Unlocking the Secrets: Exploring the Biochemical Correlates of Suicidal Thoughts and Behaviors in Adults with Autism Spectrum Conditions
Abstract
1. Introduction
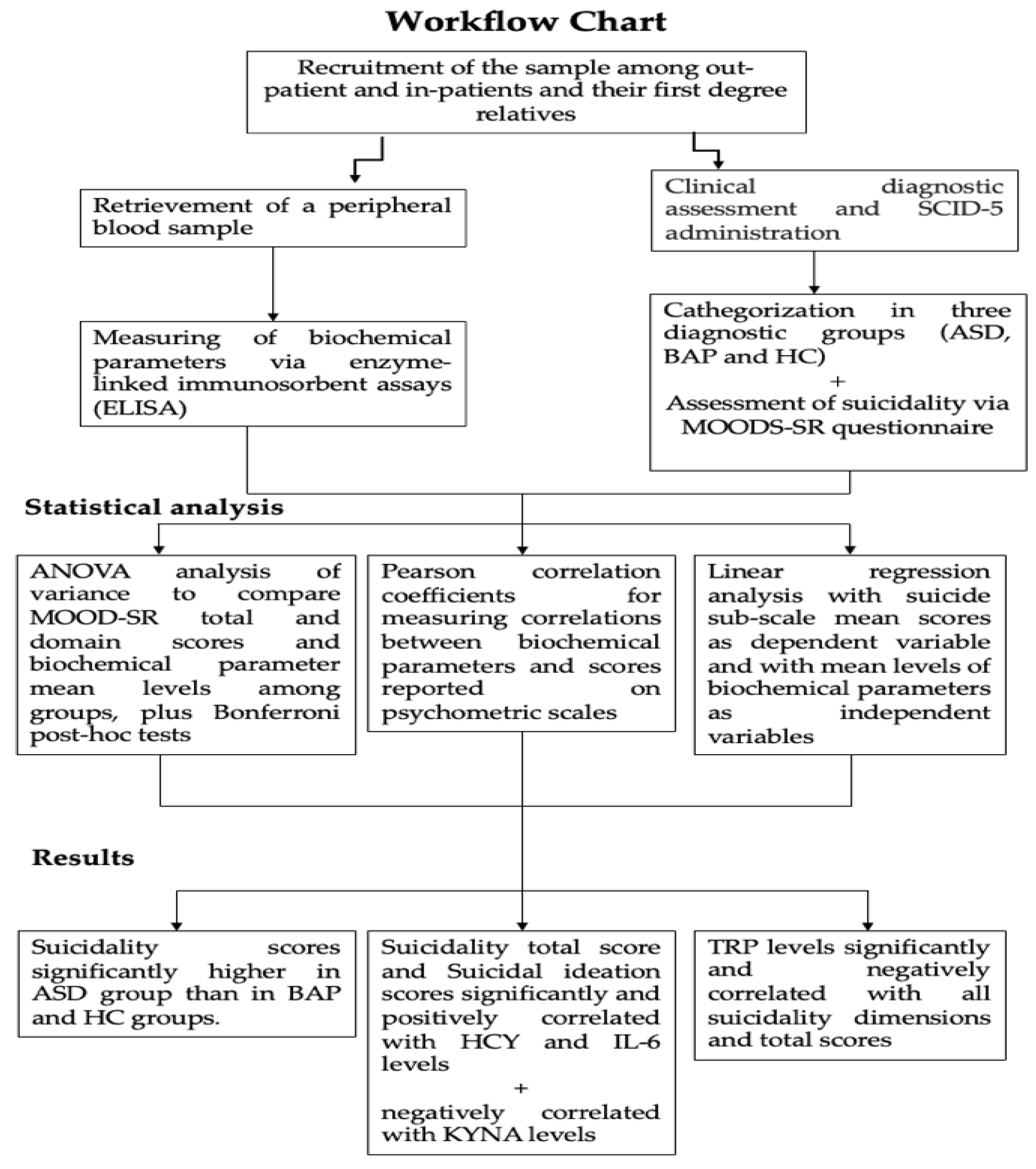
2. Materials and Methods
2.1. Recruitment Procedures
2.2. Psychometric Instruments
2.2.1. The Structured Clinical Interview for DSM-5 Disorders (SCID-5)
2.2.2. The Mood Spectrum Questionnaire (MOODS-SR)
2.3. Biochemical Evaluations
2.4. Statistical Analysis
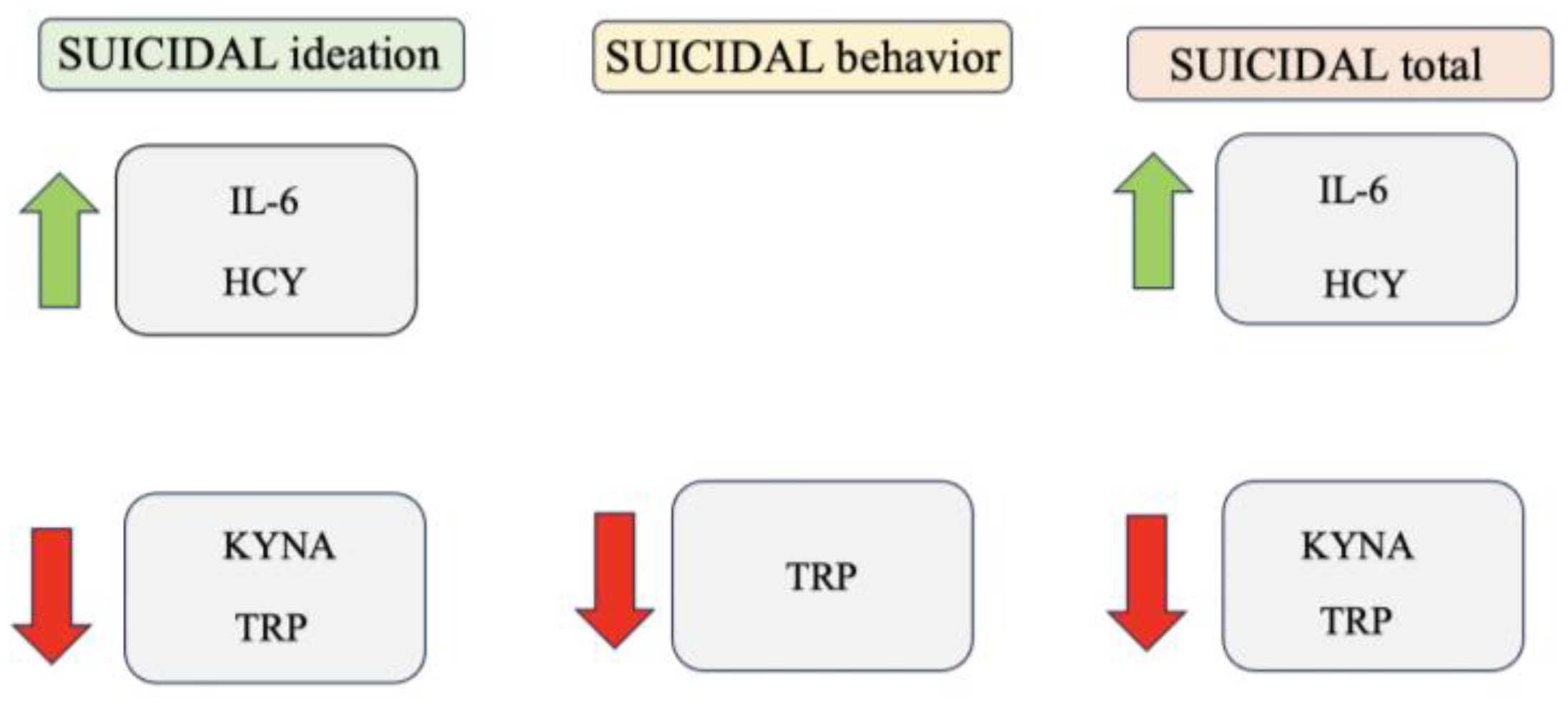
3. Results
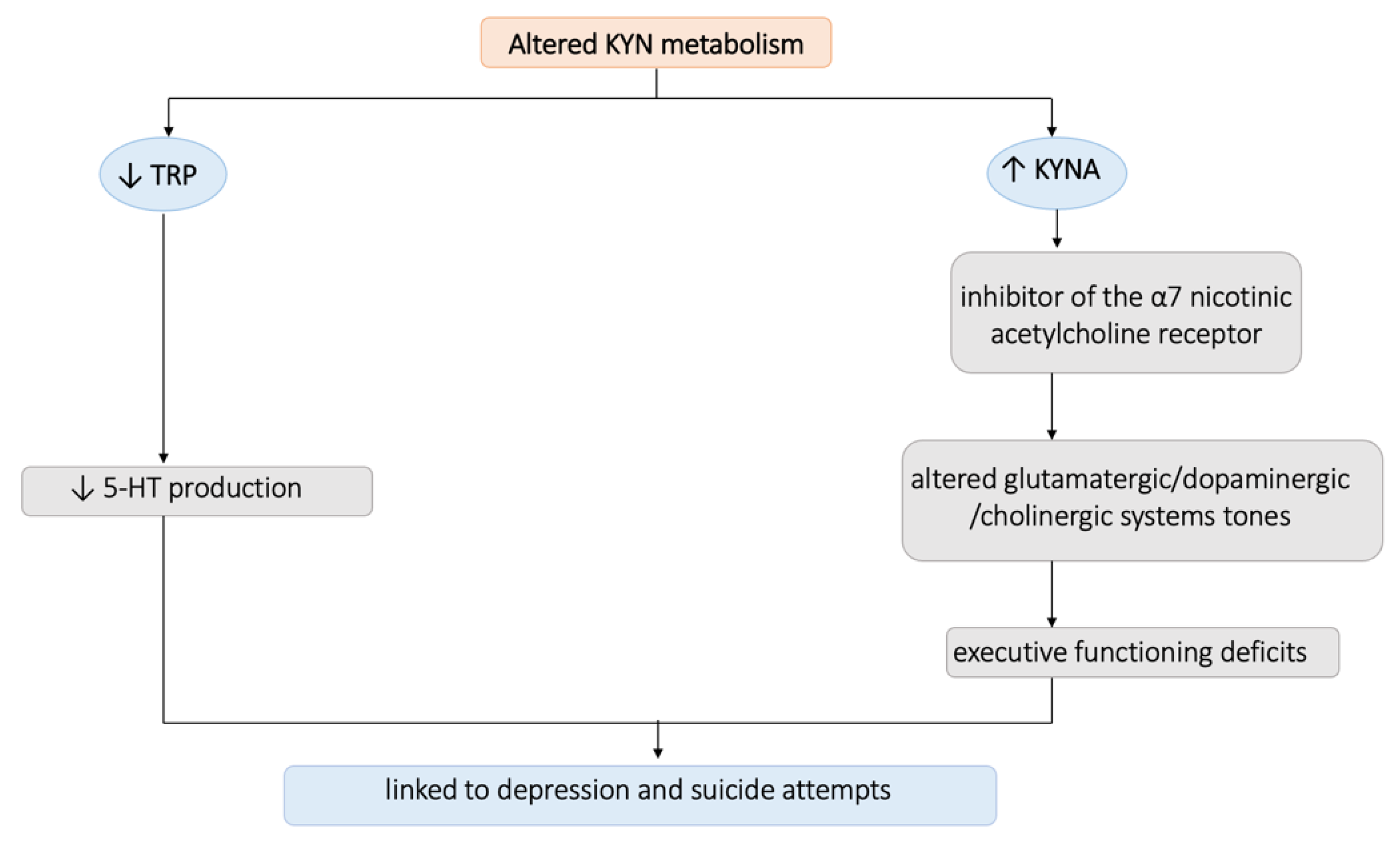
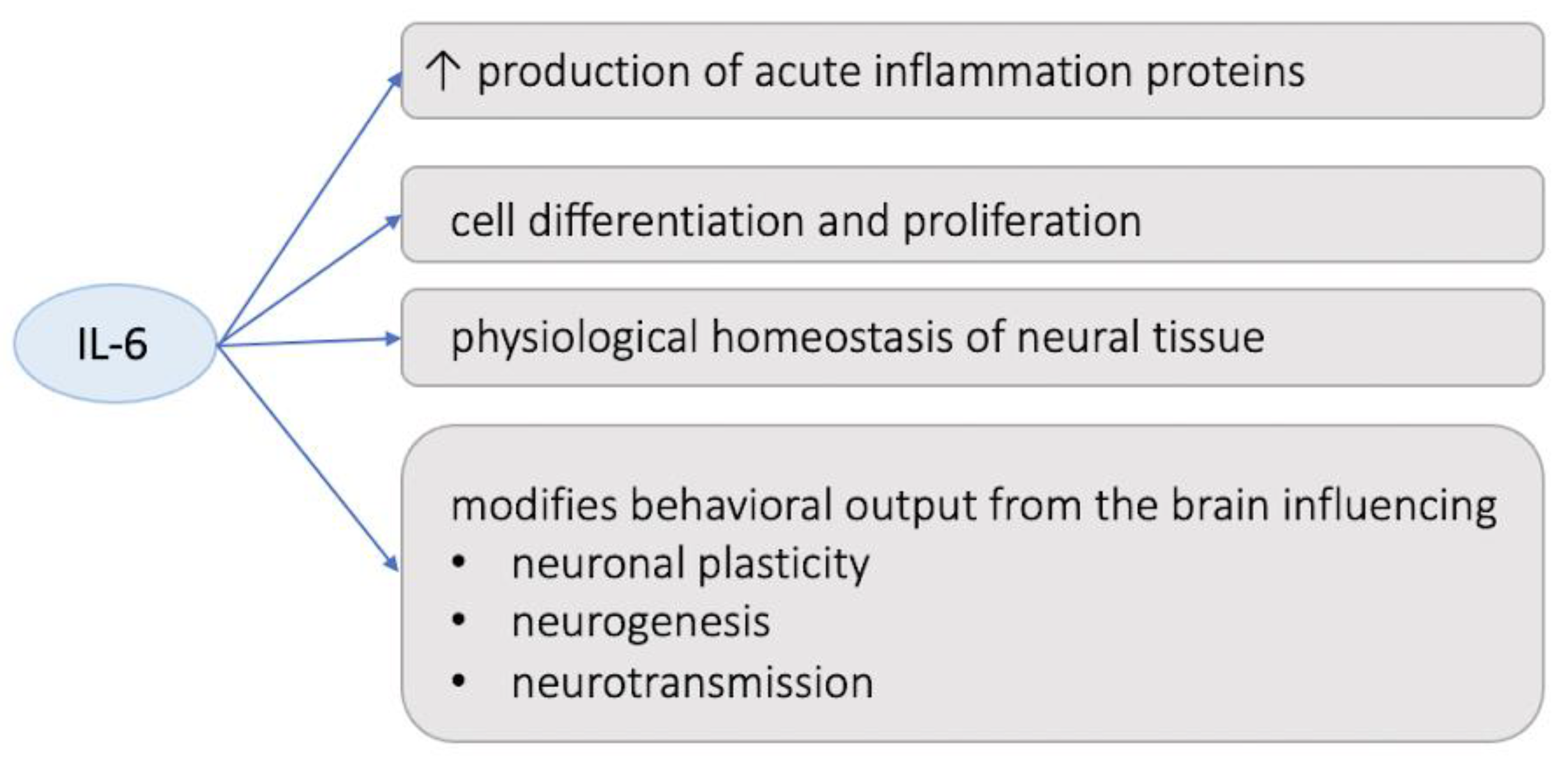
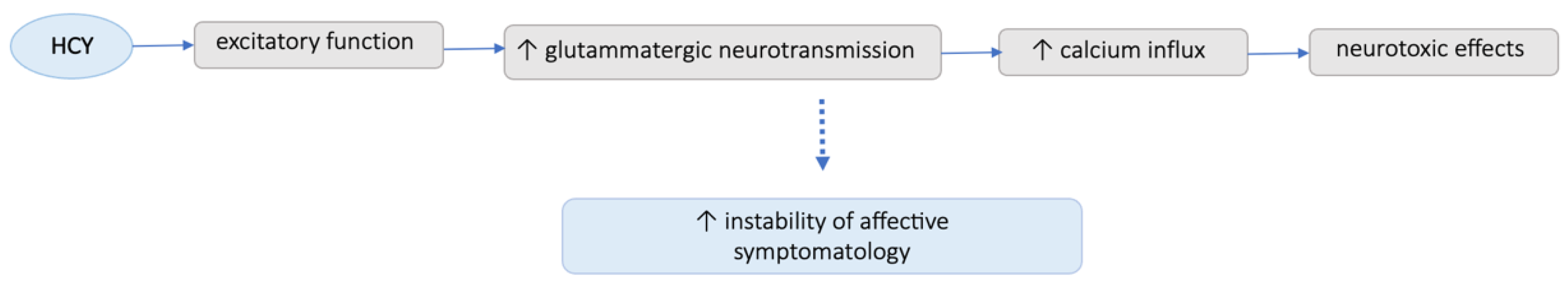
4. Discussion
5. Limits and Further Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Suicide in the World: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- O’Carroll, P.W.; Berman, A.L.; Maris, R.W.; Moscicki, E.K.; Tanney, B.L.; Silverman, M.M. Beyond the Tower of Babel: A nomenclature for suicidology. Suicide Life Threat. Behav. 1996, 26, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Malafosse, A. Genetics of suicidal behavior. Am. J. Med. Genet. C. Semin. Med. Genet. 2005, 133, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.K.; Pellmar, T.C.; Kleinman, A.M.; Bunney, W.E. Reducing Suicide: A National Imperative; The National Academies Press: Washington, DC, USA, 2002. [Google Scholar]
- Turecki, G.; Ernst, C.; Jollant, F.; Labonté, B.; Mechawar, N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012, 35, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.N.; Campbell, D.; Caruncho, H.J.; Henter, I.D.; Ballard, E.D.; A Zarate, C. Suicide Biomarkers to Predict Risk, Classify Diagnostic Subtypes, and Identify Novel Therapeutic Targets: 5 Years of Promising Research. Int. J. Neuropsychopharmacol. 2022, 25, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Hawton, K.; i Comabella, C.C.; Haw, C.; Saunders, K. Risk factors for suicide in individuals with depression: A systematic review. J. Affect. Disord. 2013, 147, 17–28. [Google Scholar] [CrossRef]
- May, A.M.; Klonsky, E.D.; Klein, D.N. Predicting future suicide attempts among depressed suicide ideators: A 10-year longitudinal study. J. Psychiatr. Res. 2012, 46, 946–952. [Google Scholar] [CrossRef]
- Bokor, J.; Sutori, S.; Torok, D.; Gal, Z.; Eszlari, N.; Gyorik, D.; Baksa, D.; Petschner, P.; Serafini, G.; Pompili, M.; et al. Inflamed Mind: Multiple Genetic Variants of IL6 Influence Suicide Risk Phenotypes in Interaction with Early and Recent Adversities in a Linkage Disequilibrium-Based Clumping Analysis. Front. Psychiatry 2021, 12, 746206. [Google Scholar] [CrossRef]
- Davis, A.T.; Schrueder, C. The prediction of suicide. Med. J. Aust. 1990, 153, 552–554. [Google Scholar] [CrossRef]
- Blasco-Fontecilla, H.; Lopez-Castroman, J.; Giner, L.; Baca-García, E.; Oquendo, M.A. Predicting Suicidal Behavior: Are We Really that Far Along? Comment on Discovery and Validation of Blood Biomarkers for Suicidality. Curr. Psychiatry Rep. 2013, 15, 424. [Google Scholar] [CrossRef]
- Smith, E.G.; Kim, H.M.; Ganoczy, D.; Stano, C.; Pfeiffer, P.N.; Valenstein, M. Suicide Risk Assessment Received Prior to Suicide Death by Veterans Health Administration Patients with a History of Depression. J. Clin. Psychiatry 2013, 74, 226–232. [Google Scholar] [CrossRef]
- Ganança, L.; Oquendo, M.A.; Tyrka, A.R.; Cisneros-Trujillo, S.; Mann, J.J.; Sublette, M.E. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 2016, 63, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Niciu, M.J.; Mathews, D.C.; Ionescu, D.F.; Richards, E.M.; Furey, M.L.; Yuan, P.; Nugent, A.C.; Henter, I.D.; Machado-Vieira, R.; Zarate, C.A., Jr. Biomarkers in mood disorders research: Developing new and improved therapeutics. Rev. Psiquiatr. Clin. 2014, 41, 131–134. [Google Scholar] [CrossRef]
- Bryleva, E.Y.; Brundin, L. Kynurenine pathway metabolites and suicidality. Neuropharmacology 2017, 112, 324–330. [Google Scholar] [CrossRef]
- Orsolini, L.; Latini, R.; Pompili, M.; Serafini, G.; Volpe, U.; Vellante, F.; Fornaro, M.; Valchera, A.; Tomasetti, C.; Fraticelli, S.; et al. Understanding the Complex of Suicide in Depression: From Research to Clinics. Psychiatry Investig. 2020, 17, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Amitai, M.; Taler, M.; Ben-Baruch, R.; Lebow, M.; Rotkopf, R.; Apter, A.; Fennig, S.; Weizman, A.; Chen, A. Increased circulatory IL-6 during 8-week fluoxetine treatment is a risk factor for suicidal behaviors in youth. Brain Behav. Immun. 2020, 87, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan–Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in psychiatric disorders: What comes first? Ann. N. Y. Acad. Sci. 2018, 1437, 57–67. [Google Scholar] [CrossRef]
- Wichers, M.C.; Koek, G.H.; Robaeys, G.; Verkerk, R.; Scharpé, S.; Maes, M. IDO and interferon-α-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry 2005, 10, 538–544. [Google Scholar] [CrossRef]
- Salas-Magaña, M.; Tovilla-Zárate, C.A.; González-Castro, T.B.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Rodríguez-Pérez, J.M.; Bello, J.R. Decrease in brain-derived neurotrophic factor at plasma level but not in serum concentrations in suicide behavior: A systematic review and meta-analysis. Brain Behav. 2017, 7, e00706. [Google Scholar] [CrossRef]
- Carpita, B.; Betti, L.; Palego, L.; Bartolommei, N.; Chico, L.; Pasquali, L.; Siciliano, G.; Monzani, F.; Franchi, R.; Rogani, S.; et al. Plasma redox and inflammatory patterns during major depressive episodes: A cross-sectional investigation in elderly patients with mood disorders. CNS Spectr. 2020, 26, 416–426. [Google Scholar] [CrossRef]
- Marazziti, D.; Abelli, M.; Baroni, S.; Carpita, B.; Piccinni, A.; Dell’Osso, L. Recent findings on the pathophysiology of social anxiety disorder. Clin. Neuropsych. 2014, 11, 91–100. [Google Scholar]
