Elevated Serum Urea-to-Creatinine Ratio and In-Hospital Death in Patients with Hyponatremia Hospitalized for COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definition of Covariates
2.3. Outcomes
2.4. Statistical Analysis
3. Results
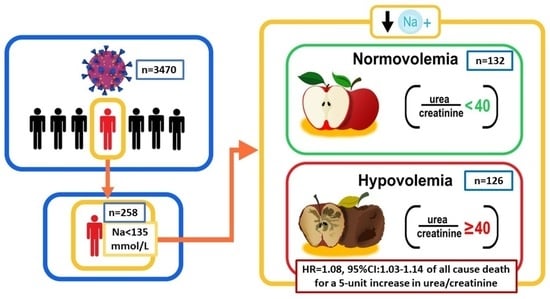
3.1. General Characteristics of Hyponatremic COVID-19 Patients
3.2. General Characteristics of Hyponatremic COVID-19 Patients Partitioned Based on a UCR < or ≥40 at Baseline
3.3. Relationship of Serum Urea-to-Creatinine Ratio at Admission and the Incidence of In-Hospital Death or Admission to Intensive Care Unit in Hyponatremic COVID-19 Patients
3.4. Relationship between Changes in the Urea/Creatinine Ratio and Outcomes in Hyponatremic COVID-19 Patients
3.5. Prognostic Role of Changes in Serum Sodium Values in Hyponatremic Patients Partitioned Based on a UCR < or ≥40 at Baseline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schrier, R.W.; Sharma, S.; Shchekochikhin, D. Hyponatraemia: More than Just a Marker of Disease Severity? Nat. Rev. Nephrol. 2013, 9, 37–50. [Google Scholar] [CrossRef]
- Wald, R.; Jaber, B.L.; Price, L.L.; Upadhyay, A.; Madias, N.E. Impact of Hospital-Associated Hyponatremia on Selected Outcomes. Arch. Intern. Med. 2010, 170, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Schefold, J.C.; Guignard, V.; Exadaktylos, A.K.; Pfortmueller, C.A. Hyponatraemia Is Independently Associated with In-Hospital Mortality in Patients with Pneumonia. Eur. J. Intern. Med. 2018, 54, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Exuzides, A.; Spalding, J.; Foreman, A.; Jones, A.G.; Colby, C.; Shorr, A.F. Hyponatremia and Hospital Outcomes among Patients with Pneumonia: A Retrospective Cohort Study. BMC Pulm. Med. 2008, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Palin, K.; Moreau, M.L.; Sauvant, J.; Orcel, H.; Nadjar, A.; Duvoid-Guillou, A.; Dudit, J.; Rabié, A.; Moos, F. Interleukin-6 Activates Arginine Vasopressin Neurons in the Supraoptic Nucleus during Immune Challenge in Rats. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1289–E1299. [Google Scholar] [CrossRef]
- Atila, C.; Monnerat, S.; Bingisser, R.; Siegemund, M.; Lampart, M.; Rueegg, M.; Zellweger, N.; Osswald, S.; Rentsch, K.; Christ-Crain, M.; et al. Inverse Relationship between IL-6 and Sodium Levels in Patients with COVID-19 and Other Respiratory Tract Infections: Data from the COVIVA Study. Endocr. Connect. 2022, 11, e220171. [Google Scholar] [CrossRef]
