Pathogenesis, Intervention, and Current Status of Drug Development for Sarcopenia: A Review
Abstract
:1. Introduction
2. Epidemiology and Pathophysiology
3. Risk Factors for Primary Sarcopenia
3.1. Lack of Exercise
3.2. Imbalance in Hormones and Cytokines
3.3. Insufficient Protein Synthesis
3.4. Dysfunction of Motor Units
3.5. Lifestyle of Individual
3.6. Physical Condition at Birth
3.7. Nutritional Status
4. Risk Factors for Secondary Sarcopenia
4.1. Cachexia
4.2. Frailty
4.3. Sarcopenic Obesity
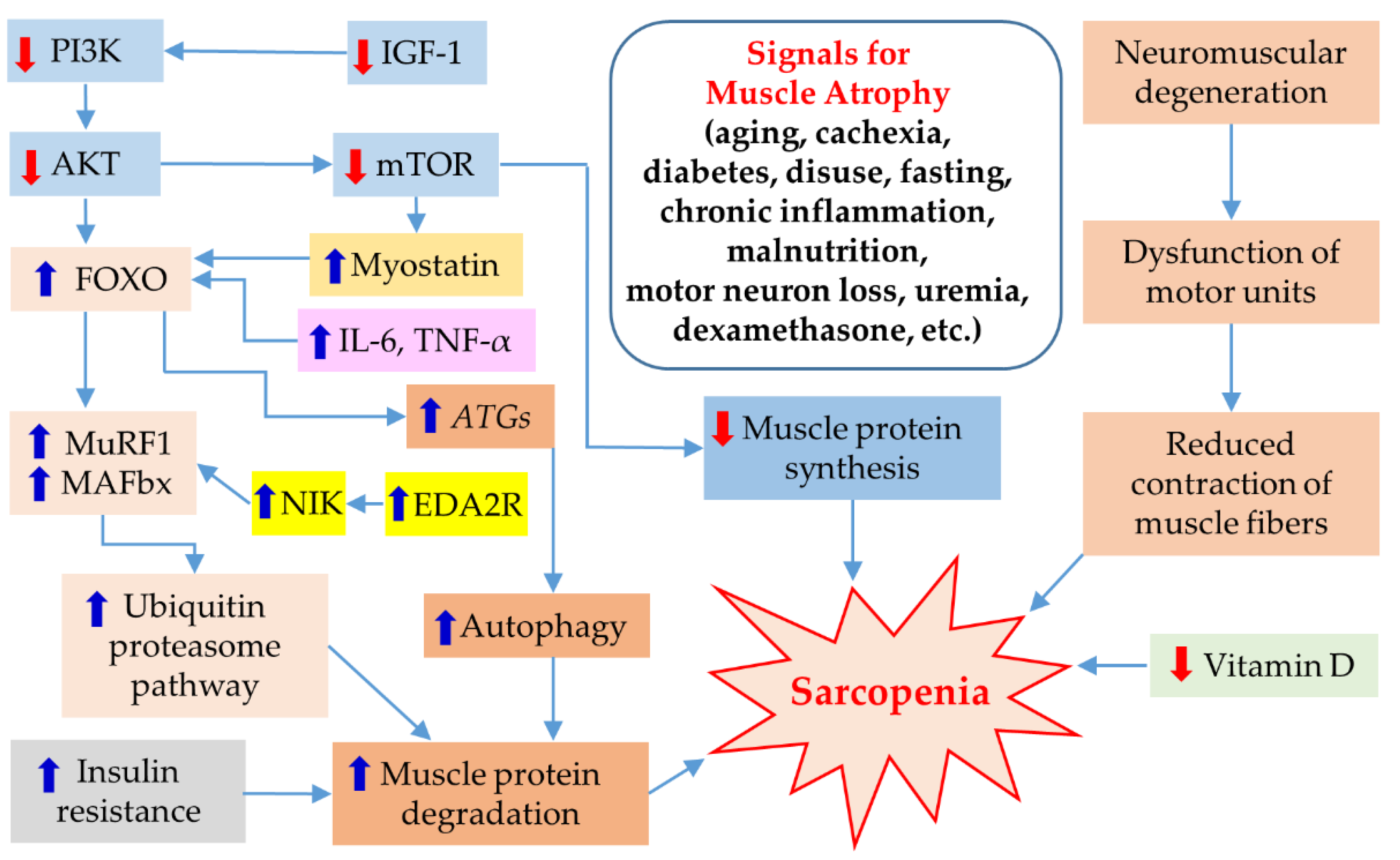
5. Pathogenesis
6. Diagnosis of Sarcopenia
7. Histopathology
8. Intervention
8.1. Non-Pharmacologic Treatment
8.2. Pharmacological Therapies
9. Exercise Mimetics
10. Herbal Supplements and Nutrition
11. Conclusions and Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Roubenoff, R. Origins and clinical relevance of sarcopenia. Can. J. Appl. Physiol. 2001, 26, 78–89. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Perna, S.; Spadaccini, D.; Nichetti, M.; Avanzato, I.; Faliva, M.A.; Rondanelli, M. Osteosarcopenic Visceral Obesity and Osteosarcopenic Subcutaneous Obesity, Two New Phenotypes of Sarcopenia: Prevalence, Metabolic Profile, and Risk Factors. J. Aging Res. 2018, 2018, 6147426. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef]
- Van Ancum, J.M.; Alcazar, J.; Meskers, C.G.M.; Nielsen, B.R.; Suetta, C.; Maier, A.B. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Arch. Gerontol. Geriatr. 2020, 90, 104125. [Google Scholar] [CrossRef]
- Ramirez, E.; Salas, R.; Bouzas, C.; Pastor, R.; Tur, J.A. Comparison between Original and Reviewed Consensus of European Working Group on Sarcopenia in Older People: A Probabilistic Cross-Sectional Survey among Community-Dwelling Older People. Gerontology 2022, 68, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.V.; Paiva, A.E.G.; Silva, A.C.B.; de Castro, I.C.; Santiago, A.F.; de Oliveira, E.P.; Porto, L.C.J. Prevalence of sarcopenia according to EWGSOP1 and EWGSOP2 in older adults and their associations with unfavorable health outcomes: A systematic review. Aging Clin. Exp. Res. 2022, 34, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Kim, J.T.; Park, C.H.; Cha, Y. Diagnosis and Management of Sarcopenia after Hip Fracture Surgery: Current Concept Review. Hip Pelvis 2022, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef] [Green Version]
- McPhee, J.S.; Cameron, J.; Maden-Wilkinson, T.; Piasecki, M.; Yap, M.H.; Jones, D.A.; Degens, H. The Contributions of Fiber Atrophy, Fiber Loss, In Situ Specific Force, and Voluntary Activation to Weakness in Sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1287–1294. [Google Scholar] [CrossRef]
- Edström, E.; Altun, M.; Bergman, E.; Johnson, H.; Kullberg, S.; Ramírez-León, V.; Ulfhake, B. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol. Behav. 2007, 92, 129–135. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef] [Green Version]
- Walrand, S.; Zangarelli, A.; Guillet, C.; Salles, J.; Soulier, K.; Giraudet, C.; Patrac, V.; Boirie, Y. Effect of fast dietary proteins on muscle protein synthesis rate and muscle strength in ad libitum-fed and energy-restricted old rats. Br. J. Nutr. 2011, 106, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.H.; Hood, D.A. Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life 2009, 61, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Exercise at old age: Does it increase or alleviate oxidative stress? Ann. N. Y. Acad. Sci. 2001, 928, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Di Iorio, A.; Di Renzo, D.; Paganelli, R.; Saggini, R.; Abate, G. Frailty in the elderly: The physical dimension. Eur. Medicophys. 2007, 43, 407–415. [Google Scholar]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 9, 213–228. [Google Scholar] [CrossRef]
- Ying, L.; Zhang, Q.; Yang, Y.M.; Zhou, J.Y. A Combination of Serum Biomarkers in Elderly Patients with Sarcopenia: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2022, 2022, 4026940. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Booth, F.W.; Chakravarthy, M.V.; Spangenburg, E.E. Exercise and gene expression: Physiological regulation of the human genome through physical activity. J. Physiol. 2002, 543, 399–411. [Google Scholar] [CrossRef]
- Sayer, A.A.; Syddall, H.E.; Gilbody, H.J.; Dennison, E.M.; Cooper, C. Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M930–M934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayer, A.A.; Dennison, E.M.; Syddall, H.E.; Jameson, K.; Martin, H.J.; Cooper, C. The developmental origins of sarcopenia: Using peripheral quantitative computed tomography to assess muscle size in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.P.; Jameson, K.A.; Syddall, H.E.; Martin, H.J.; Stewart, C.E.; Cooper, C.; Sayer, A.A. Developmental influences, muscle morphology, and sarcopenia in community-dwelling older men. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, M.; Ishida, J.; von Haehling, S.; Anker, S.D.; Springer, J. Nutrition in cachexia: From bench to bedside. J. Cachexia Sarcopenia Muscle 2016, 7, 107–109. [Google Scholar] [CrossRef] [Green Version]