- Rengasamy, M.; Zhong, Y.; Marsland, A.; Chen, K.; Douaihy, A.; Brent, D.; Melhem, N.M. Signaling networks in inflammatory pathways and risk for suicidal behavior. Brain Behav. Immun. Health 2020, 7, 100122. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Kim, B.S.; Im, H.-I. Pathophysiological Role of Neuroinflammation in Neurodegenerative Diseases and Psychiatric Disorders. Int. Neurourol. J. 2016, 20, S2–S7. [Google Scholar] [CrossRef]
- Wang, A.K.; Miller, B.J. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef]
- Lindqvist, D.; Janelidze, S.; Hagell, P.; Erhardt, S.; Samuelsson, M.; Minthon, L.; Hansson, O.; Björkqvist, M.; Träskman-Bendz, L.; Brundin, L. Interleukin-6 Is Elevated in the Cerebrospinal Fluid of Suicide Attempters and Related to Symptom Severity. Biol. Psychiatry 2009, 66, 287–292. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattei, D.; Westrin, A.; Träskman-Bendz, L.; Brundin, L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav. Immun. 2011, 25, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Janelidze, S.; Erhardt, S.; Träskman-Bendz, L.; Engström, G.; Brundin, L. CSF biomarkers in suicide attempters-a principal component analysis. Acta Psychiatr. Scand. 2011, 124, 52–61. [Google Scholar] [CrossRef]
- Martinez, J.M.; Garakani, A.; Yehuda, R.; Gorman, J.M. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress. Anxiety 2011, 29, 32–38. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Ren, X.; Fareed, J.; Hoppensteadt, D.A.; Roberts, R.C.; Conley, R.R.; Dwivedi, Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J. Psychiatr. Res. 2012, 46, 57–63. [Google Scholar] [CrossRef]
- Hoyo-Becerra, C.; Huebener, A.; Trippler, M.; Lutterbeck, M.; Liu, Z.J.; Truebner, K.; Bajanowski, T.; Gerken, G.; Hermann, D.M.; Schlaak, J.F. Concomitant Interferon Alpha Stimulation and TLR3 Activation Induces Neuronal Expression of Depression-Related Genes That Are Elevated in the Brain of Suicidal Persons. PLoS ONE 2013, 8, e83149. [Google Scholar] [CrossRef]
- O’Donovan, A.; Rush, G.; Hoatam, G.; Hughes, B.M.; McCrohan, A.; Kelleher, C.; O’Farrelly, C.; Malone, K.M. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress. Anxiety 2013, 30, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.N.; Rizavi, H.S.; Zhang, H.; Bhaumik, R.; Ren, X. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J. Psychiatry Neurosci. 2018, 43, 376–385. [Google Scholar] [CrossRef]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A Specific Inflammatory Profile Underlying Suicide Risk? Systematic Review of the Main Literature Findings. Int. J. Environ. Res. Public. Health 2020, 17, 2393. [Google Scholar] [CrossRef]
- Miná, V.A.; Lacerda-Pinheiro, S.F.; Maia, L.C.; Pinheiro, R.F., Jr.; Meireles, C.B.; de Souza, S.I.; Reis, A.O.; Bianco, B.; Rolim, M.L. The influence of inflammatory cytokines in physiopathology of suicidal behavior. J. Affect. Disord. 2015, 172, 219–230. [Google Scholar] [CrossRef]
- Niculescu, A.B.; Levey, D.; Phalen, P.; Le-Niculescu, H.; Dainton-Howard, H.; Jain, N.; Belanger, E.; James, A.; George, S.; Weber, H.; et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol. Psychiatry 2015, 20, 1266–1285. [Google Scholar] [CrossRef]
- Brundin, L.; Sellgren, C.M.; Lim, C.K.; Grit, J.; Pålsson, E.; Landén, M.; Samuelsson, M.; Lundgren, K.; Brundin, P.; Fuchs, D.; et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl. Psychiatry 2016, 6, e865. [Google Scholar] [CrossRef]
- Niculescu, A.B.; Le-Niculescu, H.; Levey, D.F.; Phalen, P.L.; Dainton, H.L.; Roseberry, K.; Niculescu, E.M.; Niezer, J.O.; Williams, A.; Graham, D.L.; et al. Precision medicine for suicidality: From universality to subtypes and personalization. Mol. Psychiatry 2017, 22, 1250–1273. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Dempke, W.C. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Khairova, R.A.; Machado-Vieira, R.; Du, J.; Manji, H.K. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 2009, 12, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflammation 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Hodes, G.E.; Ménard, C.; Russo, S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress 2016, 4, 15–22. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Tovilla-Zárate, C.A.; López-Narváez, M.L.; Genis-Mendoza, A.D.; Juárez-Rojop, I.E. Interleukin-6 Levels in Serum, Plasma, and Cerebral Spinal Fluid in Individuals with Suicide Behavior: Systematic Review and Meta-Analysis with Meta-Regression. J. Interf. Cytokine Res. 2021, 41, 258–267. [Google Scholar] [CrossRef]
- Fernández-Sevillano, J.; González-Ortega, I.; MacDowell, K.; Zorrilla, I.; López, M.P.; Courtet, P.; Gabilondo, A.; Martínez-Cengotitabengoa, M.; Leza, J.C.; Sáiz, P.; et al. Inflammation biomarkers in suicide attempts and their relation to abuse, global functioning and cognition. World J. Biol. Psychiatry 2021, 23, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Amitai, M.; Taler, M.; Lebow, M.; Ben-Baruch, R.; Apter, A.; Fennig, S.; Weizman, A.; Chen, A. An increase in IL-6 levels at 6-month follow-up visit is associated with SSRI-emergent suicidality in high-risk children and adolescents treated with fluoxetine. Eur. Neuropsychopharmacol. 2020, 40, 61–69. [Google Scholar] [CrossRef]
- Bryleva, E.Y.; Brundin, L. Suicidality and Activation of the Kynurenine Pathway of Tryptophan Metabolism. Curr. Top Behav. Neurosci. 2016, 31, 269–284. [Google Scholar] [CrossRef]
- Erhardt, S.; Lim, C.K.; Linderholm, K.R.; Janelidze, S.; Lindqvist, D.; Samuelsson, M.; Lundberg, K.; Postolache, T.T.; Träskman-Bendz, L.; Guillemin, G.J.; et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2012, 38, 743–752. [Google Scholar] [CrossRef]
