The Role of Extracellular Heat Shock Proteins in Cardiovascular Diseases
Abstract
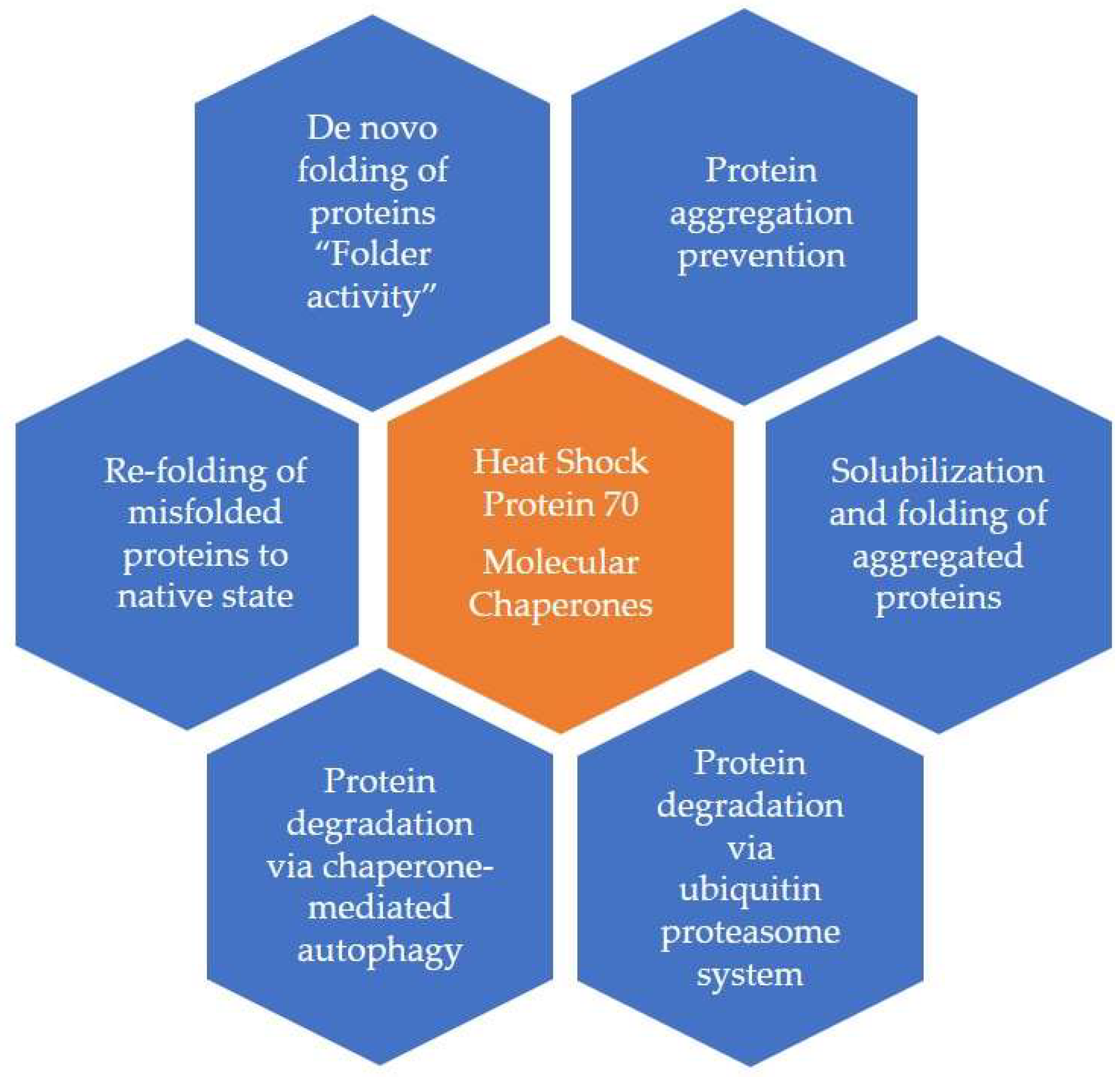
:1. Introduction
2. Biochemistry and Types of HSPs and Related Molecules
2.1. Extracellular Heat Shock Proteins [Ec-HSPs]
2.2. Other HSP70-like Molecules
2.3. Co-Chaperones
2.4. Small HSPs
3. Role in Non-Cardiac Disorders
4. Ec-HSPs and Cardiovascular Diseases
| Family | Important Members | Major Role | Reference |
|---|---|---|---|
| Hsp90 | Hsp90α (HSPC2) | Role in cytoprotection, vascular relaxation, atherosclerosis, and systemic lupus erythematosus. | [17,39,51] |
| Hsp90β (HSPC3) | |||
| Grp94 (HSPC4) | |||
| Hsp70 and HSP70- like molecules | Hsp70 (Hsp72) (HSPA1) | Role in atherosclerosis, heart failure, and hypertension; possible auto-antigen | [17,50,52,53,54,55,56,57] |
| Hsp70 (Hsp73) (HSPA8) | |||
| Grp78 (BIP) (HSPA5) | |||
| Utp (Grp75) (HSPA9) | |||
| Hsp60 | Hsp60—mostly intracellular | Released during cell necrosis; role in atherosclerosis, heart failure, rheumatoid arthritis, diabetes mellitus, and central nervous system disorders. | [10,11,58,59,60,61] |
| Hsp40 | Hsp40 (Dnaj) (DNAJB1) | Collagen preservation | [62] |
| Small Hsp | αCrystallin (HSPB4) | Hsp27 has a protective role in atherosclerosis; antioxidant functions; elevated in various cancers; inhibits apoptotic pathways. Hsp22 has recently been shown to play an important role in cardiomyopathies and age-related cardiac affections. | [63,64] |
| Hsp25 (HSPB1) | |||
| Hsp27 (HSPB2) | |||
| Hsp20 (HSPB6) | |||
| Hsp22 (HSPB8) | |||
| Co-chaperones | CHIP, BAG 3, Hsp40, GrapE, BAG1, BAG2 | Interact with HSP70 and HSP90 during stress. | [17] |
5. Heart Failure
6. HSP70
7. HSP60
8. HSP90
9. HSP 22
10. Cachexia
11. Atherosclerosis and Coronary Artery Disease
12. Acute Coronary Syndromes
13. Hypertension
14. Chronic Atrial Fibrillation
15. Emerging Diagnostic/Therapeutic Possibilities
16. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritossa, F. Discovery of the heat shock response. Cell Stress Chaperones 1996, 1, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 1996, 381, 571–579. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Bolhassani, A.; Gazali, A.; Stebbing, J.; Srivastava, P.; Knowlton, A.; Srivatsa, U.; Leishman, S.J.; Ford, P.J.; et al. Heat-shock proteins in diagnosis and treatment: An overview of different biochemical and immunological functions. Immunotherapy 2019, 11, 215–239. [Google Scholar] [CrossRef]
- De Maio, A. Heat shock proteins: Facts, thoughts, and dreams. Shock 1999, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009, 16, 574–581. [Google Scholar] [CrossRef]
- Dattilo, S.; Mancuso, C.; Koverech, G.; Di Mauro, P.; Ontario, M.L.; Petralia, C.C.; Petralia, A.; Maiolino, L.; Serra, A.; Calabrese, E.J.; et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing 2015, 12, 20. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hightower, L.E.; Guidon, P.T., Jr. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J. Cell. Physiol. 1989, 138, 257–266. [Google Scholar] [CrossRef]
- Zhou, F.; Xing, D.; Chen, W.R. Regulation of HSP70 on activating macrophages using PDT-induced apoptotic cells. Int. J. Cancer 2009, 125, 1380–1389. [Google Scholar] [CrossRef]