- Govender, N.; Khaliq, O.; Moodley, J.; Naicker, T. Unravelling the Mechanistic Role of ACE2 and TMPRSS2 in Hypertension: A Risk Factor for COVID-19. Curr. Hypertens. Rev. 2022, 18, 130–137. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, J.G.; Núñez-Gil, I.J.; Cuesta, M.; Rubio, M.A.; Maroun-Eid, C.; Arroyo-Espliguero, R.; Romero, R.; Becerra-Muñoz, V.M.; Uribarri, A.; Feltes, G.; et al. Prognostic Impact of Hyponatremia and Hypernatremia in COVID-19 Pneumonia. A HOPE-COVID-19 (Health Outcome Predictive Evaluation for COVID-19) Registry Analysis. Front. Endocrinol. 2020, 11, 599255. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Uppal, N.N.; Sharma, P.; Khanin, Y.; Shah, H.H.; Malieckal, D.A.; Bellucci, A.; Sachdeva, M.; Rondon-Berrios, H.; Jhaveri, K.D.; et al. Prevalence and Outcomes of Hyponatremia and Hypernatremia in Patients Hospitalized with COVID-19. Nephrol. Dial. Transpl. 2021, 36, 1135–1138. [Google Scholar] [CrossRef]
- Frontera, J.A.; Valdes, E.; Huang, J.; Lewis, A.; Lord, A.S.; Zhou, T.; Kahn, D.E.; Melmed, K.; Czeisler, B.M.; Yaghi, S.; et al. Prevalence and Impact of Hyponatremia in Patients With Coronavirus Disease 2019 in New York City. Crit. Care Med. 2020, 48, e1211–e1217. [Google Scholar] [CrossRef]
- Tzoulis, P.; Waung, J.A.; Bagkeris, E.; Hussein, Z.; Biddanda, A.; Cousins, J.; Dewsnip, A.; Falayi, K.; McCaughran, W.; Mullins, C.; et al. Dysnatremia Is a Predictor for Morbidity and Mortality in Hospitalized Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, S.; Regolisti, G.; Rebora, P.; Occhino, G.; Belli, M.; Molon, G.; Citerio, G.; Beltrame, A.; Maloberti, A.; Generali, E.; et al. Negative Prognostic Impact of Electrolyte Disorders in Patients Hospitalized for Covid-19 in a Large Multicenter Study. J. Nephrol. 2022, 36, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Bhasin-Chhabra, B.; Veitla, V.; Weinberg, S.; Koratala, A. Demystifying Hyponatremia: A Clinical Guide to Evaluation and Management. Nutr. Clin. Pr. 2022, 37, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.; Abernethy, W.B.; Simel, D.L. The Rational Clinical Examination. Is This Patient Hypovolemic? JAMA 1999, 281, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.B.; Owen, J.A.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is This Elderly Patient Dehydrated? Diagnostic Accuracy of Hydration Assessment Using Physical Signs, Urine, and Saliva Markers. J. Am. Med. Dir. Assoc. 2015, 16, 221–228. [Google Scholar] [CrossRef]
- Stookey, J.D.; Pieper, C.F.; Cohen, H.J. Is the Prevalence of Dehydration among Community-Dwelling Older Adults Really Low? Informing Current Debate over the Fluid Recommendation for Adults Aged 70+years. Public Health Nutr. 2005, 8, 1275–1285. [Google Scholar] [CrossRef]
- Liamis, G.; Tsimihodimos, V.; Doumas, M.; Spyrou, A.; Bairaktari, E.; Elisaf, M. Clinical and Laboratory Characteristics of Hypernatraemia in an Internal Medicine Clinic. Nephrol. Dial. Transpl. 2008, 23, 136–143. [Google Scholar] [CrossRef]
- Schrier, R.W. Blood Urea Nitrogen and Serum Creatinine: Not Married in Heart Failure. Circ. Heart Fail. 2008, 1, 2–5. [Google Scholar] [CrossRef]
