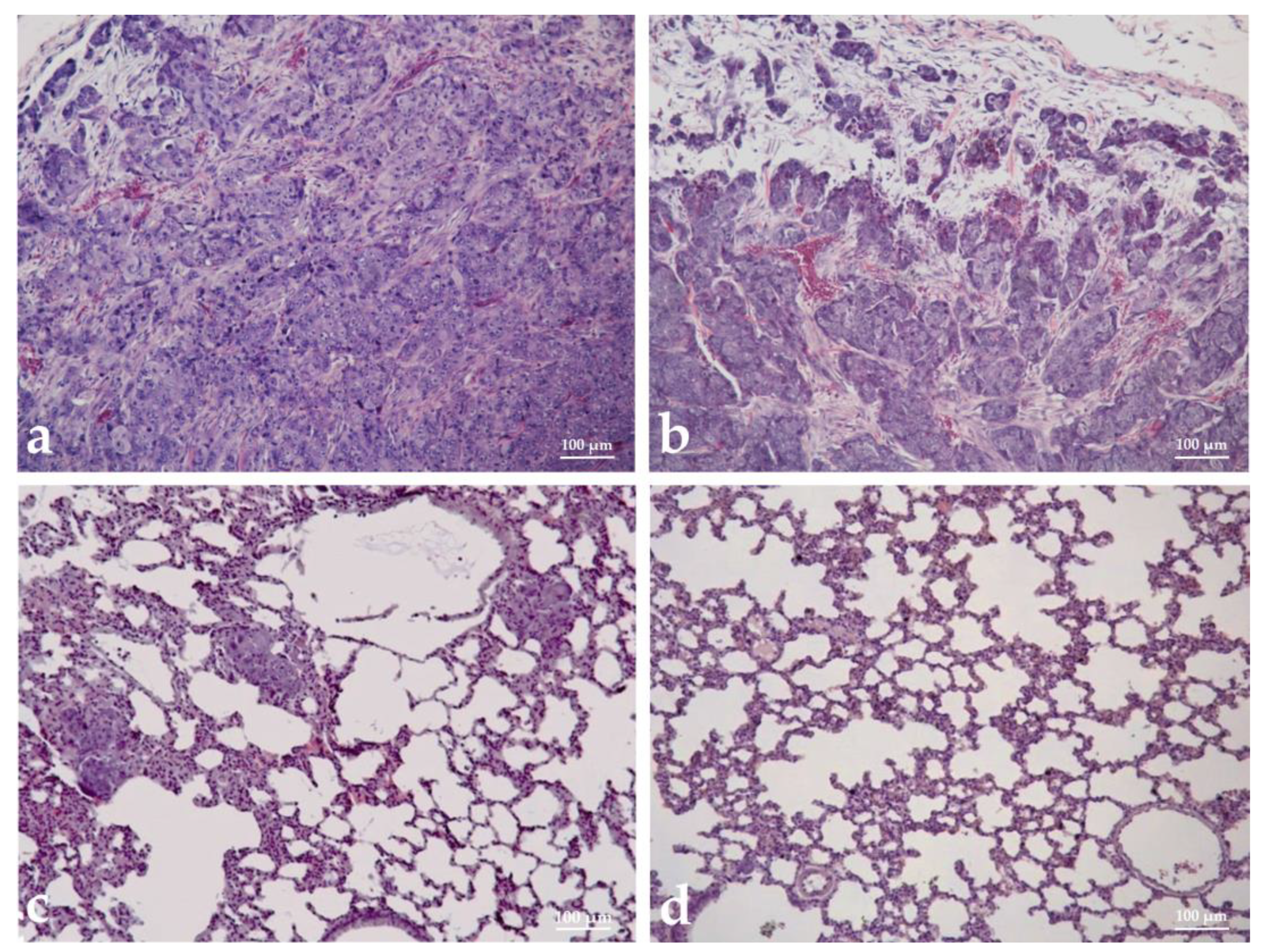
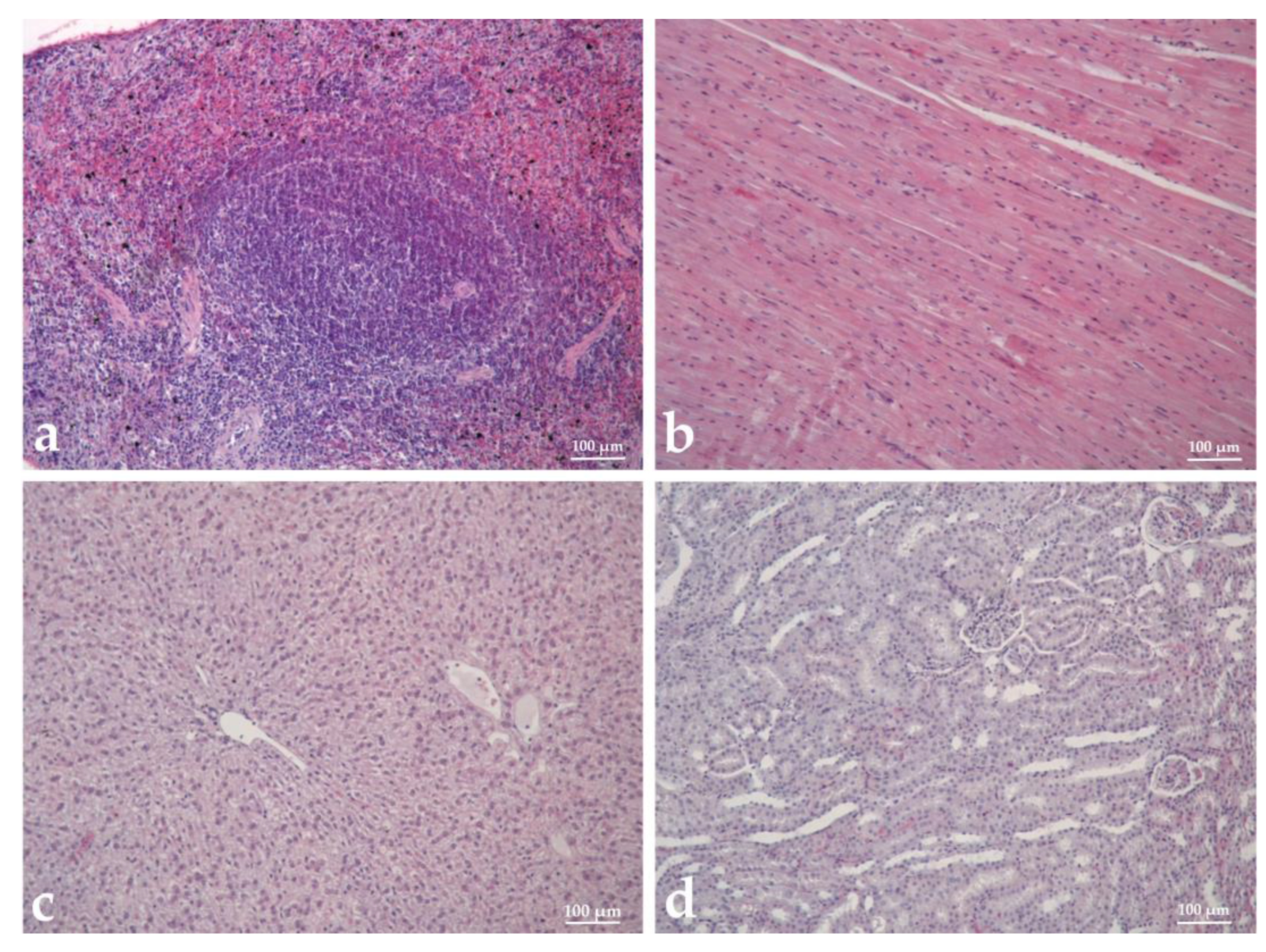
3.9. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kato, S.; Matsui, T.; Gatsogiannis, C.; Tanaka, Y. Molluscan hemocyanin: Structure, evolution, and physiology. Biophys. Rev. 2018, 10, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Decker, H. Immunological properties of oxygen-transport proteins: Hemoglobin, hemocyanin and hemerythrin. Cell. Mol. Life Sci. 2017, 74, 293–317. [Google Scholar] [CrossRef]
- Coates, C.J.; Costa-Paiva, E.M. Multifunctional roles of hemocyanins. Subcell Biochem. 2020, 94, 233–250. [Google Scholar] [PubMed]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Van Holde, K.E.; Miller, K.I.; Decker, H. Hemocyanins and invertebrate evolution. J. Biol. Chem. 2001, 276, 15563–15566. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Espinoza, C.; Salazar, F.; Del Campo, M.; Tampe, R.; Zhong, T.Y.; De Ioannes, P.; Moltedo, B.; Ferreira, J.; Lavelle, E.C.; et al. A novel immunomodulatory hemocyanin from the limpet Fissurella latimarginata promotes potent anti-tumor activity in melanoma. PLoS ONE 2014, 9, e87240. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Nova, E.; Del Campo, M.; Manubens, A.; De Ioannes, A.; Ferreira, J.; Becker, M.I. The oxygen-binding properties of hemocyanin from the mollusk Concholepas concholepas. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1746–1757. [Google Scholar] [CrossRef]
- Salazar, M.L.; Jiménez, J.M.; Villar, J.; Rivera, M.; Báez, M.; Manubens, A.; Becker, M.I. N-Glycosylation of mollusk hemocyanins contributes to their structural stability and immunomodulatory properties in mammals. J. Biol. Chem. 2019, 294, 19546–19564. [Google Scholar] [CrossRef]