- Pockley, A.G.; Wu, R.; Lemne, C.; Kiessling, R.; de Faire, U.; Frostegard, J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension 2000, 36, 303–307. [Google Scholar] [CrossRef]
- Lewthwaite, J.; Owen, N.; Coates, A.; Henderson, B.; Steptoe, A. Circulating human heat shock protein 60 in the plasma of British civil servants: Relationship to physiological and psychosocial stress. Circulation 2002, 106, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Pockley, A.G. Proteotoxic stress and circulating cell stress proteins in the cardiovascular diseases. Cell Stress Chaperones 2012, 17, 303–311. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Torigoe, T.; Kutomi, G.; Hirata, K.; Sato, N. New paradigm for intrinsic function of heat shock proteins as endogenous ligands in inflammation and innate immunity. Curr. Mol. Med. 2012, 12, 1198–1206. [Google Scholar] [CrossRef]
- De Maio, A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: A form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 2011, 16, 235–249. [Google Scholar] [CrossRef]
- Ficker, E.; Dennis, A.T.; Wang, L.; Brown, A.M. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 2003, 92, e87–e100. [Google Scholar] [CrossRef]
- Liao, W.C.; Wu, M.S.; Wang, H.P.; Tien, Y.W.; Lin, J.T. Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas 2009, 38, 422–426. [Google Scholar] [CrossRef]
- Mehta, T.A.; Greenman, J.; Ettelaie, C.; Venkatasubramaniam, A.; Chetter, I.C.; McCollum, P.T. Heat shock proteins in vascular disease--a review. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 395–402. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug. Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A.K.; Iqbal, H.M.N.; Dhama, K. Heat Shock Proteins: Therapeutic Perspectives in Inflammatory Disorders. Recent. Pat. Inflamm. Allergy Drug. Discov. 2017, 10, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Zilaee, M.; Ferns, G.A.; Ghayour-Mobarhan, M. Heat shock proteins and cardiovascular disease. Adv. Clin. Chem. 2014, 64, 73–115. [Google Scholar] [PubMed]
- Larche, J.; Azoulay, E.; Fieux, F.; Mesnard, L.; Moreau, D.; Thiery, G.; Darmon, M.; Le Gall, J.R.; Schlemmer, B. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003, 29, 1688–1695. [Google Scholar] [CrossRef]
- Hunter-Lavin, C.; Davies, E.L.; Bacelar, M.M.; Marshall, M.J.; Andrew, S.M.; Williams, J.H. Hsp70 release from peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 2004, 324, 511–517. [Google Scholar] [CrossRef]
- Henderson, B.; Pockley, A.G. Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J. Leukoc. Biol. 2010, 88, 445–462. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Neckers, L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007, 98, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Yuan, Y.; Li, D.; Liao, S.J.; Yan, B.; Wei, J.J.; Zhou, Y.H.; Zhu, J.H.; Zhang, G.M.; Feng, Z.H. Extracellular HSPA1A promotes the growth of hepatocarcinoma by augmenting tumor cell proliferation and apoptosis-resistance. Cancer Lett. 2012, 317, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Parcellier, A.; Gurbuxani, S.; Schmitt, E.; Solary, E.; Garrido, C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem. Biophys. Res. Commun. 2003, 304, 505–512. [Google Scholar] [CrossRef]