- Shen, S.; Yan, X.; Xu, B. The Blood Urea Nitrogen/Creatinine (BUN/Cre) Ratio Was U-Shaped Associated with All-Cause Mortality in General Population. Ren. Fail. 2022, 44, 184–190. [Google Scholar] [CrossRef]
- Sujino, Y.; Nakano, S.; Tanno, J.; Shiraishi, Y.; Goda, A.; Mizuno, A.; Nagatomo, Y.; Kohno, T.; Muramatsu, T.; Nishimura, S.; et al. Clinical Implications of the Blood Urea Nitrogen/Creatinine Ratio in Heart Failure and Their Association with Haemoconcentration. ESC Heart Fail. 2019, 6, 1274–1282. [Google Scholar] [CrossRef]
- Zhen, Z.; Liang, W.; Tan, W.; Dong, B.; Wu, Y.; Liu, C.; Xue, R. Prognostic Significance of Blood Urea Nitrogen/Creatinine Ratio in Chronic HFpEF. Eur. J. Clin. Investig. 2022, 52, e13761. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Hammerman, H.; Beyar, R.; Yalonetsky, S.; Kapeliovich, M.; Markiewicz, W.; Goldberg, A. Serum Blood Urea Nitrogen and Long-Term Mortality in Acute ST-Elevation Myocardial Infarction. Int. J. Cardiol. 2008, 127, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guo, N.; Duan, X.; Zhou, Q.; Zhang, Z.; Luo, L.; Ge, L. Association between the Blood Urea Nitrogen to Creatinine Ratio and In-hospital Mortality among Patients with Acute Myocardial Infarction: A Retrospective Cohort Study. Exp. Med. 2023, 25, 36. [Google Scholar] [CrossRef]
- Schrock, J.W.; Glasenapp, M.; Drogell, K. Elevated Blood Urea Nitrogen/Creatinine Ratio Is Associated with Poor Outcome in Patients with Ischemic Stroke. Clin. Neurol. Neurosurg. 2012, 114, 881–884. [Google Scholar] [CrossRef]
- Rowat, A.; Graham, C.; Dennis, M. Dehydration in Hospital-Admitted Stroke Patients: Detection, Frequency, and Association. Stroke 2012, 43, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Renner, C.J.; Kasner, S.E.; Bath, P.M.; Bahouth, M.N. VISTA Acute Steering Committee [Link] Stroke Outcome Related to Initial Volume Status and Diuretic Use. J. Am. Heart Assoc. 2022, 11, e026903. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yang, H.; Li, H.; Chen, R.; Yu, J. Relationship between the Blood Urea Nitrogen to Creatinine Ratio and In-Hospital Mortality in Non-Traumatic Subarachnoid Hemorrhage Patients: Based on Propensity Score Matching Method. J. Clin. Med. 2022, 11, 7031. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Zhao, X.; Wang, L.; Liu, F.; Wang, T.; Ye, D.; Lv, Y. Diagnostic Performance of a Blood Urea Nitrogen to Creatinine Ratio-Based Nomogram for Predicting In-Hospital Mortality in COVID-19 Patients. Risk. Manag. Health Policy 2021, 14, 117–128. [Google Scholar] [CrossRef]
- Hosten, A.O. BUN and Creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, NJ, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Schrier, R.W. Blood Urea Nitrogen. J. Am. Coll. Cardiol. 2011, 58, 383–385. [Google Scholar] [CrossRef]