- Relph, W.L. Addressing the nutritional needs of older patients. Nurs. Older People 2016, 28, 16–19. [Google Scholar] [CrossRef]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26, 389–399. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Morley, J.E.; Anker, S.D.; Evans, W.J. Cachexia and aging: An update based on the Fourth International Cachexia Meeting. J. Nutr. Health Aging 2009, 13, 47–55. [Google Scholar] [CrossRef]
- Durham, W.J.; Dillon, E.L.; Sheffield-Moore, M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Kaiser, M.J.; Sieber, C.C. Sarcopenia in nursing home residents. J. Am. Med. Dir. Assoc. 2008, 9, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Waters, D.L.; Baumgartner, R.N. Sarcopenia and obesity. Clin. Geriatr. Med. 2011, 27, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Kritchevsky, S.B.; Newman, A.B.; Taaffe, D.R.; Nicklas, B.J.; Visser, M.; Lee, J.S.; Nevitt, M.; Tylavsky, F.A.; Rubin, S.M.; et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am. J. Clin. Nutr. 2007, 85, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.Y.; Ruts, E.; Kim, J.; Janumala, I.; Heymsfield, S.; Gallagher, D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am. J. Clin. Nutr. 2004, 79, 874–880. [Google Scholar] [CrossRef] [Green Version]
- Hughes, V.A.; Roubenoff, R.; Wood, M.; Frontera, W.R.; Evans, W.J.; Fiatarone Singh, M.A. Anthropometric assessment of 10-y changes in body composition in the elderly. Am. J. Clin. Nutr. 2004, 80, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Karalaki, M.; Fili, S.; Philippou, A.; Koutsilieris, M. Muscle regeneration: Cellular and molecular events. In Vivo 2009, 23, 779–796. [Google Scholar] [PubMed]
- Ceafalan, L.C.; Popescu, B.O.; Hinescu, M.E. Cellular players in skeletal muscle regeneration. BioMed Res. Int. 2014, 2014, 957014. [Google Scholar] [CrossRef]
- Grefte, S.; Kuijpers-Jagtman, A.M.; Torensma, R.; Von den Hoff, J.W. Skeletal muscle development and regeneration. Stem Cells Dev. 2007, 16, 857–868. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, T.W.; Oke, S.M.; Patel, H.; Smith, T.R. Getting to grips with sarcopenia: Recent advances and practical management for the gastroenterologist. Frontline Gastroenterol. 2021, 12, 53–61. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Riuzzi, F.; Sorci, G.; Arcuri, C.; Giambanco, I.; Bellezza, I.; Minelli, A.; Donato, R. Cellular and molecular mechanisms of sarcopenia: The S100B perspective. J. Cachexia Sarcopenia Muscle 2018, 9, 1255–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgic, S.N.; Domaniku, A.; Toledo, B.; Agca, S.; Weber, B.Z.C.; Arabaci, D.H.; Ozornek, Z.; Lause, P.; Thissen, J.P.; Loumaye, A.; et al. EDA2R-NIK signalling promotes muscle atrophy linked to cancer cachexia. Nature 2023, 617, 827–834. [Google Scholar] [CrossRef]
- Tintignac, L.A.; Lagirand, J.; Batonnet, S.; Sirri, V.; Leibovitch, M.P.; Leibovitch, S.A. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J. Biol. Chem. 2005, 280, 2847–2856. [Google Scholar] [CrossRef] [Green Version]
- Morel, S.; Hugon, G.; Vitou, M.; Védère, M.; Fons, F.; Rapior, S.; Saint, N.; Carnac, G. A Bioassay-Guided Fractionation of Rosemary Leaf Extract Identifies Carnosol as a Major Hypertrophy Inducer in Human Skeletal Muscle Cells. Nutrients 2021, 13, 4190. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auyeung, T.W.; Lee, J.S.; Leung, J.; Kwok, T.; Woo, J. Adiposity to muscle ratio predicts incident physical limitation in a cohort of 3,153 older adults--an alternative measurement of sarcopenia and sarcopenic obesity. Age 2013, 35, 1377–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Choi, S.H.; Lim, S.; Yoon, J.W.; Kang, S.M.; Kim, K.W.; Lim, J.Y.; Cho, N.H.; Jang, H.C. Sarcopenia and obesity: Gender-different relationship with functional limitation in older persons. J. Korean Med. Sci. 2013, 28, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, K.; May, C.R.; Patel, H.P.; Baxter, M.; Sayer, A.A.; Roberts, H.C. Implementation of grip strength measurement in medicine for older people wards as part of routine admission assessment: Identifying facilitators and barriers using a theory-led intervention. BMC Geriatr. 2018, 18, 79. [Google Scholar] [CrossRef] [Green Version]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Masanés, F.; Rojano, I.L.X.; Salvà, A.; Serra-Rexach, J.A.; Artaza, I.; Formiga, F.; Cuesta, F.; López Soto, A.; Ruiz, D.; Cruz-Jentoft, A.J. Cut-off Points for Muscle Mass—Not Grip Strength or Gait Speed—Determine Variations in Sarcopenia Prevalence. J. Nutr. Health Aging 2017, 21, 825–829. [Google Scholar] [CrossRef]
- Treviño-Aguirre, E.; López-Teros, T.; Gutiérrez-Robledo, L.; Vandewoude, M.; Pérez-Zepeda, M. Availability and use of dual energy X-ray absorptiometry (DXA) and bio-impedance analysis (BIA) for the evaluation of sarcopenia by Belgian and Latin American geriatricians. J. Cachexia Sarcopenia Muscle 2014, 5, 79–81. [Google Scholar] [CrossRef]