- Lebret, T.; Watson, R.W.; Molinie, V.; O’Neill, A.; Gabriel, C.; Fitzpatrick, J.M.; Botto, H. Heat shock proteins HSP27, HSP60, HSP70, and HSP90: Expression in bladder carcinoma. Cancer 2003, 98, 970–977. [Google Scholar] [CrossRef]
- So, A.; Hadaschik, B.; Sowery, R.; Gleave, M. The role of stress proteins in prostate cancer. Curr. Genom. 2007, 8, 252–261. [Google Scholar]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X.; et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Rong, B.; Zhao, C.; Liu, H.; Ming, Z.; Cai, X.; Gao, W.; Yang, S. Identification and verification of Hsp90-beta as a potential serum biomarker for lung cancer. Am. J. Cancer Res. 2014, 4, 874–885. [Google Scholar]
- Gunther, S.; Ostheimer, C.; Stangl, S.; Specht, H.M.; Mozes, P.; Jesinghaus, M.; Vordermark, D.; Combs, S.E.; Peltz, F.; Jung, M.P.; et al. Correlation of Hsp70 Serum Levels with Gross Tumor Volume and Composition of Lymphocyte Subpopulations in Patients with Squamous Cell and Adeno Non-Small Cell Lung Cancer. Front. Immunol. 2015, 6, 556. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Luo, S.; Xu, F.; Zou, G.; Xu, G.; He, J.; Huang, Y.; Zhu, H.; Li, Y. The expression of DAMP proteins HSP70 and cancer-testis antigen SPAG9 in peripheral blood of patients with HCC and lung cancer. Cell Stress Chaperones 2017, 22, 237–244. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Zhang, Y.; Ni, X.; Zhang, G.; Cui, X.; Liu, M.; Xu, C.; Zhang, Q.; Zhu, H.; et al. ZIP4 Promotes Muscle Wasting and Cachexia in Mice With Orthotopic Pancreatic Tumors by Stimulating RAB27B-Regulated Release of Extracellular Vesicles From Cancer Cells. Gastroenterology 2019, 156, 722–734.e6. [Google Scholar] [CrossRef]
- Niu, M.; Song, S.; Su, Z.; Wei, L.; Li, L.; Pu, W.; Zhao, C.; Ding, Y.; Wang, J.; Cao, W.; et al. Inhibition of heat shock protein (HSP) 90 reverses signal transducer and activator of transcription (STAT) 3-mediated muscle wasting in cancer cachexia mice. Br. J. Pharmacol. 2021, 178, 4485–4500. [Google Scholar] [CrossRef]
- Zhang, G.; Anderson, L.J.; Gao, S.; Sin, T.K.; Zhang, Z.; Wu, H.; Jafri, S.H.; Graf, S.A.; Wu, P.C.; Dash, A.; et al. Weight Loss in Cancer Patients Correlates With p38beta MAPK Activation in Skeletal Muscle. Front. Cell. Dev. Biol. 2021, 9, 784424. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Sobkoviak, R.; Jockheck-Clark, A.R.; Shi, B.; Mandelin, A.M., 2nd; Tak, P.P.; Haines, G.K., 3rd; Nicchitta, C.V.; Pope, R.M. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J. Immunol. 2009, 182, 4965–4973. [Google Scholar] [CrossRef]
- Shukla, H.D.; Pitha, P.M. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012, 2012, 728605. [Google Scholar] [CrossRef]
- Yuan, J.; Dunn, P.; Martinus, R.D. Detection of Hsp60 in saliva and serum from type 2 diabetic and non-diabetic control subjects. Cell Stress Chaperones 2011, 16, 689–693. [Google Scholar] [CrossRef]
- Oglesbee, M.J.; Herdman, A.V.; Passmore, G.G.; Hoffman, W.H. Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kDa heat shock protein. Clin. Biochem. 2005, 38, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ock, C.Y.; Kim, S.J.; Hahm, K.B. Heat shock protein: Hard worker or bad offender for gastric diseases. Int. J. Proteom. 2010, 2010, 259163. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Sun, W.; Taldone, T.; Rodina, A.; Chiosis, G. Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegener. 2010, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.K. Targeted activation of heat shock proteins by natural bioactive compounds to prevent neurodegenerative diseases. J. Anc. Dis. Prev. Rem. 2014, 2, e113. [Google Scholar] [CrossRef]