- Pandey, G.N.; Dwivedi, Y. Peripheral Biomarkers for Suicide. In The Neurobiological Basis of Suicide; Dwivedi, Y., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Millton, UK, 2012. [Google Scholar]
- Arango, V.; Underwood, M.D.; Mann, J. Chapter 35 Serotonin brain circuits involved in major depression and suicide. Prog. Brain Res. 2002, 136, 443–453. [Google Scholar] [CrossRef]
- Azmitia, E.C. Evolution of Serotonin: Sunlight to Suicide. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 3–22. [Google Scholar]
- Pandey, G.N. Biological basis of suicide and suicidal behavior. Bipolar Disord. 2013, 15, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Sudol, K.; Mann, J.J. Biomarkers of Suicide Attempt Behavior: Towards a Biological Model of Risk. Curr. Psychiatry Rep. 2017, 19, 31. [Google Scholar] [CrossRef]
- Lybech, L.K.M.; Calabró, M.; Briuglia, S.; Drago, A.; Crisafulli, C. Suicide Related Phenotypes in a Bipolar Sample: Genetic Underpinnings. Genes 2021, 12, 1482. [Google Scholar] [CrossRef] [PubMed]
- Voracek, M.; Loibl, L.M. Genetics of suicide: A systematic review of twin studies. Wien. Klin. Wochenschr. 2007, 119, 463–475. [Google Scholar] [CrossRef]
- Brent, D.A.; Mann, J.J. Family genetic studies, suicide, and suicidal behavior. Am. J. Med. Genet. Part C Semin. Med. Genet. 2005, 133, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mullins, N.; Bigdeli, T.B.; Børglum, A.D.; Coleman, J.R.; Demontis, D.; Mehta, D.; Power, R.A.; Ripke, S.; Stahl, E.A.; Starnawska, A.; et al. GWAS of Suicide Attempt in Psychiatric Disorders and Association with Major Depression Polygenic Risk Scores. Am. J. Psychiatry 2019, 176, 651–660. [Google Scholar] [CrossRef]
- Ruderfer, D.M.; Walsh, C.G.; Aguirre, M.W.; Tanigawa, Y.; Ribeiro, J.D.; Franklin, J.C.; Rivas, M.A. Significant shared heritability underlies suicide attempt and clinically predicted probability of attempting suicide. Mol. Psychiatry 2019, 25, 2422–2430. [Google Scholar] [CrossRef]
- Stein, M.B.; Ware, E.B.; Mitchell, C.; Chen, C.-Y.; Borja, S.; Cai, T.; Dempsey, C.L.; Fullerton, C.S.; Gelernter, J.; Heeringa, S.G.; et al. Genomewide association studies of suicide attempts in US soldiers. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Mullins, N.; Perroud, N.; Uher, R.; Butler, A.W.; Cohen-Woods, S.; Rivera, M.; Malki, K.; Euesden, J.; Power, R.A.; Tansey, K.E.; et al. Genetic relationships between suicide attempts, suicidal ideation and major psychiatric disorders: A genome-wide association and polygenic scoring study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 428–437. [Google Scholar] [CrossRef]
- Galfalvy, H.; Haghighi, F.; Hodgkinson, C.; Goldman, D.; Oquendo, M.A.; Burke, A.; Huang, Y.-Y.; Giegling, I.; Rujescu, D.; Bureau, A.; et al. A genome-wide association study of suicidal behavior. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 557–563. [Google Scholar] [CrossRef]
- Erlangsen, A.; Appadurai, V.; Wang, Y.; Turecki, G.; Mors, O.; Werge, T.; Mortensen, P.B.; Starnawska, A.; Børglum, A.D.; Schork, A.; et al. Genetics of suicide attempts in individuals with and without mental disorders: A population-based genome-wide association study. Mol. Psychiatry 2018, 25, 2410–2421. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Cremone, I.M.; Amatori, G.; Cappelli, A.; Cuomo, A.; Barlati, S.; Massimetti, G.; Vita, A.; Fagiolini, A.; Carmassi, C.; et al. Investigating the Relationship between Autistic Traits, Ruminative Thinking, and Suicidality in a Clinical Sample of Subjects with Bipolar Disorder and Borderline Personality Disorder. Brain Sci. 2021, 11, 621. [Google Scholar] [CrossRef]
- Pelton, M.K.; Cassidy, S.A. Are autistic traits associated with suicidality? A test of the interpersonal-psychological theory of suicide in a non-clinical young adult sample. Autism Res. 2017, 10, 1891–1904. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Gau, S.S. Suicidality in Children with Elevated Autistic Traits. Autism Res. 2020, 13, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carpita, B.; Muti, D.; Morelli, V.; Salarpi, G.; Salerni, A.; Scotto, J.; Massimetti, G.; Gesi, C.; Ballerio, M.; et al. Mood symptoms and suicidality across the autism spectrum. Compr. Psychiatry 2019, 91, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules 2023, 28, 1889. [Google Scholar] [CrossRef]
- Sultan, S. Translating neuroimaging changes to neuro-endophenotypes of autistic spectrum disorder: A narrative review. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 139. [Google Scholar] [CrossRef]
- Just, M.A.; Keller, T.A.; Malave, V.L.; Kana, R.K.; Varma, S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 2012, 36, 1292–1313. [Google Scholar] [CrossRef] [PubMed]
- Just, M.; Minshew, N.; Williams, D.; Cherkassky, V.; Kana, R.; Keller, T.; Damarla, S. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010, 5, 273–279. [Google Scholar] [CrossRef]
- Kana, R.K.; Keller, T.A.; Minshew, N.J.; Just, M.A. Inhibitory Control in High-Functioning Autism: Decreased Activation and Underconnectivity in Inhibition Networks. Biol. Psychiatry 2007, 62, 198–206. [Google Scholar] [CrossRef]
- Kana, R.K.; Keller, T.A.; Cherkassky, V.L.; Minshew, N.J.; Just, M.A. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain 2006, 129, 2484–2493. [Google Scholar] [CrossRef]
- Kana, R.K.; Keller, T.A.; Cherkassky, V.L.; Minshew, N.J.; Just, M. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc. Neurosci. 2009, 4, 135–152. [Google Scholar] [CrossRef]
- Koshino, H.; Carpenter, P.A.; Minshew, N.J.; Cherkassky, V.L.; Keller, T.A.; Just, M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage 2005, 24, 810–821. [Google Scholar] [CrossRef]