- Testani, J.M.; Cappola, T.P.; Brensinger, C.M.; Shannon, R.P.; Kimmel, S.E. Interaction Between Loop Diuretic-Associated Mortality and Blood Urea Nitrogen Concentration in Chronic Heart Failure. J. Am. Coll. Cardiol. 2011, 58, 375–382. [Google Scholar] [CrossRef]
- Kajimoto, K.; Minami, Y.; Sato, N.; Takano, T. Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry Serum Sodium Concentration, Blood Urea Nitrogen, and Outcomes in Patients Hospitalized for Acute Decompensated Heart Failure. Int. J. Cardiol. 2016, 222, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mohmand, H.K.; Issa, D.; Ahmad, Z.; Cappuccio, J.D.; Kouides, R.W.; Sterns, R.H. Hypertonic Saline for Hyponatremia: Risk of Inadvertent Overcorrection. Clin. J. Am. Soc. Nephrol. 2007, 2, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Sterns, R.H.; Nigwekar, S.U.; Hix, J.K. The Treatment of Hyponatremia. Semin. Nephrol. 2009, 29, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.W.; Fowler, A.J.; Wan, Y.I.; Flower, L.; Heyland, D.K.; Day, A.; Pearse, R.M.; Prowle, J.R.; Puthucheary, Z. Catabolism in Critical Illness: A Reanalysis of the REducing Deaths Due to OXidative Stress (REDOXS) Trial*. Crit. Care Med. 2022, 50, 1072–1082. [Google Scholar] [CrossRef]
- Chapela, S.P.; Simancas-Racines, D.; Montalvan, M.; Frias-Toral, E.; Simancas-Racines, A.; Muscogiuri, G.; Barrea, L.; Sarno, G.; Martínez, P.I.; Reberendo, M.J.; et al. Signals for Muscular Protein Turnover and Insulin Resistance in Critically Ill Patients: A Narrative Review. Nutrients 2023, 15, 1071. [Google Scholar] [CrossRef]



| Overall | Urea/Creatinine < 40 | Urea/Creatinine ≥ 40 | p | Missing (%) | |
|---|---|---|---|---|---|
| n | 258 | 126 | 132 | ||
| Male (n, %) | 178 (69.0) | 89 (70.6) | 89 (67.4) | 0.673 | 0 |
| Age (years, median [Q1–Q3]) | 69 [59, 78] | 65 [52, 75] | 71 [65, 80] | <0.001 | 0 |
| Hematocrit (%, median [Q1–Q3]) | 37.8 [34.0, 41.0] | 38.2 [34.3, 41.5] | 36.9 [34.0, 40.6] | 0.432 | 2.7 |
| Hemoglobin (g/dL, median [Q1–Q3]) | 12.8 [11.3, 13.9] | 12.9 [11.3, 13.9] | 12.5 [11.2, 14.0] | 0.477 | 2.7 |
| White blood cell count (103/µL, median [Q1–Q3]) | 6.38 [4.75, 10.10] | 6.00 [4.45, 9.60] | 7.00 [4.84, 10.44] | 0.213 | 3.9 |
| Urea (mg/dL, median [Q1–Q3]) | 41.5 [30.0, 63.8] | 31.0 [24.0, 43.7] | 52.0 [40.0, 78.7] | <0.001 | 0 |
| Creatinine (mg/dL, median [Q1–Q3]) | 1.00 [0.82, 1.39] | 1.00 [0.82, 1.33] | 1.01 [0.82, 1.41] | 0.879 | 0 |
| Urea/Creatinine (median [Q1–Q3]) | 40.6 [31.1, 51.1] | 31.0 [25.9, 34.8] | 50.5 [44.5, 59.4] | - | 0 |
| Sodium (mmol/L, median [Q1–Q3]) | 133 [131, 134] | 133 [131, 134] | 133 [131, 134] | 0.412 | 0.4 |
| Potassium (mmol/L, median [Q1–Q3]) | 4.11 [3.76, 4.57] | 4.02 [3.66, 4.38] | 4.22 [3.92, 4.70] | 0.002 | 0.4 |