- Currie, R.W.; Karmazyn, M.; Kloc, M.; Mailer, K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ. Res. 1988, 63, 543–549. [Google Scholar] [CrossRef]
- Pattison, J.S.; Sanbe, A.; Maloyan, A.; Osinska, H.; Klevitsky, R.; Robbins, J. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation 2008, 117, 2743–2751. [Google Scholar] [CrossRef]
- Ranek, M.J.; Stachowski, M.J.; Kirk, J.A.; Willis, M.S. The role of heat shock proteins and co-chaperones in heart failure. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2018, 373, 20160530. [Google Scholar] [CrossRef]
- Saha, A.; Ahmed, S. The Link Between Heat Shock Proteins, Renin-Angiotensin System, and the Coagulation Cascade in the Pathogenesis of the Coronavirus-19 Disease. Adv. Exp. Med. Biol. 2022, 18, 161–171. [Google Scholar]
- Zheng, Y.; Im, C.N.; Seo, J.S. Inhibitory effect of Hsp70 on angiotensin II-induced vascular smooth muscle cell hypertrophy. Exp. Mol. Med. 2006, 38, 509–518. [Google Scholar] [CrossRef]
- Brundel, B.J.; Henning, R.H.; Ke, L.; van Gelder, I.C.; Crijns, H.J.; Kampinga, H.H. Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation. J. Mol. Cell. Cardiol. 2006, 41, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Walley, K.R.; Wang, Y.; Indrambarya, T.; Boyd, J.H. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ. J. 2011, 75, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Kapadia, S.; Torre-Amione, G.; Durand, J.B.; Bies, R.; Young, J.; Mann, D.L. Differential expression of heat shock proteins in normal and failing human hearts. J. Mol. Cell. Cardiol. 1998, 30, 811–818. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Watanabe, T.; Nitobe, J.; Miyashita, T.; Miyamoto, T.; Kitahara, T.; Suzuki, S.; Sasaki, T.; Bilim, O.; et al. Relation of serum heat shock protein 60 level to severity and prognosis in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2008, 102, 606–610. [Google Scholar] [CrossRef] [PubMed]
- St Rammos, K.; Koullias, G.J.; Hassan, M.O.; Argyrakis, N.P.; Voucharas, C.G.; Scarupa, S.J.; Cowte, T.G. Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc. Surg. 2002, 10, 228–232. [Google Scholar] [CrossRef]
- Tamura, S.; Marunouchi, T.; Tanonaka, K. Heat-shock protein 90 modulates cardiac ventricular hypertrophy via activation of MAPK pathway. J. Mol. Cell. Cardiol. 2019, 127, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Lord, R.S. Expression of heat shock protein-70 by dendritic cells in the arterial intima and its potential significance in atherogenesis. J. Vasc. Surg. 2002, 35, 368–375. [Google Scholar] [CrossRef]
- Sanbe, A.; Osinska, H.; Saffitz, J.E.; Glabe, C.G.; Kayed, R.; Maloyan, A.; Robbins, J. Desmin-related cardiomyopathy in transgenic mice: A cardiac amyloidosis. Proc. Natl. Acad. Sci. USA 2004, 101, 10132–10136. [Google Scholar] [CrossRef]