- Koshino, H.; Kana, R.K.; Keller, T.A.; Cherkassky, V.L.; Minshew, N.J.; Just, M.A. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb. Cortex 2008, 18, 289–300. [Google Scholar] [CrossRef]
- Mason, R.A.; Williams, D.L.; Kana, R.K.; Minshew, N.; Just, M.A. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 2008, 46, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, A.; Liu, Y.; Williams, D.L.; Keller, T.A.; Minshew, N.J.; Just, M. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain 2011, 134, 2422–2435. [Google Scholar] [CrossRef]
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Minshew, N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 2004, 127, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.; Luthert, P.; Dean, A.; Harding, B.; Janota, I.; Montgomery, M.; Rutter, M.; Lantos, P. A clinicopathological study of autism. Brain 1998, 121, 889–905. [Google Scholar] [CrossRef]
- Bauman, M.; Kemper, T.L. Histoanatomic observations of the brain in early infantile autism. Neurology 1985, 35, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Karns, C.M.; Davis, H.R.; Ziccardi, R.; Carper, R.A.; Tigue, Z.D.; Chisum, H.J.; Moses, P.; Pierce, K.; Lord, C.; et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 2001, 57, 245–254. [Google Scholar] [CrossRef]
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of Brain Overgrowth in the First Year of Life in Autism. JAMA 2003, 290, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, H.C.; Poe, M.; Gerig, G.; Smith, R.G.; Provenzale, J.; Ross, A.; Gilmore, J.; Piven, J. Magnetic Resonance Imaging and Head Circumference Study of Brain Size in Autism. Arch. Gen. Psychiatry 2005, 62, 1366–1376. [Google Scholar] [CrossRef]
- Castelli, F.; Frith, C.; Happé, F.; Frith, U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002, 125, 1839–1849. [Google Scholar] [CrossRef]
- Frith, C. What Do Imaging Studies Tell Us About the Neural Basis of Autism? In Autism: Neural Basis and Treatment Possibilities: Novartis Foundation Symposium; Bock, G., Goode, J., Eds.; John Wiley & Sons: Chichester, UK, 2003; Volume 251, pp. 149–176. [Google Scholar]
- Schultz, R.T.; Klin, A. Genetics of Childhood Disorders: XLIII. Autism, Part 2: Neural Foundations. J. Am. Acad. Child. Adolesc. Psychiatry 2002, 41, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Aylward, E.H.; Minshew, N.J.; Field, K.; Sparks, B.F.; Singh, N. Effects of age on brain volume and head circumference in autism. Neurology 2002, 59, 175–183. [Google Scholar] [CrossRef]
- Piven, J.; Arndt, S.; Bailey, J.; Havercamp, S.; Andreasen, N.C.; Palmer, P. An MRI study of brain size in autism. Am. J. Psychiatry 1995, 152, 1145–1149. [Google Scholar] [CrossRef]
- Carper, R.A.; Courchesne, E. Localized enlargement of the frontal cortex in early autism. Biol. Psychiatry 2005, 57, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Thabault, M.; Turpin, V.; Maisterrena, A.; Jaber, M.; Egloff, M.; Galvan, L. Cerebellar and Striatal Implications in Autism Spectrum Disorders: From Clinical Observations to Animal Models. Int. J. Mol. Sci. 2022, 23, 2294. [Google Scholar] [CrossRef]
- Khadem-Reza, Z.K.; Zare, H. Evaluation of brain structure abnormalities in children with autism spectrum disorder (ASD) using structural magnetic resonance imaging. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 135. [Google Scholar] [CrossRef]
- Carper, R.A.; Moses, P.; Tigue, Z.D.; Courchesne, E. Cerebral Lobes in Autism: Early Hyperplasia and Abnormal Age Effects. Neuroimage 2002, 16, 1038–1051. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Filipek, P.A.; Kemper, T.L.; Normandin, J.J.; Sanders, H.A.; Kennedy, D.N.; Caviness, V.S. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004, 55, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Calderoni, S.; Conti, E.; Gesi, C.; Carmassi, C.; Dell’Osso, L.; Cioni, G.; Muratori, F.; Guzzetta, A. The Broad Autism (Endo)Phenotype: Neurostructural and Neurofunctional Correlates in Parents of Individuals with Autism Spectrum Disorders. Front. Neurosci. 2016, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Redcay, E.; Kennedy, D.P. The autistic brain: Birth through adulthood. Curr. Opin. Neurol. 2004, 17, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Waiter, G.D.; Williams, J.H.; Murray, A.; Gilchrist, A.; Perrett, D.; Whiten, A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: A voxel-based investigation. Neuroimage 2005, 24, 455–461. [Google Scholar] [CrossRef]
- Carpita, B.; Carmassi, C.; Calderoni, S.; Muti, D.; Muscarella, A.; Massimetti, G.; Cremone, I.M.; Gesi, C.; Conti, E.; Muratori, F.; et al. The broad autism phenotype in real-life: Clinical and functional correlates of autism spectrum symptoms and rumination among parents of patients with autism spectrum disorder. CNS Spectr. 2020, 25, 765–773. [Google Scholar] [CrossRef]
- Carpita, B.; Muti, D.; Muscarella, A.; Dell’Oste, V.; Diadema, E.; Massimetti, G.; Signorelli, M.S.; Fusar Poli, L.; Gesi, C.; Aguglia, E.; et al. Sex Differences in the Relationship between PTSD Spectrum Symptoms and Autistic Traits in a Sample of University Students. Clin. Pract. Epidemiol. Ment. Health 2019, 15, 110–119. [Google Scholar] [CrossRef]