- Becker, M.I.; Arancibia, S.; Salazar, F.; Del Campo, M.; De Ioannes, A. Mollusk Hemocyanins as natural immunostimulants in biomedical applications. In Immune Response Activation; IntechOpen Press: London, UK, 2014; pp. 45–72. [Google Scholar] [CrossRef]
- Lammers, R.J.; Witjes, W.P.; Janzing-Pastors, M.H.; Caris, C.T.; Witjes, J.A. Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: Results from a prospective randomized phase III trial. J. Clin. Oncol. 2012, 30, 2273–2279. [Google Scholar] [CrossRef]
- Jiménez, J.M.; Salazar, M.L.; Arancibia, S.; Villar, J.; Salazar, F.; Brown, G.D.; Lavelle, E.C.; Martínez-Pomares, L.; Ortiz-Quintero, J.; Lavandero, S.; et al. TLR4, but neither Dectin-1 nor Dectin-2, participates in the mollusk hemocyanin-induced proinflammatory effects in antigen-presenting cells from mammals. Front. Immunol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Velkova, L.; Dolashka, P.; Van Beeumen, J.; Devreese, B. N-glycan structures of β-HlH subunit of Helix lucorum hemocyanin. Carbohydr. Res. 2017, 449, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Salazar, F.; Becker, M.I. Hemocyanins in the immunotherapy of superficial bladder cancer. In Bladder Cancer—From Basic to Robotic Surgery; InTech: Rijeka, Croatia, 2012; pp. 221–242. [Google Scholar]
- Zhong, T.Y.; Arancibia, S.; Born, R.; Tampe, R.; Villar, J.; Del Campo, M.; Manubens, A.; Becker, M.I. Hemocyanins stimulate innate immunity by inducing different temporal patterns of proinflammatory cytokine expression in macrophages. J. Immunol. 2016, 196, 4650–4662. [Google Scholar] [CrossRef]