- Genth-Zotz, S.; Bolger, A.P.; Kalra, P.R.; von Haehling, S.; Doehner, W.; Coats, A.J.; Volk, H.D.; Anker, S.D. Heat shock protein 70 in patients with chronic heart failure: Relation to disease severity and survival. Int. J. Cardiol. 2004, 96, 397–401. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; Xing, R.; Yu, H.; Zhang, Y.; Li, Z.; Gao, W. Heat shock protein 70 acts as a potential biomarker for early diagnosis of heart failure. PLoS ONE 2013, 8, e67964. [Google Scholar] [CrossRef]
- Von Haehling, S.; Anker, M.S.; Anker, S.D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: Facts and numbers update 2016. J. Cachexia Sarcopenia Muscle 2016, 7, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Sapra, G.; Tham, Y.K.; Cemerlang, N.; Matsumoto, A.; Kiriazis, H.; Bernardo, B.C.; Henstridge, D.C.; Ooi, J.Y.; Pretorius, L.; Boey, E.J.; et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat. Commun. 2014, 5, 5705. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iturbe, B.; Lanaspa, M.A.; Johnson, R.J. The role of autoimmune reactivity induced by heat shock protein 70 in the pathogenesis of essential hypertension. Br. J. Pharmacol. 2019, 176, 1829–1838. [Google Scholar] [CrossRef]
- Xiaonan, S.; Sharadhi, S.; Amirah, H.; Hongyu, Q. Heat Shock Protein 22 in Physiological and Pathological Hearts: Small Molecule, Large Potentials. Cells 2022, 11, 1–14. [Google Scholar]
- Hofmann, C.; Katus, H.A.; Doroudgar, S. Protein Misfolding in Cardiac Disease. Circulation 2019, 139, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Dybdahl, B.; Wahba, A.; Lien, E.; Flo, T.H.; Waage, A.; Qureshi, N.; Sellevold, O.F.; Espevik, T.; Sundan, A. Inflammatory response after open heart surgery: Release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation 2002, 105, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Jenei, Z.M.; Gombos, T.; Forhecz, Z.; Pozsonyi, Z.; Karadi, I.; Janoskuti, L.; Prohaszka, Z. Elevated extracellular HSP70 (HSPA1A) level as an independent prognostic marker of mortality in patients with heart failure. Cell Stress Chaperones 2013, 18, 809–813. [Google Scholar] [CrossRef]
- Kim, S.C.; Stice, J.P.; Chen, L.; Jung, J.S.; Gupta, S.; Wang, Y.; Baumgarten, G.; Trial, J.; Knowlton, A.A. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ. Res. 2009, 105, 1186–1195. [Google Scholar] [CrossRef]
- Hochleitner, B.W.; Hochleitner, E.O.; Obrist, P.; Eberl, T.; Amberger, A.; Xu, Q.; Margreiter, R.; Wick, G. Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 617–623. [Google Scholar] [CrossRef]
- Lin, L.; Kim, S.C.; Wang, Y.; Gupta, S.; Davis, B.; Simon, S.I.; Torre-Amione, G.; Knowlton, A.A. HSP60 in heart failure: Abnormal distribution and role in cardiac myocyte apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2238–H2247. [Google Scholar] [CrossRef]
- Bonanad, C.; Nunez, J.; Sanchis, J.; Bodi, V.; Chaustre, F.; Chillet, M.; Minana, G.; Forteza, M.J.; Palau, P.; Nunez, E.; et al. Serum heat shock protein 60 in acute heart failure: A new biomarker? Congest. Heart Fail. 2013, 19, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Sakao, S.; Tatsumi, K. Vascular remodeling in pulmonary arterial hypertension: Multiple cancer-like pathways and possible treatment modalities. Int. J. Cardiol. 2011, 147, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Skorzynska-Dziduszko, K.E.; Olszewska, A.; Prendecka, M.; Malecka-Massalska, T. Serum Heat Shock Protein 90 Alpha: A New Marker of Hypertension-Induced Endothelial Injury? Adv. Clin. Exp. Med. 2016, 25, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Deng, S.; Lian, Z.; Yu, K. Novel Drugs with High Efficacy against Tumor Angiogenesis. Int. J. Mol. Sci. 2022, 23, 6934. [Google Scholar] [CrossRef]