- Carpita, B.; Cremone, I.M.; Amatori, G.; Cappelli, A.; Salerni, A.; Massimetti, G.; Borgioli, D.; Carmassi, C.; Massai, R.; Dell’osso, L. Investigating the relationship between orthorexia nervosa and autistic traits in a university population. CNS Spectr. 2022, 27, 613–620. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carpita, B.; Bertelloni, C.A.; Diadema, E.; Barberi, F.M.; Gesi, C.; Carmassi, C. Subthreshold autism spectrum in bipolar disorder: Prevalence and clinical correlates. Psychiatry Res. 2019, 281, 112605. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Cremone, I.M.; Carpita, B.; Fagiolini, A.; Massimetti, G.; Bossini, L.; Vita, A.; Barlati, S.; Carmassi, C.; Gesi, C. Correlates of autistic traits among patients with borderline personality disorder. Compr. Psychiatry 2018, 83, 7–11. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carpita, B.; Cremone, I.M.; Gesi, C.; D’Eermo, A.; De Iorio, G.; Massimetti, G.; Aguglia, E.; Bucci, P.; Carpiniello, B.; et al. Autism spectrum in patients with schizophrenia: Correlations with real-life functioning, resilience, and coping styles. CNS Spectr. 2021, 12, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carmassi, C.; Cremone, I.M.; Muti, D.; Salerni, A.; Barberi, F.M.; Massimetti, E.; Gesi, C.; Politi, P.; Aguglia, E.; et al. Defining the Optimal Threshold Scores for Adult Autism Subthreshold Spectrum (AdAS Spectrum) in Clinical and General Population. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Conversano, C.; Corsi, M.; Bertelloni, C.A.; Cremone, I.M.; Carpita, B.; Carbone, M.G.; Gesi, C.; Carmassi, C. Polysubstance and Behavioral Addictions in a Patient with Bipolar Disorder: Role of Lifetime Subthreshold Autism Spectrum. Case Rep. Psychiatry 2018, 2018, 1547975. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Bertelloni, C.A.; Di Paolo, M.; Avella, M.T.; Carpita, B.; Gori, F.; Pompili, M.; Carmassi, C. Problematic Internet Use in University Students Attending Three Superior Graduate Schools in Italy: Is Autism Spectrum Related to Suicide Risk? Int. J. Environ. Res. Public Health 2019, 16, 1098. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Lorenzi, P.; Carpita, B. The neurodevelopmental continuum towards a neurodevelopmental gradient hypothesis. J. Psychopathol. 2019, 25, 179–182. [Google Scholar]
- Cassidy, S.; Rodgers, J. Understanding and prevention of suicide in autism. Lancet Psychiatry 2017, 4, e11. [Google Scholar] [CrossRef]
- Hedley, D.; Uljarević, M. Systematic Review of Suicide in Autism Spectrum Disorder: Current Trends and Implications. Curr. Dev. Disord. Rep. 2018, 5, 65–76. [Google Scholar] [CrossRef]
- Hirvikoski, T.; Mittendorfer-Rutz, E.; Boman, M.; Larsson, H.; Lichtenstein, P.; Bölte, S. Premature mortality in autism spectrum disorder. Br. J. Psychiatry 2016, 208, 232–238. [Google Scholar] [CrossRef]
- Hwang, Y.I.; Srasuebkul, P.; Foley, K.; Arnold, S.; Trollor, J.N. Mortality and cause of death of Australians on the autism spectrum. Autism Res. 2019, 12, 806–815. [Google Scholar] [CrossRef]
- Kirby, A.V.; Bakian, A.V.; Zhang, Y.; Bilder, D.A.; Keeshin, B.R.; Coon, H. A 20-year study of suicide death in a statewide autism population. Autism Res. 2019, 12, 658–666. [Google Scholar] [CrossRef]
- Mayes, S.D.; Gorman, A.A.; Hillwig-Garcia, J.; Syed, E. Suicide ideation and attempts in children with autism. Res. Autism Spectr. Disord. 2013, 7, 109–119. [Google Scholar] [CrossRef]
- Paquette-Smith, M.; Weiss, J.; Lunsky, Y. History of Suicide Attempts in Adults with Asperger Syndrome. Crisis 2014, 35, 273–277. [Google Scholar] [CrossRef]
- Segers, M.; Rawana, J. What Do We Know About Suicidality in Autism Spectrum Disorders? A Systematic Review. Autism Res. 2014, 7, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Abu-Akel, A.; Chisholm, K.; Lin, A.; Zahid, S.; Pelton, M.; Apperly, I.; Hansen, P.C.; Wood, S.J. Autism and psychosis: Clinical implications for depression and suicide. Schizophr. Res. 2018, 195, 80–85. [Google Scholar] [CrossRef]
- Cassidy, S.; Bradley, L.; Shaw, R.; Baron-Cohen, S. Risk markers for suicidality in autistic adults. Mol. Autism 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Kenny, R.; Griffiths, S.; Allison, C.; Mosse, D.; Holt, R.; O’connor, R.C.; Cassidy, S.; Baron-Cohen, S. Autistic traits in adults who have attempted suicide. Mol. Autism 2019, 10, 26. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.; Karg, R.S.; Spitzer, R.L. SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders, Clinician Version; American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Dell’Osso, L.; Cremone, I.M.; Carpita, B.; Dell’Oste, V.; Muti, D.; Massimetti, G.; Barlati, S.; Vita, A.; Fagiolini, A.; Carmassi, C.; et al. Rumination, posttraumatic stress disorder, and mood symptoms in borderline personality disorder. Neuropsychiatr. Dis. Treat. 2019, 15, 1231–1238. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Carpita, B.; Nardi, B.; Palego, L.; Cremone, I.M.; Massimetti, G.; Carmassi, C.; Betti, L.; Giannaccini, G.; Dell’osso, L. Kynurenine pathway and autism spectrum phenotypes: An investigation among adults with autism spectrum disorder and their first-degree relatives. CNS Spectr. 2022, 28, 374–385. [Google Scholar] [CrossRef]
- Carpita, B.; Stagnari, R.; Palego, L.; Baroni, D.; Massimetti, G.; Nardi, B.; Cremone, I.M.; Betti, L.; Giannaccini, G.; Dell’Osso, L. Circulating levels of 5-HT and BDNF in Adults with Autism Spectrum Conditions: An Investigation in a Sample of Subjects with Autism Spectrum Disorder, their first-degree Relatives and Controls. Curr. Med. Chem. 2023. ahead of print. [Google Scholar] [CrossRef]
- Carpita, B.; Massoni, L.; Battaglini, S.; Palego, L.; Cremone, I.M.; Massimetti, G.; Betti, L.; Giannaccini, G.; Dell’osso, L. IL-6, homocysteine, and autism spectrum phenotypes: An investigation among adults with autism spectrum disorder and their first-degree relatives. CNS Spectr. 2023, 1–9. [Google Scholar] [CrossRef]
- Hedley, D.; Uljarević, M.; Foley, K.-R.; Richdale, A.; Trollor, J. Risk and protective factors underlying depression and suicidal ideation in Autism Spectrum Disorder. Depress. Anxiety 2018, 35, 648–657. [Google Scholar] [CrossRef]
- Jobe, L.E.; Williams White, S. Loneliness, social relationships, and a broader autism phenotype in college students. Pers. Individ Differ. 2007, 42, 1479–1489. [Google Scholar] [CrossRef]