- Villar, J.; Salazar, M.L.; Jiménez, J.M.; Campo, M.D.; Manubens, A.; Gleisner, M.A.; Ávalos, I.; Salazar-Onfray, F.; Salazar, F.; Mitchell, D.A.; et al. C-type lectin receptors MR and DC-SIGN are involved in recognition of hemocyanins, shaping their immunostimulatory effects on human dendritic cells. Eur. J. Immunol. 2021, 51, 1715–1731. [Google Scholar] [CrossRef]
- Pizarro-Bauerle, J.; Maldonado, I.; Sosoniuk-Roche, E.; Vallejos, G.; López, M.N.; Salazar-Onfray, F.; Aguilar-Guzmán, L.; Valck, C.; Ferreira, A.; Becker, M.I. Molluskan hemocyanins activate the classical pathway of the human complement system through natural antibodies. Front. Immunol. 2017, 8, 188. [Google Scholar] [CrossRef]
- Sarker, M.M.R.; Zhong, M. Keyhole limpet hemocyanin augmented the killing activity, cytokine production and proliferation of NK cells, and inhibited the proliferation of Meth A sarcoma cells in vitro. Indian J. Pharmacol. 2014, 46, 40. [Google Scholar] [CrossRef]
- Antonova, O.; Yossifova, L.; Staneva, R.; Stevanovic, S.; Dolashka, P.; Toncheva, D. Changes in the gene expression profile of the bladder cancer cell lines after treatment with Helix lucorum and Rapana venosa hemocyanin. J. Buon. 2015, 20, 180–187. [Google Scholar] [PubMed]
- Dolashki, A.; Dolashka, P.; Stenzl, A.; Stevanovic, S.; Aicher, W.K.; Velkova, L.; Velikova, R.; Voelter, W. Antitumour activity of Helix hemocyanin against bladder carcinoma permanent cell lines. Biotechnol. Biotechnol. Equip. 2019, 33, 20–32. [Google Scholar] [CrossRef]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Toshkova, R.; Velkova, L.; Dolashki, A.; Dolashka, P. Hemocyanins from Helix and Rapana snails exhibit in vitro antitumor effects in human colorectal adenocarcinoma. Biomedicines 2020, 8, 194. [Google Scholar] [CrossRef]