- Bahat, G.; Yilmaz, O.; Kılıç, C.; Oren, M.M.; Karan, M.A. Performance of SARC-F in Regard to Sarcopenia Definitions, Muscle Mass and Functional Measures. J. Nutr. Health Aging 2018, 22, 898–903. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Takeuchi, T.; Goto, M.; Ogura, T.; Nakamura, S.; Kakimoto, K.; Miyazaki, T.; Nishiguchi, S.; et al. Screening Tools for Sarcopenia. In Vivo 2021, 35, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Gryglewska, B.; Gąsowski, J. The usefulness of SARC-F. Aging Clin. Exp. Res. 2021, 33, 2307. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Tanaka, T.; Shibasaki, K.; Ouchi, Y.; Kikutani, T.; Higashiguchi, T.; Obuchi, S.P.; Ishikawa-Takata, K.; Hirano, H.; Kawai, H.; et al. Development of a simple screening test for sarcopenia in older adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 93–101. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Fielding, R.; Visser, M.; van Loon, L.J.; Rolland, Y.; Orwoll, E.; Reid, K.; Boonen, S.; Dere, W.; Epstein, S.; et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013, 93, 201–210. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Peters, K.W.; Shardell, M.D.; McLean, R.R.; Dam, T.T.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Janssen, I.; Heymsfield, S.B.; Ross, R. Relation between whole-body and regional measures of human skeletal muscle. Am. J. Clin. Nutr. 2004, 80, 1215–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baracos, V.E.; Reiman, T.; Mourtzakis, M.; Gioulbasanis, I.; Antoun, S. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 2010, 91, 1133s–1137s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef]
- Landi, F.; Onder, G.; Russo, A.; Liperoti, R.; Tosato, M.; Martone, A.M.; Capoluongo, E.; Bernabei, R. Calf circumference, frailty and physical performance among older adults living in the community. Clin. Nutr. 2014, 33, 539–544. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Peel, N.M.; Kuys, S.S.; Klein, K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 39–46. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Maggio, M.; Ceda, G.P.; Ticinesi, A.; De Vita, F.; Gelmini, G.; Costantino, C.; Meschi, T.; Kressig, R.W.; Cesari, M.; Fabi, M.; et al. Instrumental and Non-Instrumental Evaluation of 4-Meter Walking Speed in Older Individuals. PLoS ONE 2016, 11, e0153583. [Google Scholar] [CrossRef] [Green Version]
- Rydwik, E.; Bergland, A.; Forsén, L.; Frändin, K. Investigation into the reliability and validity of the measurement of elderly people’s clinical walking speed: A systematic review. Physiother. Theory Pract. 2012, 28, 238–256. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Fielding, R.A.; Pahor, M.; Goodpaster, B.; Hellerstein, M.; van Kan, G.A.; Anker, S.D.; Rutkove, S.; Vrijbloed, J.W.; Isaac, M.; et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Chen, L.K.; Lee, W.J.; Peng, L.N.; Liu, L.K.; Arai, H.; Akishita, M. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2016, 17, 767.e1–767.e7. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef] [Green Version]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Doherty, T.J.; Brown, W.F. Age-related changes in the twitch contractile properties of human thenar motor units. J. Appl. Physiol. 1997, 82, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, B.E.; Irving, D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J. Neurol. Sci. 1977, 34, 213–219. [Google Scholar] [CrossRef]
- Yarasheski, K.E. Exercise, aging, and muscle protein metabolism. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M918–M922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, S.M.; Ferrell, R.F.; Hurley, B.F. Strength training for the prevention and treatment of sarcopenia. J. Nutr. Health Aging 2000, 4, 143–155. [Google Scholar] [PubMed]
- Supriya, R.; Singh, K.P.; Gao, Y.; Gu, Y.; Baker, J.S. Effect of Exercise on Secondary Sarcopenia: A Comprehensive Literature Review. Biology 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [CrossRef] [Green Version]
- McKendry, J.; Currier, B.S.; Lim, C.; McLeod, J.C.; Thomas, A.C.Q.; Phillips, S.M. Nutritional Supplements to Support Resistance Exercise in Countering the Sarcopenia of Aging. Nutrients 2020, 12, 2057. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Sarcopenia and age-related endocrine function. Int. J. Endocrinol. 2012, 2012, 127362. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, H.; Sakuma, K. Comprehensive approach to sarcopenia treatment. Curr. Clin. Pharmacol. 2014, 9, 171–180. [Google Scholar] [CrossRef]
- Christiansen, A.R.; Lipshultz, L.I.; Hotaling, J.M.; Pastuszak, A.W. Selective androgen receptor modulators: The future of androgen therapy? Transl. Androl. Urol. 2020, 9, S135–S148. [Google Scholar] [CrossRef]
- GTx-024 as a Treatment for Stress Urinary Incontinence in Women. 2018. Available online: https://ClinicalTrials.gov/show/NCT02658448 (accessed on 31 May 2023).