- Holt, M.K.; Vivolo-Kantor, A.M.; Polanin, J.R.; Holland, K.M.; DeGue, S.; Matjasko, J.L.; Wolfe, M.; Reid, G. Bullying and Suicidal Ideation and Behaviors: A Meta-Analysis. Pediatrics 2015, 135, e496–e509. [Google Scholar] [CrossRef]
- Klomek, A.B.; Marrocco, F.; Kleinman, M.; Schonfeld, I.S.; Gould, M.S. Peer Victimization, Depression, and Suicidiality in Adolescents. Suicide Life-Threat. Behav. 2008, 38, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, A.; Schuller, B.; Marchi, E.; Piana, S.; Camurri, A.; Lassalle, A.; Baron-Cohen, S. An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol. Autism 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.A.; Gould, K.; Townsend, E.; Pelton, M.; Robertson, A.E.; Rodgers, J. Is Camouflaging Autistic Traits Associated with Suicidal Thoughts and Behaviours? Expanding the Interpersonal Psychological Theory of Suicide in an Undergraduate Student Sample. J. Autism Dev. Disord. 2020, 50, 3638–3648. [Google Scholar] [CrossRef]
- Cremone, I.M.; Carpita, B.; Nardi, B.; Casagrande, D.; Stagnari, R.; Amatori, G.; Dell’osso, L. Measuring Social Camouflaging in Individuals with High Functioning Autism: A Literature Review. Brain Sci. 2023, 13, 469. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Chen, L.-M.; Bao, C.-H.; Wu, Y.; Liang, S.-H.; Di Wang, D.; Wu, L.-Y.; Huang, Y.; Liu, H.-R.; Wu, H.-G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflammation 2021, 18, 135. [Google Scholar] [CrossRef]
- Lapin, I.; Oxenkrug, G. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1969, 293, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Tryptophan-kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: The serotonin hypothesis revisited 40 years later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56–63. [Google Scholar] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Rodríguez-Núñez, M.; Marco, M.-P.; Mir, M.; Samitier, J. Kynurenic Acid Electrochemical Immunosensor: Blood-Based Diagnosis of Alzheimer’s Disease. Biosensors 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Sublette, M.E.; Galfalvy, H.C.; Fuchs, D.; Lapidus, M.; Grunebaum, M.F.; Oquendo, M.A.; Mann, J.J.; Postolache, T.T. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav. Immun. 2011, 25, 1272–1278. [Google Scholar] [CrossRef]
- Bradley, K.A.; Case, J.A.; Khan, O.; Ricart, T.; Hanna, A.; Alonso, C.M.; Gabbay, V. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 2015, 227, 206–212. [Google Scholar] [CrossRef]
- Almeida-Montes, L.G.; Valles-Sanchez, V.; Moreno-Aguilar, J.; A Chavez-Balderas, R.; A García-Marín, J.; Sotres, J.F.C.; Hheinze-Martin, G. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J. Psychiatry Neurosci. 2000, 25, 371–377. [Google Scholar]
- Clark, D.B. Serum tryptophan ratio and suicidal behavior in adolescents: A prospective study. Psychiatry Res. 2003, 119, 199–204. [Google Scholar] [CrossRef]
- Pfeffer, C.R.; McBride, P.; Anderson, G.M.; Kakuma, T.; Fensterheim, L.; Khait, V. Peripheral serotonin measures in prepubertal psychiatric inpatients and normal children: Associations with suicidal behavior and its risk factors. Biol. Psychiatry 1998, 44, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.-M.; Kim, Y.K.; Verkerk, R.; Scharpé, S.; Steinbusch, H.; Leonard, B. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J. Affect. Disord. 2007, 98, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Carlborg, A.; Jokinen, J.; Jönsson, E.G.; Erhardt, S.; Nordström, P. CSF kynurenic acid and suicide risk in schizophrenia spectrum psychosis. Psychiatry Res. 2013, 205, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Meltzer, H.Y.; Scharpè, S.; Bosmans, E.; Suy, E.; De Meester, I.; Calabrese, J.; Cosyns, P. Relationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res. 1993, 49, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Neurobiology of suicidal behaviour. Nat. Rev. Neurosci. 2003, 4, 819–828. [Google Scholar] [CrossRef]
- Müller, N.; Schwarz, M.J. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol. Psychiatry 2007, 12, 988–1000. [Google Scholar] [CrossRef]
- Amori, L.; Wu, H.-Q.; Marinozzi, M.; Pellicciari, R.; Guidetti, P.; Schwarcz, R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience 2009, 159, 196–203. [Google Scholar] [CrossRef]
- Poeggeler, B.; Rassoulpour, A.; Wu, H.-Q.; Guidetti, P.; Roberts, R.; Schwarcz, R. Dopamine receptor activation reveals a novel, kynurenate-sensitive component of striatal N-methyl-d-aspartate neurotoxicity. Neuroscience 2007, 148, 188–197. [Google Scholar] [CrossRef]
- Schwarcz, R.; Pellicciari, R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002, 303, 1–10. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The Brain Metabolite Kynurenic Acid Inhibits α7 Nicotinic Receptor Activity and Increases Non-α7 Nicotinic Receptor Expression: Physiopathological Implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Chess, A.C.; Simoni, M.K.; Alling, T.E.; Bucci, D.J. Elevations of Endogenous Kynurenic Acid Produce Spatial Working Memory Deficits. Schizophr. Bull. 2007, 33, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Keilp, J.G.; Sackeim, H.A.; Brodsky, B.S.; Oquendo, M.A.; Malone, K.M.; Mann, J.J. Neuropsychological Dysfunction in Depressed Suicide Attempters. Am. J. Psychiatry 2001, 158, 735–741. [Google Scholar] [CrossRef]
- Marzuk, P.M.; Hartwell, N.; Leon, A.C.; Portera, L. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr. Scand. 2005, 112, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.R.; Muñiz, P.E.U.; Ayala, T.B.; Cervantes, G.I.V.; Huitrón, R.L.; Pineda, B.; Esquivel, D.F.G.; de la Cruz, G.P.; Chaverrí, J.P.; Chapul, L.S.; et al. On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants 2022, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, R.C.-M.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef]