| C-reactive protein (mg/L, median [Q1–Q3]) | 84.6 [41.2, 133.0] | 86.3 [46.5, 132.2] | 81.4 [40.6, 133.0] | 0.721 | 9.3 |
| Ischemic heart disease (n, %) | 45 (17.5) | 14 (11.2) | 31 (23.5) | 0.015 | 0.4 |
| Heart failure (n, %) | 14 (5.4) | 5 (4.0) | 9 (6.8) | 0.472 | 0.4 |
| Peripheral vascular disease (n, %) | 24 (9.3) | 7 (5.6) | 17 (12.9) | 0.073 | 0.4 |
| History of stroke (n, %) | 24 (9.3) | 11 (8.8) | 13 (9.8) | 0.941 | 0.4 |
| Dementia (n, %) | 13 (5.1) | 6 (4.8) | 7 (5.3) | 1 | 0.4 |
| COPD (n, %) | 26 (10.1) | 4 (3.2) | 22 (16.7) | 0.001 | 0.4 |
| Liver failure (n, %) | 19 (7.4) | 6 (4.8) | 13 (9.8) | 0.191 | 0.4 |
| eGFR (mL/min/1.73 m2, median [Q1–Q3]) | 69.7 [47.3, 87.8] | 72.9 [51.6, 89.3] | 67.1 [44.2, 84.5] | 0.097 | 2.7 |
| CKD (eGFR < 60 mL/min/1.73 m2, n, %) | 90 (35.9) | 39 (31.5) | 51 (40.2) | 0.191 | 2.7 |
| Cancer (n, %) | 35 (13.8) | 18 (14.5) | 17 (13.1) | 0.88 | 1.6 |
| Diabetes mellitus (n, %) | 82 (31.9) | 33 (26.4) | 49 (37.1) | 0.087 | 0.4 |
| Charlson Comorbidity Index (median [Q1–Q3]) | 2 [0, 3] | 1 [0, 3] | 2 [1, 3] | 0.001 | 0 |
| Systolic blood pressure (mmHg, median [Q1–Q3]) | 130 [120, 145] | 130 [120, 147] | 130 [120, 145] | 0.78 | 2.7 |
| Diastolic blood pressure (mmHg, median [Q1–Q3]) | 75 [67, 80] | 75 [67, 80] | 75 [67, 80] | 0.89 | 2.7 |
| Heart rate (bpm, median [Q1–Q3]) | 88 [75, 102] | 87 [75, 100] | 89 [75, 105] | 0.259 | 33.7 |
| RAAS inhibitors (n, %) | 102 (42.9) | 40 (33.9) | 62 (51.7) | 0.008 | 7.8 |
| Beta blockers (n, %) | 86 (34.3) | 31 (25.4) | 55 (42.6) | 0.006 | 2.7 |
| Anticoagulants (n, %) | 28 (11.0) | 12 (9.8) | 16 (12.2) | 0.671 | 1.6 |
| Antiarrhythmics (n, %) | 11 (4.3) | 4 (3.3) | 7 (5.4) | 0.601 | 1.9 |
| Model A (n = 258, Deaths = 52) | Model B (n = 255, Deaths = 52) | Model C (n = 247, Deaths = 49) | ||||
|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| 5-point increment of urea/creatinine | 1.06 (1.02–1.12) | 0.0093 | 1.07 (1.02–1.12) | 0.0082 | 1.08 (1.03–1.14) | 0.0011 |
| Age (years) | 1.04 (1.01–1.07) | 0.0109 | 1.04 (1.01–1.07) | 0.0131 | 1.03 (1.01–1.06) | 0.0187 |
| Male (yes vs. no) | 1.40 (0.75–2.62) | 0.2913 | 1.41 (0.75–2.63) | 0.2884 | 1.68 (0.87–3.23) | 0.1220 |
| Charlson Comorbidity Index | 1.46 (1.24–1.71) | <0.001 | 1.46 (1.23–1.74) | <0.001 | 1.18 (0.95–1.46) | 0.1471 |
| Diuretic during hospitalization (yes vs. no) | 0.97 (0.52–1.80) | 0.9246 | 1.12 (0.59–2.12) | 0.7368 | ||
| Corticosteroid during hospitalization (yes vs. no) | 0.90 (0.51–1.59) | 0.7216 | 0.53 (0.28–1.01) | 0.0519 | ||
| Potassium at admission (mmol/L) | 1.61 (1.04–2.48) | 0.0346 | ||||
| eGFR at admission (mL/min/1.73 m2) | 0.97 (0.96–0.99) | 0.0007 | ||||
| Model A (n = 258, ICU = 37) | Model B (n = 255, ICU = 37) | Model C (n = 247, ICU = 37) | ||||