- Xu, Q.; Fawcett, T.W.; Udelsman, R.; Holbrook, N.J. Activation of heat shock transcription factor 1 in rat aorta in response to high blood pres-sure. Hypertension 1996, 28, 53–57. [Google Scholar] [CrossRef]
- Qi, J.; Yang, P.; Yi, B.; Huo, Y.; Chen, M.; Zhang, J.; Sun, J. Heat shock protein 90 inhibition by 17-DMAG attenuates abdominal aortic aneurysm formation in mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H841–H852. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, J.; Chen, J.; Lai, J.; Zhu, F.; Xu, X.; Wang, D.W. CYP2J2-Derived EETs Attenuated Angiotensin II-Induced Adventitial Remodeling via Reduced Inflammatory Response. Cell. Physiol. Biochem. 2016, 39, 721–739. [Google Scholar] [CrossRef]
- Nakamura, T.; Hinagata, J.; Tanaka, T.; Imanishi, T.; Wada, Y.; Kodama, T.; Doi, T. HSP90, HSP70, and GAPDH directly interact with the cytoplasmic domain of macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2002, 290, 858–864. [Google Scholar] [CrossRef]
- Xu, Q. Role of heat shock proteins in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1547–1559. [Google Scholar] [CrossRef]
- Mandal, K.; Jahangiri, M.; Xu, Q. Autoimmunity to heat shock proteins in atherosclerosis. Autoimmun. Rev. 2004, 3, 31–37. [Google Scholar] [CrossRef]
- Pockley, A.G.; Bulmer, J.; Hanks, B.M.; Wright, B.H. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones 1999, 4, 29–35. [Google Scholar] [CrossRef]
- Xiao, Q.; Mandal, K.; Schett, G.; Mayr, M.; Wick, G.; Oberhollenzer, F.; Willeit, J.; Kiechl, S.; Xu, Q. Association of serum-soluble heat shock protein 60 with carotid atherosclerosis: Clinical significance determined in a follow-up study. Stroke 2005, 36, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Holme, E.R.; McKay, I.C.; Hood, S.; McColl, K.E.; Hillis, W.S. Association between antibodies to heat shock protein 65 and coronary atherosclerosis. Possible mechanism of action of Helicobacter pylori and other bacterial infections in increasing cardiovascular risk. Eur. Heart J. 1998, 19, 387–394. [Google Scholar] [CrossRef]
- Jeroudi, M.O.; Hartley, C.J.; Bolli, R. Myocardial reperfusion injury: Role of oxygen radicals and potential therapy with antioxidants. Am. J. Cardiol. 1994, 73, B2–B7. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K.; Torsney, E.; Poloniecki, J.; Camm, A.J.; Xu, Q.; Jahangiri, M. Association of high intracellular, but not serum, heat shock protein 70 with postoperative atrial fibrillation. Ann. Thorac. Surg. 2005, 79, 865–871, discussion 871. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, J.; Nakai, A.; Matsuda, K.; Komeda, M.; Ban, T.; Nagata, K. Reactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heart. Circulation 1999, 99, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Moulin, D.; Hupont, S.; Koufany, M.; Netter, P.; Reboul, P.; Jouzeau, J.Y. Oxidative stress-induced expression of HSP70 contributes to the inhibitory effect of 15d-PGJ2 on inducible prostaglandin pathway in chondrocytes. Free Radic. Biol. Med. 2014, 76, 114–126. [Google Scholar] [CrossRef]