- Maes, M.; Scharpé, S.; Meltzer, H.Y.; Bosmans, E.; Suy, E.; Calabrese, J.; Cosyns, P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993, 49, 11–27. [Google Scholar] [CrossRef]
- Rush, G.; O’donovan, A.; Nagle, L.; Conway, C.; McCrohan, A.; O’farrelly, C.; Lucey, J.V.; Malone, K.M. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. J. Affect. Disord. 2016, 205, 60–68. [Google Scholar] [CrossRef]
- Yang, C.; Tiemessen, K.M.; Bosker, F.J.; Wardenaar, K.J.; Lie, J.; Schoevers, R.A. Interleukin, tumor necrosis factor-α and C-reactive protein profiles in melancholic and non-melancholic depression: A systematic review. J. Psychosom. Res. 2018, 111, 58–68. [Google Scholar] [CrossRef]
- E Thase, M. Recognition and diagnosis of atypical depression. J. Clin. Psychiatry 2007, 68, 11–16. [Google Scholar]
- Rudolf, S.; Greggersen, W.; Kahl, K.G.; Hüppe, M.; Schweiger, U. Elevated IL-6 levels in patients with atypical depression but not in patients with typical depression. Psychiatry Res. 2014, 217, 34–38. [Google Scholar] [CrossRef]
- Morris, M.S.; Fava, M.; Jacques, P.F.; Selhub, J.; Rosenberg, I.H. Depression and Folate Status in the US Population. Psychother. Psychosom. 2003, 72, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Borus, J.S.; E Alpert, J.; A Nierenberg, A.; Rosenbaum, J.F.; Bottiglieri, T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am. J. Psychiatry 1997, 154, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Tell, G.S.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Folate, Vitamin B12, Homocysteine, and the MTHFR 677C→T Polymorphism in Anxiety and Depression. Arch. Gen. Psychiatry 2003, 60, 618–626. [Google Scholar] [CrossRef]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.P.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Lautenschlager, N.; Flicker, L.; Leedman, P.; Vasikaran, S.; Gelavis, A.; Ludlow, J. Association Between Homocysteine, Depression, and Cognitive Function in Community-Dwelling Older Women from Australia. J. Am. Geriatr. Soc. 2004, 52, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Nurk, E.; Smith, A.D.; Ueland, P.M.; Gjesdal, C.G.; Bjelland, I.; Tverdal, A.; Tell, G.S.; Nygård, O.; Vollset, S.E. The Hordaland Homocysteine Study: A Community-Based Study of Homocysteine, Its Determinants, and Associations with Disease. J. Nutr. 2006, 136, 1731–1740. [Google Scholar] [CrossRef]
- Moradi, F.; Lotfi, K.; Armin, M.; Clark, C.C.; Askari, G.; Rouhani, M.H. The association between serum homocysteine and depression: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Investig. 2021, 51, e13486. [Google Scholar] [CrossRef]
- Kim, J.-M.; Kang, H.-J.; Kim, J.-W.; Choi, W.; Lee, J.-Y.; Kim, S.-W.; Shin, I.-S.; Kim, M.-G.; Chun, B.J.; Stewart, R. Multiple serum biomarkers for predicting suicidal behaviours in depressive patients receiving pharmacotherapy. Psychol. Med. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Ho, P.I.; Ortiz, D.; Rogers, E.; Shea, T.B. Multiple aspects of homocysteine neurotoxicity: Glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 2002, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.; Yamamoto, C. Gamma-D-glutamylglycine and cis-2,3-piperidine dicarboxylate as antagonists of excitatory amino acids in the hippocampus. Exp. Brain Res. 1984, 55, 351–358. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Parslow, R.A.; Lux, O.; Salonikas, C.; Wen, W.; Naidoo, D.; Christensen, H.; Jorm, A.F. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol. Med. 2005, 35, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Matsushita, Y.; Sasaki, S.; Ohta, M.; Sato, M.; Mishima, N. Serum folate and homocysteine and depressive symptoms among Japanese men and women. Eur. J. Clin. Nutr. 2010, 64, 289–296. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Shroff, M.R.; Beydoun, H.A.; Zonderman, A.B. Serum Folate, Vitamin B-12, and Homocysteine and Their Association with Depressive Symptoms among U.S. Adults. Psychosom. Med. 2010, 72, 862–873. [Google Scholar] [CrossRef]
- Elstgeest, L.E.M.; A Brouwer, I.; Penninx, B.W.H.; van Schoor, N.M.; Visser, M. Vitamin B12, homocysteine and depressive symptoms: A longitudinal study among older adults. Eur. J. Clin. Nutr. 2017, 71, 468–475. [Google Scholar] [CrossRef]
- Fraguas, R.; Papakostas, G.I.; Mischoulon, D.; Bottiglieri, T.; Alpert, J.; Fava, M. Anger Attacks in Major Depressive Disorder and Serum Levels of Homocysteine. Biol. Psychiatry 2006, 60, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, J.R.; Chalmers, A.H.; Winefield, A.; Blake-Mortimer, J.S. Changes in Clinically Relevant Metabolites with Psychological Stress Parameters. Behav. Med. 2003, 29, 52–59. [Google Scholar] [CrossRef]
- Stoney, C.M.; Engebretson, T.O. Plasma homocysteine concentrations are positively associated with hostility and anger. Life Sci. 2000, 66, 2267–2275. [Google Scholar] [CrossRef]
- Levine, J.; Sela, B.-A.; Osher, Y.; Belmaker, R. High homocysteine serum levels in young male schizophrenia and bipolar patients and in an animal model. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2005, 29, 1181–1191. [Google Scholar] [CrossRef]
- Brundin, L.; Erhardt, S.; Bryleva, E.Y.; Achtyes, E.D.; Postolache, T.T. The role of inflammation in suicidal behaviour. Acta Psychiatr. Scand. 2015, 132, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Carpita, B.; Marazziti, D.; Palego, L.; Giannaccini, G.; Betti, L.; Dell’Osso, L. Microbiota, Immune System and Autism Spectrum Disorders: An Integrative Model towards Novel Treatment Options. Curr. Med. Chem. 2020, 27, 5119–5136. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Hassan, A.; Zenz, G.; Holzer, P. Diabesity and mood disorders: Multiple links through the microbiota-gut-brain axis. Mol. Asp. Med. 2018, 66, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.R.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Safadi, J.M.; Quinton, A.M.G.; Lennox, B.R.; Burnet, P.W.J.; Minichino, A. Gut dysbiosis in severe mental illness and chronic fatigue: A novel trans-diagnostic construct? A systematic review and meta-analysis. Mol. Psychiatry 2021, 27, 141–153. [Google Scholar] [CrossRef]