- Study to Assess Enobosarm (GTx-024) in Postmenopausal Women With Stress Urinary Incontinence. 2018. Available online: https://ClinicalTrials.gov/show/NCT03241342 (accessed on 31 May 2023).
- Durability Extension Study to Assess Clinical Activity and Safety of Enobosarm (GTx-024) in Stress Urinary Incontinence. 2018. Available online: https://ClinicalTrials.gov/show/NCT03508648 (accessed on 31 May 2023).
- Study of GTx-024 on Muscle Wasting (Cachexia) Cancer. 2008. Available online: https://ClinicalTrials.gov/show/NCT00467844 (accessed on 31 May 2023).
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.A.; Steiner, M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Add-on Study for Protocol G200802 (NCT02463032): Effect of GTx-024 on Maximal Neuromuscular Function and Lean Body Mass. 2018. Available online: https://ClinicalTrials.gov/show/NCT02746328 (accessed on 31 May 2023).
- Effect of GTx-024 on Muscle Wasting in Patients With Non-Small Cell Lung Cancer (NSCLC) on First Line Platinum. 2013. Available online: https://ClinicalTrials.gov/show/NCT01355497 (accessed on 31 May 2023).
- Crawford, J.; Prado, C.M.; Johnston, M.A.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef] [Green Version]
- Phase III Study of the Effect of GTx-024 on Muscle Wasting in Patients With Non-Small Cell Lung Cancer (NSCLC). 2013. Available online: https://ClinicalTrials.gov/show/NCT01355484 (accessed on 31 May 2023).
- Enobosarm and Anastrozole in Pre-Menopausal Women with High Mammographic Breast Density. 2018. Available online: https://ClinicalTrials.gov/show/NCT03264651 (accessed on 31 May 2023).
- Study to Evaluate the Safety and Efficacy of 13 Weeks of the Selective Androgen Receptor Modulator (SARM) GSK2881078 in Chronic Obstructive Pulmonary Disease (COPD). 2019. Available online: https://ClinicalTrials.gov/show/NCT03359473 (accessed on 31 May 2023).
- Mohan, D.; Rossiter, H.; Watz, H.; Fogarty, C.; Evans, R.A.; Man, W.; Tabberer, M.; Beerahee, M.; Kumar, S.; Millns, H.; et al. Selective androgen receptor modulation for muscle weakness in chronic obstructive pulmonary disease: A randomised control trial. Thorax 2023, 78, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.J.; Bae, G.U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharmacal Res. 2021, 44, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Lee, S.J. Myostatin: A Skeletal Muscle Chalone. Annu. Rev. Physiol. 2023, 85, 269–291. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef]
- Dao, T.; Green, A.E.; Kim, Y.A.; Bae, S.J.; Ha, K.T.; Gariani, K.; Lee, M.R.; Menzies, K.J.; Ryu, D. Sarcopenia and Muscle Aging: A Brief Overview. Endocrinol. Metab. 2020, 35, 716–732. [Google Scholar] [CrossRef] [PubMed]
- A Phase 2 Study to Evaluate the Safety, Efficacy, Pharmacokinetics and Pharmacodynamics of PF-06252616 in Duchenne Muscular Dystrophy. 2018. Available online: https://ClinicalTrials.gov/show/NCT02310763 (accessed on 31 May 2023).
- Wagner, K.R.; Abdel-Hamid, H.Z.; Mah, J.K.; Campbell, C.; Guglieri, M.; Muntoni, F.; Takeshima, Y.; McDonald, C.M.; Kostera-Pruszczyk, A.; Karachunski, P.; et al. Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy. Neuromuscul. Disord. 2020, 30, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, S.P.; Zhang, Y.; Binks, M.; Marraffino, S. Quantitative muscle MRI biomarkers in Duchenne muscular dystrophy: Cross-sectional correlations with age and functional tests. Biomark. Med. 2021, 15, 761–773. [Google Scholar] [CrossRef]
- Wagner, K.R.; Guglieri, M.; Ramaiah, S.K.; Charnas, L.; Marraffino, S.; Binks, M.; Vaidya, V.S.; Palmer, J.; Goldstein, R.; Muntoni, F. Safety and disease monitoring biomarkers in Duchenne muscular dystrophy: Results from a Phase II trial. Biomark. Med. 2021, 15, 1389–1396. [Google Scholar] [CrossRef]
- Sherlock, S.P.; Palmer, J.; Wagner, K.R.; Abdel-Hamid, H.Z.; Bertini, E.; Tian, C.; Mah, J.K.; Kostera-Pruszczyk, A.; Muntoni, F.; Guglieri, M.; et al. Quantitative magnetic resonance imaging measures as biomarkers of disease progression in boys with Duchenne muscular dystrophy: A phase 2 trial of domagrozumab. J. Neurol. 2022, 269, 4421–4435. [Google Scholar] [CrossRef]
- Muntoni, F.; Guglieri, M.; Mah, J.K.; Wagner, K.R.; Brandsema, J.F.; Butterfield, R.J.; McDonald, C.M.; Mayhew, A.G.; Palmer, J.P.; Marraffino, S.; et al. Novel approaches to analysis of the North Star Ambulatory Assessment (NSAA) in Duchenne muscular dystrophy (DMD): Observations from a phase 2 trial. PLoS ONE 2022, 17, e0272858. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, J.; Purohit, V.S.; Harnisch, L.O.; Dua, P.; Tan, B.; Nicholas, T. Population PK and PD Analysis of Domagrozumab in Pediatric Patients with Duchenne Muscular Dystrophy. Clin. Pharmacol. Ther. 2022, 112, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, S.P.; Palmer, J.; Wagner, K.R.; Abdel-Hamid, H.Z.; Tian, C.; Mah, J.K.; Muntoni, F.; Guglieri, M.; Butterfield, R.J.; Charnas, L.; et al. Dual-energy X-ray absorptiometry measures of lean body mass as a biomarker for progression in boys with Duchenne muscular dystrophy. Sci. Rep. 2022, 12, 18762. [Google Scholar] [CrossRef]
- An Open-Label Extension Study to Evaluate Safety of PF-06252616 in Boys with Duchenne Muscular Dystrophy. 2018. Available online: https://ClinicalTrials.gov/show/NCT02907619 (accessed on 31 May 2023).
- Efficacy and Safety of Apitegromab in Patients with Later-Onset Spinal Muscular Atrophy Treated with Nusinersen or Risdiplam. 2024. Available online: https://ClinicalTrials.gov/show/NCT05156320 (accessed on 31 May 2023).