- Velkova, L.; Dimitrov, I.; Schwarz, H.; Stevanovic, S.; Voelter, W.; Salvato, B.; Dolashka-Angelova, P. Structure of hemocyanin from garden snail Helix lucorum. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Dolashki, A.; Velkova, L.; Daskalova, E.; Zheleva, N.; Topalova, Y.; Atanasov, V.; Voelter, W.; Dolashka, P. Antimicrobial Activities of Different Fractions from Mucus of the Garden Snail Cornu aspersum. Biomedicines 2020, 8, 315. [Google Scholar] [CrossRef]
- Vassilev, N.G.; Simova, S.D.; Dangalov, M.; Velkova, L.; Atanasov, V.; Dolashki, A.; Dolashka, P. An 1H NMR- and MS-Based Study of Metabolites Profiling of Garden Snail Helix aspersa Mucus. Metabolites 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Gardeva, E.; Toshkova, R.; Yossifova, L.; Minkova, K.; Ivanova, N.; Gigova, L. Antitumor activity of C-phycocyanin from Arthronema africanum (Cyanophyceae). Braz. Arch. Biol. Technol. 2014, 57, 675–684. [Google Scholar] [CrossRef]
- Mossmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meths. 1983, 65, 55. [Google Scholar] [CrossRef]
- Wahab, I.; Abdul, A.; Alzubairi, A.; Elhassan, M.; Mohan, S. In vitro ultramorphological assessment of apoptosis induced by zerumbone on (HeLa). J. Biomed. Biotechnol. 2009, 2009, 769568. [Google Scholar] [PubMed]
- Ignatova, M.G.; Manolova, N.E.; Toshkova, R.A.; Rashkov, I.B.; Gardeva, E.G.; Yossifova, L.S.; Alexandrov, M.T. Electrospun nanofibrous mats containing quaternized chitosan and polylactide with in vitro antitumor activity against HeLa cells. Biomacromolecules 2010, 116, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Toshkova, R.; Manolova, N.; Gardeva, E.; Ignatova, M.; Yossifova, L.; Rashkov, I.; Alexandrov, M. Antitumor activity of quaternized chitosan-based electrospun implants against Graffi myeloid tumor. Int. J. Pharm. 2010, 400, 221–233. [Google Scholar] [CrossRef]
- Gilewski, T.A.; Ragupathi, G.; Dickler, M.; Powell, S.; Bhuta, S.; Panageas, K.; Koganty, R.R.; Chin-Eng, J.; Hudis, C.; Norton, L.; et al. Immunization of high-risk breast cancer patients with clustered sTn-KLH conjugate plus the immunologic adjuvant QS-21. Clin. Cancer Res. 2007, 13, 2977–2985. [Google Scholar] [CrossRef]
- McFadden, D.W.; Riggs, D.R.; Jackson, B.J.; Vona-Davis, L. Keyhole limpet hemocyanin, a novel immune stimulant with promising anticancer activity in Barrett’s esophageal adenocarcinoma. Am. J. Surg. 2003, 186, 552–555. [Google Scholar] [CrossRef]
- Boyanova, O.; Dolashka, P.; Toncheva, D.; Rammensee, H.-G.; Stevanovic, S. In vitro Effect of Molluscan Hemocyanins on CAL-29 and T-24 Bladder Cancer Cell Lines. Biomed. Rep. 2013, 1, 235–238. [Google Scholar] [CrossRef]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Toshkova, R.; Velkova, L.; Atanasov, V.; Dolashki, A.; et al. In vitro antitumour activity of hemocyanins isolated from Helix Aspersa and Helix Lucorum in human bladder carcinoma cells. Comptes Rendus L’académie Bulg. Sci. 2021, 74, 1346–1353. [Google Scholar] [CrossRef]
- Dolashki, A.; Antonova, O.; Velkova, L.; Kaynarov, D.; Voelter, W.; Dolashka, P. Selective cytotoxicity and changes in protein expression of T24 bladder carcinoma permanent cell line after treatment with hemocyanins. Curr. Med. Chem. 2022, 29, 6479–6498. [Google Scholar] [CrossRef] [PubMed]