- McCall, W.V.; Black, C.G. The Link Between Suicide and Insomnia: Theoretical Mechanisms. Curr. Psychiatry Rep. 2013, 15, 389. [Google Scholar] [CrossRef]
- McCall, W.V.; Benca, R.M.; Rosenquist, P.B.; Youssef, N.A.; McCloud, L.; Newman, J.C.; Case, D.; Rumble, M.E.; Szabo, S.T.; Phillips, M.; et al. Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT): A Randomized Clinical Trial. Am. J. Psychiatry 2019, 176, 957–965. [Google Scholar] [CrossRef]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Guo, S.; Hu, S.; Chen, J.; Ye, M.; Liu, Z. The microbiota–gut–brain axis and its modulation in the therapy of depression: Comparison of efficacy of conventional drugs and traditional Chinese medicine approaches. Pharmacol. Res. 2022, 183, 106372. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hu, Y.; Liu, W.; Zhu, G.; Zhang, R.; You, J.; Shao, Y.; Li, Y.; Zhang, Z.; Cui, J.; et al. Deciphering the Effective Constituents and Mechanisms of Portulaca oleracea L. for Treating NASH via Integrating Bioinformatics Analysis and Experimental Pharmacology. Front. Pharmacol. 2022, 12, 818227. [Google Scholar] [CrossRef]
- Tsuchiyagaito, A.; Smith, J.L.; El-Sabbagh, N.; Zotev, V.; Misaki, M.; Al Zoubi, O.; Teague, T.K.; Paulus, M.P.; Bodurka, J.; Savitz, J. Real-time fMRI neurofeedback amygdala training may influence kynurenine pathway metabolism in major depressive disorder. NeuroImage Clin. 2021, 29, 102559. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; e Cordeiro, T.M.; Suchting, R.; de Dios, C.; Leal, V.A.C.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
| MOODS-SR | ASD (n = 24) (Mean ± SD) | BAP (n = 24) (Mean ± SD) | CTL (n = 24) (Mean ± SD) | F | df | p |
|---|---|---|---|---|---|---|
| Manic component | 22.77 ± 12.20 | 14.32 ± 9.32 | 6.12 ± 5.73 | 18.13 | 2 | <0.001 * |
| Depressive component | 34.32 ± 11.84 | 16.23 ± 13.73 | 7.79 ± 5.79 | 35.39 | 2 | <0.001 * |
| Rhythmicity | 13.00 ± 5.35 | 9.24 ± 5.36 | 5.54 ± 4.30 | 12.74 | 2 | <0.001 * |
| MOODS total | 70.09 ± 23.87 | 40.62 ± 24.97 | 19.46 ± 12.68 | 33.62 | 2 | <0.001 * |
| Suicidality Sub-Scale | ASD (n = 24) (Mean ± SD) | BAP (n = 24) (Mean ± SD) | CTL (n = 24) (Mean ± SD) | F | df | p |
|---|---|---|---|---|---|---|
| Suicidal ideation | 1.87 ± 1.73 | 0.54 ± 1.25 | 0.12 ± 0.61 | 12.22 | 2 | <0.001 * |
| Suicidal behavior | 0.50 ± 0.83 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.62 | 2 | <0.001 * |
| Suicidality total | 2.37 ± 2.24 | 0.54 ± 1.25 | 0.12 ± 0.61 | 14.81 | 2 | <0.001 * |
| Manic Component | Depressive Component | Rhythmicity | MOODS Total | |
|---|---|---|---|---|
| IL-6 pg/mL | 0.233 | 0.428 ** | 0.538 ** | 0.422 * |
| HCY µM | 0.119 | 0.283 * | 0.208 | 0.232 |
| 5-HT (PPP) ng/mL | −0.137 | −0.188 | −0.134 | −0.178 |
| 5-HT (intra-platelet) ng/mg prot | −0.093 | −0.136 | −0.064 | −0.120 |
| KYN ng/mL | −0.218 | −0.099 | −0.052 | −0.146 |
| KYNA ng/mL | −0.250 * | −0.398 ** | −0.315 * | −0.357 ** |
| QUIN ng/mL | −0.069 | −0.111 | −0.100 | −0.104 |
| TRP µM | −0.269 * | −0.259 * | −0.277 * | −0.294 * |
| BDNF ng/mL | −0.021 | 0.017 | −0.113 | −0.022 |
| Suicidal Ideation | Suicidal Behavior | Suicidality Total | |
|---|---|---|---|
| IL-6 pg/mL | 0.476 ** | 0.289 | 0.487 ** |
| HCY µM | 0.297 * | 0.105 | 0.278 * |
| 5-HT (PPP) ng/mL | −0.084 | −0.124 | −0.105 |
| 5-HT (intra-platelet) ng/mg prot | −0.152 | 0.044 | −0.111 |
| KYN ng/mL | −0.077 | −0.215 | −0.127 |
| KYNA ng/mL | −0.267 * | −0.116 | −0.253 * |
| QUIN ng/mL | −0.152 | −0.159 | −0.171 |
| TRP µM | −0.276 * | −0.294 * | −0.132 ** |
| BDNF ng/mL | 0.055 | 0.114 | 0.079 |
| b (SE) | Beta | t | p | C.I.95% | |
|---|---|---|---|---|---|
| Constant | −0.74 (0.55) | −1.36 | 0.183 | −1.85; −0.37 | |
| HCY µM | 0.18 (0.05) | 0.54 | 3.79 | 0.001 | 0.08; 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremone, I.M.; Nardi, B.; Amatori, G.; Palego, L.; Baroni, D.; Casagrande, D.; Massimetti, E.; Betti, L.; Giannaccini, G.; Dell'Osso, L.; et al. Unlocking the Secrets: Exploring the Biochemical Correlates of Suicidal Thoughts and Behaviors in Adults with Autism Spectrum Conditions. Biomedicines 2023, 11, 1600. https://doi.org/10.3390/biomedicines11061600
Cremone IM, Nardi B, Amatori G, Palego L, Baroni D, Casagrande D, Massimetti E, Betti L, Giannaccini G, Dell'Osso L, et al. Unlocking the Secrets: Exploring the Biochemical Correlates of Suicidal Thoughts and Behaviors in Adults with Autism Spectrum Conditions. Biomedicines. 2023; 11(6):1600. https://doi.org/10.3390/biomedicines11061600
Chicago/Turabian StyleCremone, Ivan Mirko, Benedetta Nardi, Giulia Amatori, Lionella Palego, Dario Baroni, Danila Casagrande, Enrico Massimetti, Laura Betti, Gino Giannaccini, Liliana Dell'Osso, and et al. 2023. "Unlocking the Secrets: Exploring the Biochemical Correlates of Suicidal Thoughts and Behaviors in Adults with Autism Spectrum Conditions" Biomedicines 11, no. 6: 1600. https://doi.org/10.3390/biomedicines11061600
APA StyleCremone, I. M., Nardi, B., Amatori, G., Palego, L., Baroni, D., Casagrande, D., Massimetti, E., Betti, L., Giannaccini, G., Dell'Osso, L., & Carpita, B. (2023). Unlocking the Secrets: Exploring the Biochemical Correlates of Suicidal Thoughts and Behaviors in Adults with Autism Spectrum Conditions. Biomedicines, 11(6), 1600. https://doi.org/10.3390/biomedicines11061600