|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| 5-point increment of urea/creatinine | 1.04 (0.98–1.11) | 0.1843 | 1.06 (0.99–1.12) | 0.0664 | 1.06 (0.99–1.12) | 0.0849 |
| Age (years) | 0.97 (0.94–0.99) | 0.0112 | 0.96 (0.94–0.99) | 0.0051 | 0.97 (0.94–0.99) | 0.0108 |
| Male (yes vs. no) | 2.29 (0.95–5.51) | 0.0652 | 2.28 (0.95–5.49) | 0.0657 | 2.19 (0.90–5.36) | 0.0833 |
| Charlson Comorbidity Index | 0.96 (0.76–1.21) | 0.7272 | 0.96 (0.76–1.22) | 0.7586 | 1.07 (0.81–1.41) | 0.6273 |
| Diuretic during hospitalization (yes vs. no) | 0.93 (0.38–2.27) | 0.8754 | 0.97 (0.39–2.38) | 0.9382 | ||
| Corticosteroid during hospitalization (yes vs. no) | 0.63 (0.32–1.23) | 0.1746 | 0.73 (0.36–1.48) | 0.3814 | ||
| Potassium at admission (mmol/L) | 0.66 (0.36–1.22) | 0.1848 | ||||
| eGFR at admission (mL/min/1.73 m2) | 1.01 (0.99–1.03) | 0.3601 | ||||
| Urea/Creatinine < 40 | Urea/Creatinine ≥ 40 | |||
|---|---|---|---|---|
| Model A (n = 92, Deaths = 13) | Model B (n = 107, Deaths = 30) | |||
| Parameter | OR (95% CI) | p Value | OR (95% CI) | p Value |
| ΔNa ≥ 10 mmol/L vs. ΔNa < 10 mmol/L | 0.22 (0.01–3.37) | 0.2766 | 2.93 (1.03–8.36) | 0.0443 |
| Age (years) | 1.05 (0.99–1.12) | 0.1241 | 1.04 (0.99–1.09) | 0.1144 |
| Male (yes vs. no) | 4.57 (0.77–27.1) | 0.0936 | 0.79 (0.28–2.19) | 0.6474 |
| Charlson Comorbidity Index | 1.79 (1.09–2.92) | 0.0201 | 1.32 (1.02–1.71) | 0.0335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regolisti, G.; Rebora, P.; Occhino, G.; Lieti, G.; Molon, G.; Maloberti, A.; Algeri, M.; Giannattasio, C.; Valsecchi, M.G.; Genovesi, S. Elevated Serum Urea-to-Creatinine Ratio and In-Hospital Death in Patients with Hyponatremia Hospitalized for COVID-19. Biomedicines 2023, 11, 1555. https://doi.org/10.3390/biomedicines11061555
Regolisti G, Rebora P, Occhino G, Lieti G, Molon G, Maloberti A, Algeri M, Giannattasio C, Valsecchi MG, Genovesi S. Elevated Serum Urea-to-Creatinine Ratio and In-Hospital Death in Patients with Hyponatremia Hospitalized for COVID-19. Biomedicines. 2023; 11(6):1555. https://doi.org/10.3390/biomedicines11061555
Chicago/Turabian StyleRegolisti, Giuseppe, Paola Rebora, Giuseppe Occhino, Giulia Lieti, Giulio Molon, Alessandro Maloberti, Michela Algeri, Cristina Giannattasio, Maria Grazia Valsecchi, and Simonetta Genovesi. 2023. "Elevated Serum Urea-to-Creatinine Ratio and In-Hospital Death in Patients with Hyponatremia Hospitalized for COVID-19" Biomedicines 11, no. 6: 1555. https://doi.org/10.3390/biomedicines11061555
APA StyleRegolisti, G., Rebora, P., Occhino, G., Lieti, G., Molon, G., Maloberti, A., Algeri, M., Giannattasio, C., Valsecchi, M. G., & Genovesi, S. (2023). Elevated Serum Urea-to-Creatinine Ratio and In-Hospital Death in Patients with Hyponatremia Hospitalized for COVID-19. Biomedicines, 11(6), 1555. https://doi.org/10.3390/biomedicines11061555