- Antonova, O.; Toncheva, D.; Rammensee, H.G.; Floetenmeyer, M.; Stevanovic, S.; Dolashka, P. In vitro Antiproliferative Effect of Helix aspersa Hemocyanin on Multiple Malignant Cell Lines. Z. Naturforsch. C 2014, 69, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Dolashki, A.; Velkova, L.; Stevanovic, S.; Molin, L.; Traldi, P.; Velikova, R.; Voelter, W. Bioactive compounds isolated from garden snails. J. BioSci. Biotechnol. 2015, 147–155. [Google Scholar]
- Dolashka-Angelova, P.; Beck, A.; Dolashki, A.; Beltramini, M.; Stevanovic, S.; Salvato, B.; Voelter, W. Characterization of the carbohydrate moieties of the functional unit RvH(1)-a of Rapana venosa haemocyanin using HPLC/electrospray ionization MS and glycosidase digestion. Biochem. J. 2003, 374, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Velkova, L.; Shishkov, S.; Kostova, K.; Dolashki, A.; Dimitrov, I.; Atanasov, B.; Devreese, B.; Voelter, W.; Van Beeumen, J. Glycan structures and antiviral effect of the structural subunit RvH2 of Rapana hemocyanin. Carbohydr. Res. 2010, 345, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.R.; Jackson, B.J.; Vona-Davis, L.; Nigam, A.; McFadden, D.W. In vitro effects of keyhole limpet hemocyanin in breast and pancreatic cancer in regards to cell growth, cytokine production, and apoptosis. Am. J. Surg. 2005, 189, 680–684. [Google Scholar] [CrossRef]
- Somasundar, P.; Riggs, D.R.; Jackson, B.J.; McFadden, D.W. Inhibition of melanoma growth by hemocyanin occurs via early apoptotic pathways. Am. J. Surg. 2005, 190, 713–716. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, X.; Zhang, P.; Chen, C.; Liu, S.; Huang, R.; Zhong, M.; Wei, C.; Zhang, Y. Hemocyanin from shrimp Litopenaeus vannamei has antiproliferative effect against HeLa cell in vitro. PLoS ONE 2016, 11, e0151801. [Google Scholar] [CrossRef]