- Study Evaluating MYO-029 in Adult Muscular Dystrophy. Available online: https://ClinicalTrials.gov/show/NCT00104078 (accessed on 31 May 2023).
- An Active Treatment Study of SRK-015 in Patients with Type 2 or Type 3 Spinal Muscular Atrophy. 2021. Available online: https://ClinicalTrials.gov/show/NCT03921528 (accessed on 31 May 2023).
- Long-Term Safety & Efficacy of Apitegromab in Patients with SMA Who Completed Previous Trials of Apitegromab-ONYX. 2026. Available online: https://ClinicalTrials.gov/show/NCT05626855 (accessed on 31 May 2023).
- Study of Efficacy and Safety of Bimagrumab in Patients After Hip Fracture Surgery. 2018. Available online: https://ClinicalTrials.gov/show/NCT02152761 (accessed on 31 May 2023).
- Hofbauer, L.C.; Witvrouw, R.; Varga, Z.; Shiota, N.; Cremer, M.; Tanko, L.B.; Rooks, D.; Auberson, L.Z.; Arkuszewski, M.; Fretault, N.; et al. Bimagrumab to improve recovery after hip fracture in older adults: A multicentre, double-blind, randomised, parallel-group, placebo-controlled, phase 2a/b trial. Lancet Healthy Longev. 2021, 2, e263–e274. [Google Scholar] [CrossRef] [PubMed]
- Safety, Pharmacokinetics and Efficacy of Bimagrumab in Overweight and Obese Patients with Type 2 Diabetes. 2019. Available online: https://ClinicalTrials.gov/show/NCT03005288 (accessed on 31 May 2023).
- Heymsfield, S.B.; Coleman, L.A.; Miller, R.; Rooks, D.S.; Laurent, D.; Petricoul, O.; Praestgaard, J.; Swan, T.; Wade, T.; Perry, R.G.; et al. Effect of Bimagrumab vs Placebo on Body Fat Mass Among Adults With Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2033457. [Google Scholar] [CrossRef]
- An Extension Study of the Efficacy, Safety and Tolerability of BYM338 (Bimagrumab) in Patients with Sporadic Inclusion Body Myositis Who Previously Participated in the Core Study CBYM338B2203. 2016. Available online: https://ClinicalTrials.gov/show/NCT02573467 (accessed on 31 May 2023).
- Amato, A.A.; Hanna, M.G.; Machado, P.M.; Badrising, U.A.; Chinoy, H.; Benveniste, O.; Karanam, A.K.; Wu, M.; Tankó, L.B.; Schubert-Tennigkeit, A.A.; et al. Efficacy and Safety of Bimagrumab in Sporadic Inclusion Body Myositis: Long-term Extension of RESILIENT. Neurology 2021, 96, e1595–e1607. [Google Scholar] [CrossRef]
- Dose Range Finding Study of Bimagrumab in Sarcopenia. 2018. Available online: https://ClinicalTrials.gov/show/NCT02333331 (accessed on 31 May 2023).
- Rooks, D.; Swan, T.; Goswami, B.; Filosa, L.A.; Bunte, O.; Panchaud, N.; Coleman, L.A.; Miller, R.R.; Garcia Garayoa, E.; Praestgaard, J.; et al. Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2020836. [Google Scholar] [CrossRef]
- Study of Long-Term Safety, Efficacy Tolerability of BYM338 in Patients with Sporadic Inclusion Body Myositis. 2016. Available online: https://ClinicalTrials.gov/show/NCT02250443 (accessed on 31 May 2023).
- Sivakumar, K.; Cochrane, T.I.; Sloth, B.; Ashar, H.; Laurent, D.; Tankó, L.B.; Amato, A.A. Long-term safety and tolerability of bimagrumab (BYM338) in sporadic inclusion body myositis. Neurology 2020, 95, e1971–e1978. [Google Scholar] [CrossRef]
- Efficacy and Safety of Bimagrumab/BYM338 at 52 Weeks on Physical Function, Muscle Strength, Mobility in sIBM Patients. 2016. Available online: https://ClinicalTrials.gov/show/NCT01925209 (accessed on 31 May 2023).
- Hanna, M.G.; Badrising, U.A.; Benveniste, O.; Lloyd, T.E.; Needham, M.; Chinoy, H.; Aoki, M.; Machado, P.M.; Liang, C.; Reardon, K.A.; et al. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): A randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019, 18, 834–844. [Google Scholar] [CrossRef]
- A Multi-Center Study to Assess the Effects of BYM338 on Skeletal Muscle in Sarcopenic Adults. 2013. Available online: https://ClinicalTrials.gov/show/NCT01601600 (accessed on 31 May 2023).
- Efficacy, Safety and Tolerability of BYM338 in Patients with Sporadic Inclusion Body Myositis. 2012. Available online: https://ClinicalTrials.gov/show/NCT01423110 (accessed on 31 May 2023).
- Amato, A.A.; Sivakumar, K.; Goyal, N.; David, W.S.; Salajegheh, M.; Praestgaard, J.; Lach-Trifilieff, E.; Trendelenburg, A.U.; Laurent, D.; Glass, D.J.; et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology 2014, 83, 2239–2246. [Google Scholar] [CrossRef] [Green Version]
- A 24-Week Off-Drug Extension Study in Sarcopenic Elderly Who Completed Treatment in the 6-Month Core Study. 2018. Available online: https://ClinicalTrials.gov/show/NCT02468674 (accessed on 31 May 2023).
- BYM338 in Chronic Obstructive Pulmonary Disease (COPD) Patients with Cachexia. 2014. Available online: https://ClinicalTrials.gov/show/NCT01669174 (accessed on 31 May 2023).
- Polkey, M.I.; Praestgaard, J.; Berwick, A.; Franssen, F.M.E.; Singh, D.; Steiner, M.C.; Casaburi, R.; Tillmann, H.C.; Lach-Trifilieff, E.; Roubenoff, R.; et al. Activin Type II Receptor Blockade for Treatment of Muscle Depletion in Chronic Obstructive Pulmonary Disease. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Clinical Study of BYM338 for the Treatment of Unintentional Weight Loss in Patients with Cancer of the Lung or the Pancreas. 2014. Available online: https://ClinicalTrials.gov/show/NCT01433263 (accessed on 31 May 2023).
- A Study of LY2495655 in Older Participants Undergoing Elective Total Hip Replacement. 2014. Available online: https://ClinicalTrials.gov/show/NCT01369511 (accessed on 31 May 2023).