- Nair, S.P.; Sharma, R.K. Heat shock proteins and their expression in primary murine cardiac cell populations during ischemia and reperfusion. Mol. Cell. Biochem. 2020, 464, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.C.S.; Chandra, S.; Narang, R.; Bhatia, J.; Saluja, D. Haplotype Analysis of Heat Shock Protein70 Gene and Their Association with Essential Hypertension. Ann. Clin. Exp. Hypertens. 2016, 4, 1041. [Google Scholar]
- Cai, W.F.; Zhang, X.W.; Yan, H.M.; Ma, Y.G.; Wang, X.X.; Yan, J.; Xin, B.M.; Lv, X.X.; Wang, Q.Q.; Wang, Z.Y.; et al. Intracellular or extracellular heat shock protein 70 differentially regulates cardiac remodelling in pressure overload mice. Cardiovasc. Res. 2010, 88, 140–149. [Google Scholar] [CrossRef]
- Blake, M.J.; Klevay, L.M.; Halas, E.S.; Bode, A.M. Blood pressure and heat shock protein expression in response to acute and chronic stress. Hypertension 1995, 25 Pt 1, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Schäfler, A.E.; Kirmanoglou, K.; Balbach, J.; Pecher, P.; Hannekum, A.; Schumacher, B. The expression of heat shock protein 60 in myocardium of patients with chronic atrial fibrillation. Basic Res. Cardiol. 2002, 97, 258–261. [Google Scholar] [CrossRef]
- Kirmanoglou, K.; Hannekum, A.; Schäfler, A.E. Expression of mortalin in patients with chronic atrial fibrillation. Basic Res. Cardiol. 2004, 99, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tan, H.; Cheng, L.; He, M.; Wei, Q.; Tanguay, R.M.; Wu, T. Expression of heat shock proteins in myocardium of patients with atrial fibrillation. Cell Stress Chaperones 2007, 12, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Jenei, Z.M.; Szeplaki, G.; Merkely, B.; Karadi, I.; Zima, E.; Prohaszka, Z. Persistently elevated extracellular HSP70 (HSPA1A) level as an independent prognostic marker in post-cardiac-arrest patients. Cell Stress Chaperones 2013, 18, 447–454. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Rosati, A.; De Marco, M.; Basile, A.; D’Angelo, A.; Romano, D.; Sahebkar, A.; Falco, A.; Turco, M.C. Evaluation of BAG3 levels in healthy subjects, hypertensive patients, and hypertensive diabetic patients. J. Cell. Physiol. 2018, 233, 1791–1795. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Kanagasabai, R.; Druhan, L.J.; Ilangovan, G. Heat shock protein 25-enriched plasma transfusion preconditions the heart against doxorubicin-induced dilated cardiomyopathy in mice. J. Pharmacol. Exp. Ther. 2012, 341, 829–839. [Google Scholar] [CrossRef]
- Yamagami, K.; Yamamoto, Y.; Ishikawa, Y.; Yonezawa, K.; Toyokuni, S.; Yamaoka, Y. Effects of geranyl-geranyl-acetone administration before heat shock preconditioning for conferring tolerance against ischemia-reperfusion injury in rat livers. J. Lab. Clin. Med. 2000, 135, 465–475. [Google Scholar] [CrossRef]
- Weeks, K.L.; Gao, X.; Du, X.J.; Boey, E.J.; Matsumoto, A.; Bernardo, B.C.; Kiriazis, H.; Cemerlang, N.; Tan, J.W.; Tham, Y.K.; et al. Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ. Heart Fail. 2012, 5, 523–534. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Lopez-Franco, O.; Blanco-Colio, L.M.; Munoz-Garcia, B.; Ramos-Mozo, P.; Ortega, L.; Egido, J.; Martin-Ventura, J.L. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc. Res. 2010, 86, 330–337. [Google Scholar] [CrossRef]