- Petrova, M.; Vlahova, Z.; Schröder, M.; Todorova, J.; Tzintzarov, A.; Gospodinov, A.; Velkova, L.; Kaynarov, D.; Dolashki, A.; Dolashka, P.; et al. Antitumor Activity of Bioactive Compounds from Rapana venosa against Human Breast Cell Lines. Pharmaceuticals 2023, 16, 181. [Google Scholar] [CrossRef]
- Moltedo, B.; Faunes, F.; Haussmann, D.; De Ioannes, P.; De Ioannes, A.E.; Puente, J.; Becker, M.I. Immunotherapeutic effect of Concholepas hemocyanin in the murine bladder cancer model: Evidence for conserved antitumor properties among hemocyanins. J. Urol. 2006, 176, 2690–2695. [Google Scholar] [CrossRef]
- Gesheva, V.; Chausheva, S.; Mihaylova, N.; Manoylov, I.; Doumanova, L.; Idakieva, K.; Tchorbanov, A. Anti-cancer properties of gastropodan hemocyanins in murine model of colon carcinoma. BMC Immunol. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Mora Román, J.J.; Del Campo, M.; Villar, J.; Paolini, F.; Curzio, G.; Venuti, A.; Jara, L.; Ferreira, J.; Murgas, P.; Lladser, A.; et al. Immunotherapeutic potential of mollusk hemocyanins in combination with human vaccine adjuvants in murine models of oral cancer. J. Immunol. Res. 2019, 2019, 7076942. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Kotsopoulos, J.; Brooks, J.D.; Cheung, M.C.; Rosella, L.; Lipscombe, L.; Akbari, M.R.; Austin, P.C.; Narod, S.A. Platelet count and survival after cancer. Cancers 2022, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Wei, Z.J.; Xu, A.M.; Zang, J.H. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int. J Sur. 2018, 56, 320–327. [Google Scholar] [CrossRef]