- Woodhouse, L.; Gandhi, R.; Warden, S.J.; Poiraudeau, S.; Myers, S.L.; Benson, C.T.; Hu, L.; Ahmad, Q.I.; Linnemeier, P.; Gomez, E.V.; et al. A Phase 2 Randomized Study Investigating the Efficacy and Safety of Myostatin Antibody LY2495655 versus Placebo in Patients Undergoing Elective Total Hip Arthroplasty. J. Frailty Aging 2016, 5, 62–70. [Google Scholar] [CrossRef] [PubMed]
- A Study in Older Participants Who Have Fallen and Have Muscle Weakness. 2013. Available online: https://ClinicalTrials.gov/show/NCT01604408 (accessed on 31 May 2023).
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Study of the Safety and Efficacy of REGN1033 (SAR391786) in Patients with Sarcopenia. 2015. Available online: https://ClinicalTrials.gov/show/NCT01963598 (accessed on 31 May 2023).
- A Study to Evaluate the Efficacy and Safety of Taldefgrobep Alfa in Participants with Spinal Muscular Atrophy. 2025. Available online: https://ClinicalTrials.gov/show/NCT05337553 (accessed on 31 May 2023).
- Jerry, R.M.; Duchenne, A.; Milo, T.; Nationwide Children’s, H. Clinical Intramuscular Gene Transfer of rAAV1.CMV.huFollistatin344 Trial to Patients with Duchenne Muscular Dystrophy. 2017. Available online: https://ClinicalTrials.gov/show/NCT02354781 (accessed on 31 May 2023).
- Follistatin Gene Transfer to Patients with Becker Muscular Dystrophy and Sporadic Inclusion Body Myositis. 2017. Available online: https://ClinicalTrials.gov/show/NCT01519349 (accessed on 31 May 2023).
- Mendell, J.R.; Sahenk, Z.; Malik, V.; Gomez, A.M.; Flanigan, K.M.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Meadows, E.; Lewis, S.; et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol. Ther. 2015, 23, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [Green Version]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Cereda, E.; Pisati, R.; Rondanelli, M.; Caccialanza, R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef]
- Rondanelli, M.; Cereda, E.; Klersy, C.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Gasparri, C.; Iannello, G.; Spadaccini, D.; Infantino, V.; et al. Improving rehabilitation in sarcopenia: A randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Waterhouse, M.; Sanguineti, E.; Baxter, C.; Duarte Romero, B.; McLeod, D.S.A.; English, D.R.; Armstrong, B.K.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; et al. Vitamin D supplementation and risk of falling: Outcomes from the randomized, placebo-controlled D-Health Trial. J. Cachexia Sarcopenia Muscle 2021, 12, 1428–1439. [Google Scholar] [CrossRef]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Mellen, R.H.; Girotto, O.S.; Marques, E.B.; Laurindo, L.F.; Grippa, P.C.; Mendes, C.G.; Garcia, L.N.H.; Bechara, M.D.; Barbalho, S.M.; Sinatora, R.V.; et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines 2023, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Hersch, E.C.; Merriam, G.R. Growth hormone (GH)-releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus? Clin. Interv. Aging 2008, 3, 121–129. [Google Scholar] [PubMed]
- Dingemans, A.M.; de Vos-Geelen, J.; Langen, R.; Schols, A.M. Phase II drugs that are currently in development for the treatment of cachexia. Expert Opin. Investig. Drugs 2014, 23, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Stewart Coats, A.J.; Srinivasan, V.; Surendran, J.; Chiramana, H.; Vangipuram, S.R.; Bhatt, N.N.; Jain, M.; Shah, S.; Ali, I.A.; Fuang, H.G.; et al. The ACT-ONE trial, a multicentre, randomised, double-blind, placebo-controlled, dose-finding study of the anabolic/catabolic transforming agent, MT-102 in subjects with cachexia related to stage III and IV non-small cell lung cancer and colorectal cancer: Study design. J. Cachexia Sarcopenia Muscle 2011, 2, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Pötsch, M.S.; Tschirner, A.; Palus, S.; von Haehling, S.; Doehner, W.; Beadle, J.; Coats, A.J.; Anker, S.D.; Springer, J. The anabolic catabolic transforming agent (ACTA) espindolol increases muscle mass and decreases fat mass in old rats. J. Cachexia Sarcopenia Muscle 2014, 5, 149–158. [Google Scholar] [CrossRef]
- Pötsch, M.S.; Ishida, J.; Palus, S.; Tschirner, A.; von Haehling, S.; Doehner, W.; Anker, S.D.; Springer, J. MT-102 prevents tissue wasting and improves survival in a rat model of severe cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 594–605. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Mosti, M.P.; Stunes, A.K.; Ericsson, M.; Pullisaar, H.; Reseland, J.E.; Shabestari, M.; Eriksen, E.F.; Syversen, U. Effects of the peroxisome proliferator-activated receptor (PPAR)-δ agonist GW501516 on bone and muscle in ovariectomized rats. Endocrinology 2014, 155, 2178–2189. [Google Scholar] [CrossRef] [Green Version]
- Sahebkar, A.; Chew, G.T.; Watts, G.F. New peroxisome proliferator-activated receptor agonists: Potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin. Pharmacother. 2014, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Kadayat, T.M.; Shrestha, A.; Jeon, Y.H.; An, H.; Kim, J.; Cho, S.J.; Chin, J. Targeting Peroxisome Proliferator-Activated Receptor Delta (PPARδ): A Medicinal Chemistry Perspective. J. Med. Chem. 2020, 63, 10109–10134. [Google Scholar] [CrossRef] [PubMed]
- Višnjić, D.; Lalić, H.; Dembitz, V.; Tomić, B.; Smoljo, T. AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, D.; Moon, H.Y.; van Praag, H. Exercise in a Pill: The Latest on Exercise-Mimetics. Brain Plast. 2017, 2, 153–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.J.; Byun, S. Molecular targets of exercise mimetics and their natural activators. BMB Rep. 2021, 54, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Cento, A.S.; Leigheb, M.; Caretti, G.; Penna, F. Exercise and Exercise Mimetics for the Treatment of Musculoskeletal Disorders. Curr. Osteoporos. Rep. 2022, 20, 249–259. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Peroni, G.; Guerriero, F.; Morazzoni, P.; Riva, A.; Guido, D.; Perna, S. A Systematic Review on the Effects of Botanicals on Skeletal Muscle Health in Order to Prevent Sarcopenia. Evid. Based Complement. Altern. Med. 2016, 2016, 5970367. [Google Scholar] [CrossRef] [Green Version]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic Acid, a Rosemary Extract Polyphenol, Increases Skeletal Muscle Cell Glucose Uptake and Activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef] [Green Version]