- Qi, S.; Yi, G.; Yu, K.; Feng, C.; Deng, S. The Role of HSP90 Inhibitors in the Treatment of Cardiovascular Diseases. Cells 2022, 11, 3444. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Damato, A.; Ambrosio, M.; Falco, A.; Rosati, A.; Capunzo, M.; Madonna, M.; Turco, M.C.; Januzzi, J.L.; De Laurenzi, V.; et al. The prosurvival protein BAG3: A new participant in vascular homeostasis. Cell. Death Dis. 2016, 7, e2431. [Google Scholar] [CrossRef]
- Uchiyama, T.; Atsuta, H.; Utsugi, T.; Oguri, M.; Hasegawa, A.; Nakamura, T.; Nakai, A.; Nakata, M.; Maruyama, I.; Tomura, H.; et al. HSF1 and constitutively active HSF1 improve vascular endothelial function (heat shock proteins improve vascular endothelial function). Atherosclerosis 2007, 190, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Butler, A.E.; Banach, M.; Barreto, G.E.; Sahbekar, A. Modulation of heat shock proteins by statins. Pharmacol. Res. 2018, 134, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hoogstra-Berends, F.; Meijering, R.A.; Zhang, D.; Heeres, A.; Loen, L.; Seerden, J.P.; Kuipers, I.; Kampinga, H.H.; Henning, R.H.; Brundel, B.J. Heat shock protein-inducing compounds as therapeutics to restore proteostasis in atrial fibrillation. Trends Cardiovasc. Med. 2012, 22, 62–68. [Google Scholar] [CrossRef]
- Willis, M.S.; Patterson, C. Hold me tight: Role of the heat shock protein family of chaperones in cardiac disease. Circulation 2010, 122, 1740–1751. [Google Scholar] [CrossRef]
- Toga, W.; Tanonaka, K.; Takeo, S. Changes in Hsp60 level of the failing heart following acute myocardial infarction and the effect of long-term treatment with trandolapril. Biol. Pharm. Bull. 2007, 30, 105–110. [Google Scholar] [CrossRef]
- Traxler, D.; Lainscak, M.; Simader, E.; Ankersmit, H.J.; Jug, B. Heat shock protein 27 acts as a predictor of prognosis in chronic heart failure patients. Clin. Chim. Acta 2017, 473, 127–132. [Google Scholar] [CrossRef]

| Family | Important Members |
|---|---|
| Hsp100 | Hsp105 (HSPH1) Hsp110 (HSPH2) Grp170 (HSPH4) |
| Hsp90 | Hsp90α (HSPC2) Hsp90β (HSPC3) Grp94 (HSPC4) |
| Hsp70 | Hsp70 (Hsp72) (HSPA1) Hsc70 (Hsp73) (HSPA8) Grp78 (BIP) (HSPA5) Utp (Grp75) (HSPA9) |
| Hsp40 | Hsp40 (Dnaj) DNAJB1 |
| αCrystallin (HSPB4) | |
| Hsp25 (HSPB1) | |
| Small Hsp | Hsp27 (HSPB2) |
| Hsp20 (HSPB6) | |
| Hsp22 (HSPB8) | |
| Chaperonins | GroEL (Hsp60) (HSPD1) GroES (HSPE1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patnaik, S.; Nathan, S.; Kar, B.; Gregoric, I.D.; Li, Y.-P. The Role of Extracellular Heat Shock Proteins in Cardiovascular Diseases. Biomedicines 2023, 11, 1557. https://doi.org/10.3390/biomedicines11061557
Patnaik S, Nathan S, Kar B, Gregoric ID, Li Y-P. The Role of Extracellular Heat Shock Proteins in Cardiovascular Diseases. Biomedicines. 2023; 11(6):1557. https://doi.org/10.3390/biomedicines11061557
Chicago/Turabian StylePatnaik, Soumya, Sriram Nathan, Biswajit Kar, Igor D. Gregoric, and Yi-Ping Li. 2023. "The Role of Extracellular Heat Shock Proteins in Cardiovascular Diseases" Biomedicines 11, no. 6: 1557. https://doi.org/10.3390/biomedicines11061557
APA StylePatnaik, S., Nathan, S., Kar, B., Gregoric, I. D., & Li, Y.-P. (2023). The Role of Extracellular Heat Shock Proteins in Cardiovascular Diseases. Biomedicines, 11(6), 1557. https://doi.org/10.3390/biomedicines11061557