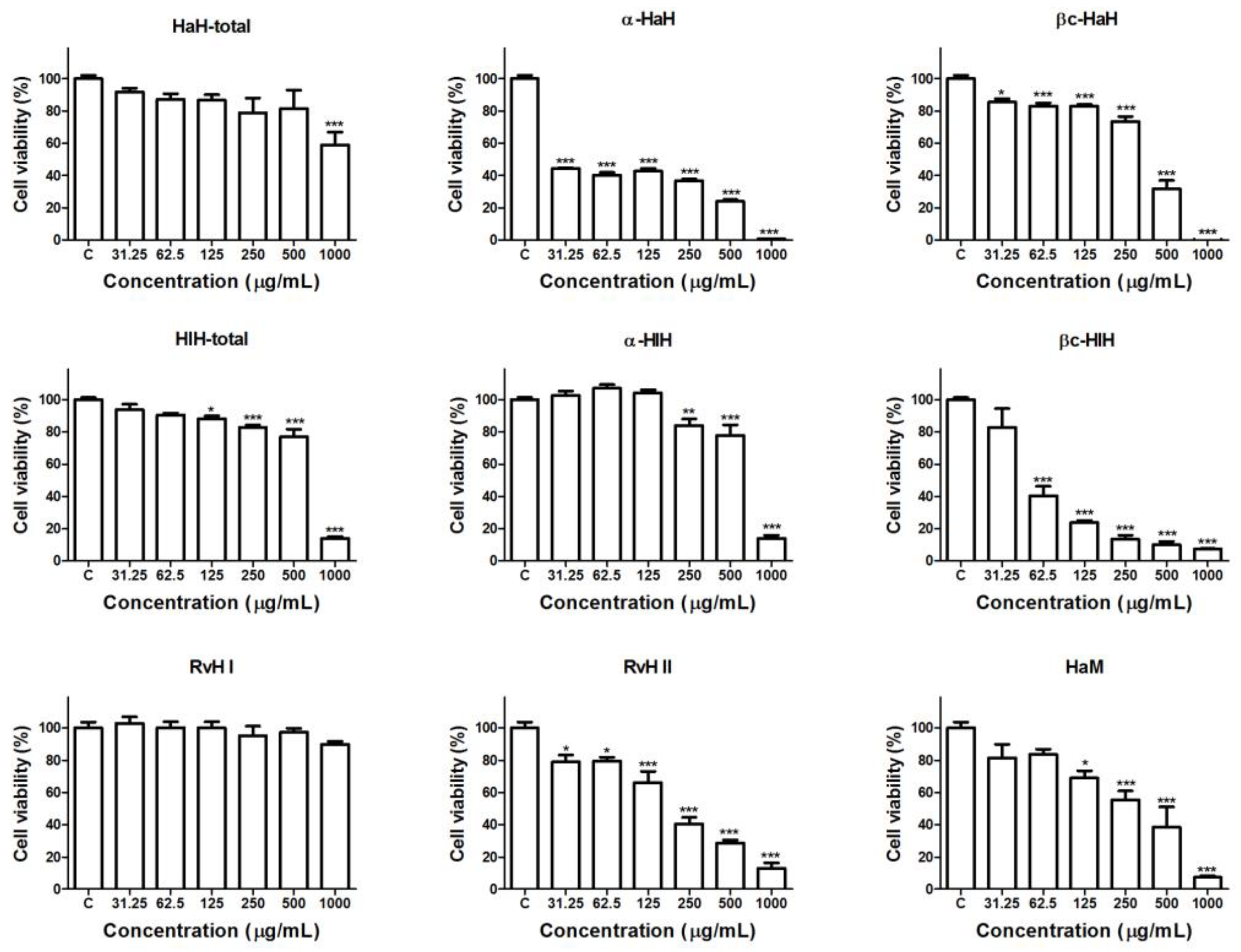

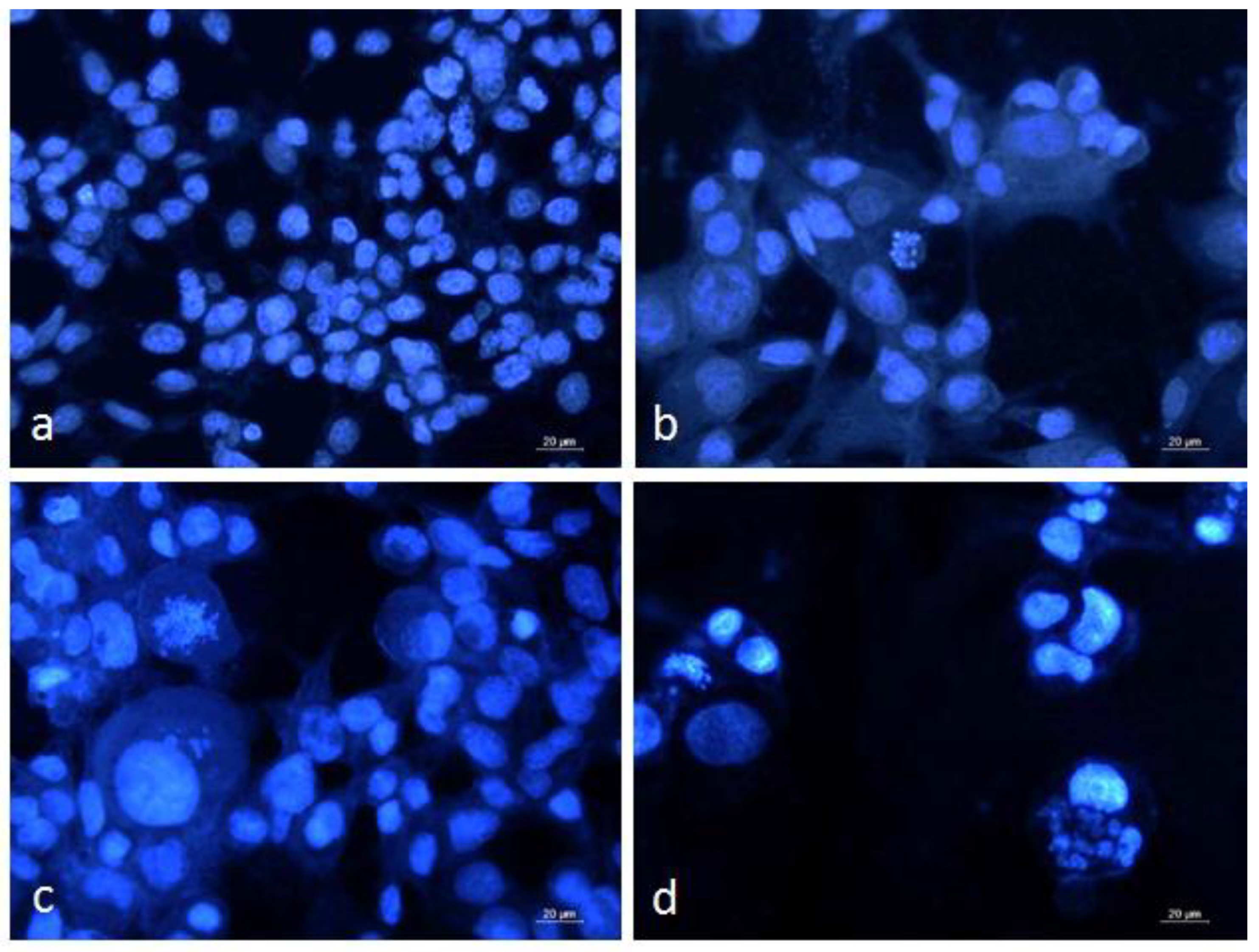
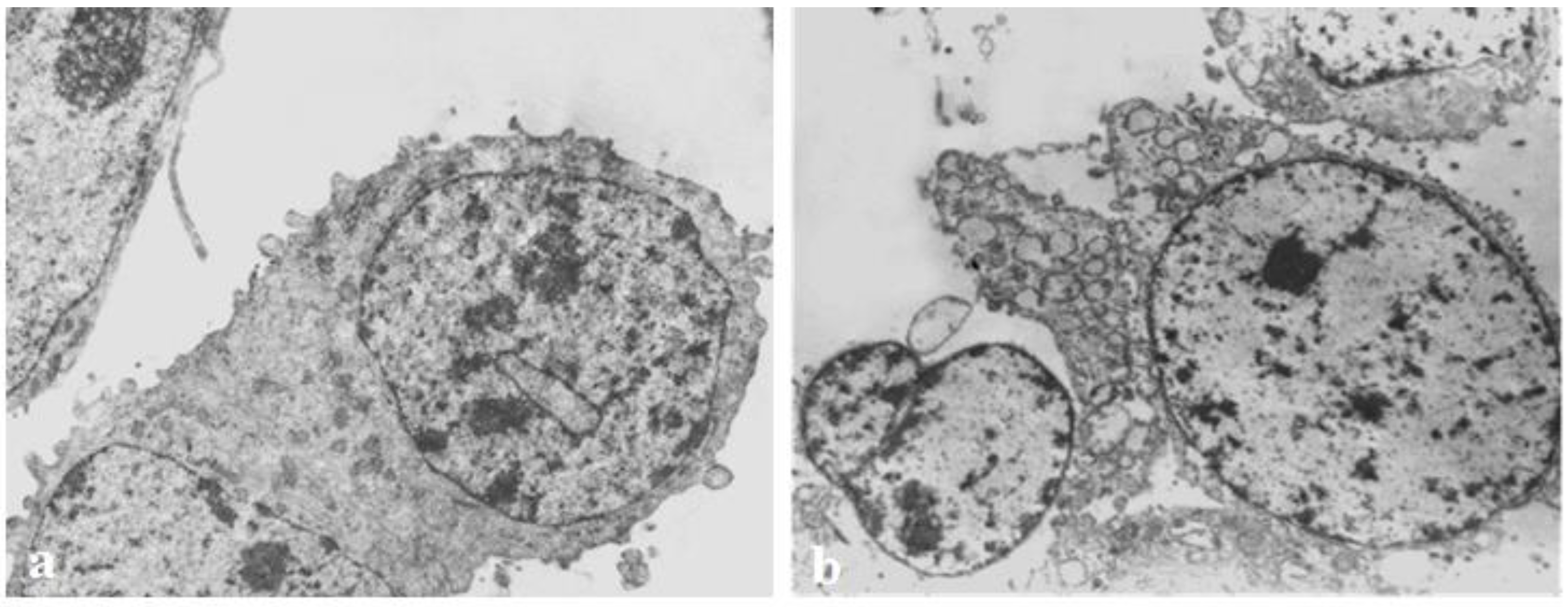
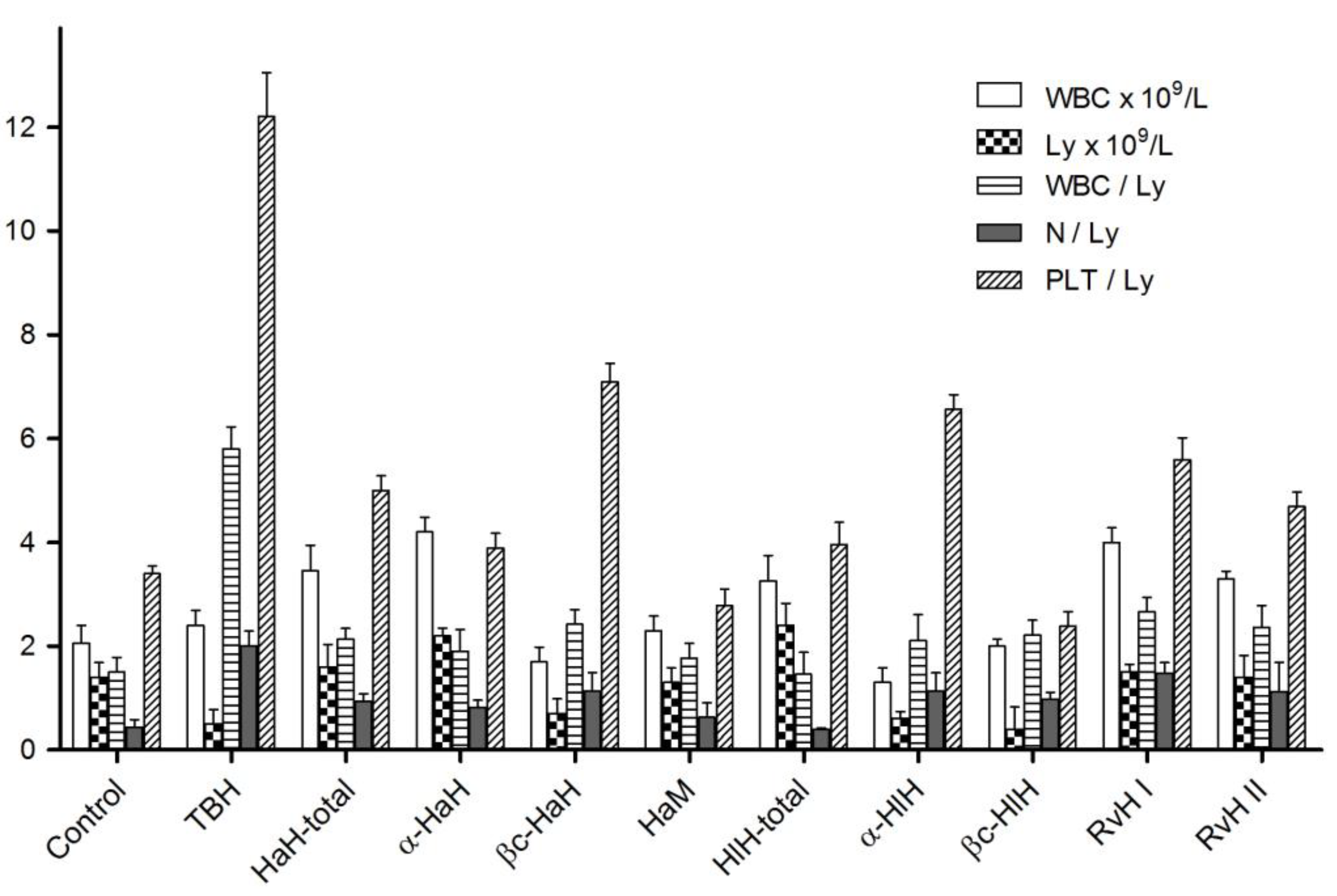
| Hemocyanins. | IC50 Values (µg/mL) |
|---|---|
| HaH-total | >1000 |
| HaH-α | <30 |
| HaH-βc | 340.2 |
| HaM | 259.5 |
| HlH-total | 650.9 |
| HlH-α | 659.6 |
| HlH-βc | 61.1 |
| RvH I | >1000 |
| RvH II | 194.1 |
| DOX | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Dolashki, A.; Velkova, L.; Dolashka, P.; Toshkova, R. Assessment of the In Vitro and In Vivo Antitumor Activity of Hemocyanins from Helix aspersa, Helix lucorum, and Rapana venosa in a Graffi Myeloid Tumor Model. Biomedicines 2023, 11, 1545. https://doi.org/10.3390/biomedicines11061545
Georgieva A, Todorova K, Iliev I, Dilcheva V, Vladov I, Petkova S, Dolashki A, Velkova L, Dolashka P, Toshkova R. Assessment of the In Vitro and In Vivo Antitumor Activity of Hemocyanins from Helix aspersa, Helix lucorum, and Rapana venosa in a Graffi Myeloid Tumor Model. Biomedicines. 2023; 11(6):1545. https://doi.org/10.3390/biomedicines11061545
Chicago/Turabian StyleGeorgieva, Ani, Katerina Todorova, Ivan Iliev, Valeria Dilcheva, Ivelin Vladov, Svetlozara Petkova, Aleksandar Dolashki, Lyudmila Velkova, Pavlina Dolashka, and Reneta Toshkova. 2023. "Assessment of the In Vitro and In Vivo Antitumor Activity of Hemocyanins from Helix aspersa, Helix lucorum, and Rapana venosa in a Graffi Myeloid Tumor Model" Biomedicines 11, no. 6: 1545. https://doi.org/10.3390/biomedicines11061545