- Kuang, J.X.; Shen, Q.; Zhang, R.Q.; Fang, Q.Y.; Deng, X.; Fan, M.; Cheng, C.R.; Zhang, X.W.; Liu, X. Carnosol attenuated atrophy of C2C12 myotubes induced by tumour-derived exosomal miR-183-5p through inhibiting Smad3 pathway activation and keeping mitochondrial respiration. Basic Clin. Pharmacol. Toxicol. 2022, 131, 500–513. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; MacPherson, R.E.K.; Tsiani, E. Carnosic Acid Attenuates the Free Fatty Acid-Induced Insulin Resistance in Muscle Cells and Adipocytes. Cells 2022, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Kojima-Yuasa, A.; Tadano, H.; Mizuno, A.; Kon, A.; Norikura, T. Ursolic acid improves the indoxyl sulfate-induced impairment of mitochondrial biogenesis in C2C12 cells. Nutr. Res. Pract. 2022, 16, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.H.; Yoo, M.A.; Kim, C.M.; Chung, H.Y.; et al. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, K.K.; Chung, K.W.; Sung, B.; Kim, M.J.; Park, C.H.; Yoon, C.; Choi, J.S.; Kim, M.K.; Kim, C.M.; Kim, N.D.; et al. Loquat (Eriobotrya japonica) extract prevents dexamethasone-induced muscle atrophy by inhibiting the muscle degradation pathway in Sprague Dawley rats. Mol. Med. Rep. 2015, 12, 3607–3614. [Google Scholar] [CrossRef] [Green Version]
- Sung, B.; Hwang, S.Y.; Kim, M.J.; Kim, M.; Jeong, J.W.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Loquat leaf extract enhances myogenic differentiation, improves muscle function and attenuates muscle loss in aged rats. Int. J. Mol. Med. 2015, 36, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.H.; Lee, S.Y.; Kim, C.M.; Kim, N.D.; Choe, S.; Lee, C.H.; Shin, J.H. Effect of Loquat Leaf Extract on Muscle Strength, Muscle Mass, and Muscle Function in Healthy Adults: A Randomized, Double-Blinded, and Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2016, 2016, 4301621. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Han, S.; Park, H. Effect of Schisandra Chinensis Extract Supplementation on Quadriceps Muscle Strength and Fatigue in Adult Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 2475. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y.; Ku, S.K.; Lee, K.W.; Song, C.H.; An, W.G. Muscle-protective effects of Schisandrae Fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 2018, 212, 175–187. [Google Scholar] [CrossRef]
- Kim, J.W.; Ku, S.K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Shang, H.; Wu, W.; Du, J.; Putheti, R. Evaluation of anti-athletic fatigue activity of Schizandra chinensis aqueous extracts in mice. Afr. J. Pharm. Pharmacol. 2009, 3, 593–597. [Google Scholar] [CrossRef]
- Kim, J.W.; Ku, S.K.; Kim, K.Y.; Kim, S.G.; Han, M.H.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. Schisandrae Fructus Supplementation Ameliorates Sciatic Neurectomy-Induced Muscle Atrophy in Mice. Oxid. Med. Cell. Longev. 2015, 2015, 872428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeon, M.; Choi, H.; Jun, H.S. Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy. Nutrients 2020, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.; Ahn, J.; Kim, M.J.; Seo, H.D.; Hahm, J.H.; Jung, C.H.; Ha, T.Y. Fruit of Schisandra chinensis and its bioactive component schizandrin B ameliorate obesity-induced skeletal muscle atrophy. Food Res. Int. 2022, 157, 111439. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Sarcopenia: Diagnosis and treatment. J. Nutr. Health Aging 2008, 12, 452–456. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 31 May 2023).
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kwon, K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Semenova, E.A.; Pranckevičienė, E.; Bondareva, E.A.; Gabdrakhmanova, L.J.; Ahmetov, I.I. Identification and Characterization of Genomic Predictors of Sarcopenia and Sarcopenic Obesity Using UK Biobank Data. Nutrients 2023, 15, 758. [Google Scholar] [CrossRef]
- Ye, Y.; Noche, R.B.; Szejko, N.; Both, C.P.; Acosta, J.N.; Leasure, A.C.; Brown, S.C.; Sheth, K.N.; Gill, T.M.; Zhao, H.; et al. A genome-wide association study of frailty identifies significant genetic correlation with neuropsychiatric, cardiovascular, and inflammation pathways. Geroscience, 2023; online ahead of print. [Google Scholar] [CrossRef]
| Title | Intervention | Conditions | Primary Outcome | Phase | Status | Clinical Trials Identifier | Refs. |
|---|---|---|---|---|---|---|---|
| GTx-024 as a treatment for stress urinary incontinence in women | GTx-024 | Stress urinary incontinence | The mean percent change in number of stress incontinence episodes/day as assessed by patient completion of the 3-day voiding diary | Phase 2 | Completed | NCT02658448 | [109] |
| Study to assess enobosarm (GTx-024) in postmenopausal women with stress urinary incontinence (ASTRID) | GTx-024, placebo | Stress urinary incontinence | Number of participants with a ≥50% reduction from baseline in the mean number of stress incontinence episodes per day at week 12 | Phase 2 | Completed | NCT03241342 | [110] |
| Durability extension study to assess clinical activity and safety of enobosarm (GTx-024) in stress urinary incontinence | GTx-024, matching placebo | Stress urinary incontinence | Durability of response, stress incontinence | Phase 2 | Terminated | NCT03508648 | [111] |
| Study of GTx-024 on muscle wasting (cachexia) cancer. | GTx-024, placebo | Cachexia | The efficacy of GTx-024 on total body lean mass | Phase 2 | Completed | NCT00467844 | [112,113] |
| Add-on study for protocol G200802 (NCT02463032): effect of GTx-024 on maximal neuromuscular function and lean body mass | GTx-024, 9 or 18 mg | ER+ and AR+ breast cancer | Maximal power production (assessed by inertial-load cycle ergometry) | Phase 2 | Completed | NCT02746328 | [114] |
| Effect of GTx-024 on muscle wasting in patients with NSCLC on first line platinum | GTx-024, placebo | Muscle wasting, NSCLC | Physical function (measure is the percentage of subjects at day 84 with stair climb power change), lean body mass (measure is the percentage of subjects at day 84 with lean body mass change) | Phase 3 | Completed | NCT01355497 | [115,116] |
| Phase III study on the effect of GTx-024 on muscle wasting in patients with NSCLC | GTx-024, placebo | Muscle wasting, NSCLC | Physical function (measure is the percentage of subjects at day 84 with stair climb power change), lean body mass (measure is the percentage of subjects at day 84 with lean body mass change) | Phase 3 | Completed | NCT01355484 | [116,117] |
| Enobosarm and anastrozole in pre-menopausal women with high mammographic breast density | Enobosarm (GTx-024) | Mammographic density | Mammographic breast density, breast tissue elasticity | Phase 1 | Completed | NCT03264651 | [118] |
| Study to evaluate the safety and efficacy of 13 weeks of the SARM GSK2881078 in chronic obstructive pulmonary disease (COPD) | GSK2881078, matching placebo | Cachexia | Change from baseline in SBP, DBP, heart rate and urinalysis parameter, number of participants with SAEs, percentage change from baseline in maximum leg press strength following 1 RM | Phase 2 | Completed | NCT03359473 | [119,120] |
| Title | Intervention | Conditions | Primary Outcome | Phase | Status | Clinical Trials Identifier | Refs. |
|---|---|---|---|---|---|---|---|
| A phase 2 study to evaluate the safety, efficacy, pharmacokinetics, and pharmacodynamics of PF-06252616 in Duchenne muscular dystrophy | PF-06252616, placebo | Duchenne muscular dystrophy | Number of participants with TEAEs by week 49, change from baseline on the 4SC as compared to placebo at weeks 17, 33, and 49 | Phase 2 | Terminated | NCT02310763 | [126,127,128,129,130,131,132,133] |
| An open-label extension study to evaluate safety of PF-06252616 in boys with Duchenne muscular dystrophy | PF-06252616 | Duchenne muscular dystrophy | Number of participants with dose reduced or temporary discontinuation due to AEs, number of participants with severe TEAEs | Phase 2 | Terminated | NCT02907619 | [127,134] |
| Efficacy and safety of apitegromab in patients with later-onset spinal muscular atrophy treated with nusinersen or risdiplam (SAPPHIRE) | Apitegromab, placebo | SMA, muscular atrophy | Main efficacy population: change from baseline in HFMSE total score | Phase 3 | Recruiting | NCT05156320 | [135] |
| Study evaluating MYO-029 in adult muscular dystrophy | MYO-029 | Becker muscular dystrophy, facioscapulohumeral muscular dystrophy, limb–girdle muscular dystrophy | Safety assessment | Phase 1, Phase 2 | Completed | NCT00104078 | [136] |
| An active treatment study of SRK-015 in patients with type 2 or type 3 spinal muscular atrophy (TOPAZ) | SRK-015 | SMA | Change from baseline in the RHS total score at day 364, change from baseline in HFMSE total score at day 364 | Phase 2 | Active, not recruiting | NCT03921528 | [137] |
| Long-term safety and efficacy of apitegromab in patients with SMA who completed previous trials of apitegromab-ONYX (ONYX) | Apitegromab | SMA | Evaluate the long-term safety and tolerability of apitegromab in patients with type 2 and type 3 SMA | Phase 3 | Not yet recruiting | NCT05626855 | [138] |
| Study of efficacy and safety of bimagrumab in patients after hip fracture surgery | Bimagrumab | Muscle wasting (Atrophy) after hip fracture surgery | Change from baseline in total lean body mass measured by DXA at weeks 12 and 24 | Phase 2 | Completed | NCT02152761 | [139,140] |
| Safety, pharmacokinetics, and efficacy of bimagrumab in overweight and obese patients with type 2 diabetes | BYM338 10 mg/kg, placebo | Diabetes mellitus type 2 | Change from baseline in total body fat mass by DXA at week 48 | Phase 2 | Completed | NCT03005288 | [141,142] |
| An extension study of the efficacy, safety, and tolerability of BYM338 (Bimagrumab) in patients with sporadic inclusion body myositis who previously participated in the core study CBYM338B2203 | Bimagrumab, placebo | sIBM | Number of participants with AEs, SAEs, and deaths, change from core study baseline in 6MWD | Phase 3 | Completed | NCT02573467 | [143,144] |
| Dose range finding study of bimagrumab in sarcopenia | Bimagrumab, placebo | Sarcopenia | Change from baseline in Total SPPB score to week 25 | Phase 2 | Completed | NCT02333331 | [145,146] |
| Study of long-term safety, efficacy tolerability of BYM338 in patients with sIBM (BYM338) | BYM338 (Bimagrumab) | sIBM | Number of participants with AE a measure of safety and tolerability | Phase 2 Phase 3 | Completed | NCT02250443 | [147,148] |
| Efficacy and safety of bimagrumab/BYM338 at 52 weeks on physical function, muscle strength, mobility in sIBM patients (RESILIENT) | BYM338/ Bimagrumab, placebo | sIBM | Change from baseline in 6MWD test at week 52 | Phase 2 Phase 3 | Completed | NCT01925209 | [149,150] |
| A multi-center study to assess the effects of BYM338 on skeletal muscle in sarcopenic adults | BYM338, placebo | Skeletal muscle | Muscle volume of the thigh (measurement gathered using MRI, magnetic resonance imaging) | Phase 2 | Completed | NCT01601600 | [151] |
| Efficacy, safety, and tolerability of BYM338 in patients with sIBM | BYM338, placebo | sIBM | Effect of BYM338 on thigh muscle volume by MRI | Phase 2 | Completed | NCT01423110 | [152,153] |
| A 24-week off-drug extension study in sarcopenic elderly who completed treatment in the 6-month core study | Bimagrumab, placebo | Sarcopenia | SPPB total score at week 49 | Phase 2 | Completed | NCT02468674 | [154] |
| BYM338 in COPD patients with cachexia | BYM338, placebo | COPD with cachexia | Percentage change from baseline of TMV by MRI Scan at week 4, 8, 16, and 24 | Phase 2 | Completed | NCT01669174 | [155,156] |
| Clinical study of BYM338 for the treatment of unintentional weight loss in patients with cancer of the lung or the pancreas | BYM338 active drug, placebo | Cachexia | Percentage change from baseline of TMV by MRI Scan at week 8 | Phase 2 | Completed | NCT01433263 | [157] |
| A study of LY2495655 in older participants undergoing elective total hip replacement | LY2495655, placebo | Muscular atrophy | Change from baseline in aLBM at week 12 | Phase 2 | Completed | NCT01369511 | [158,159] |
| A study in older participants who have fallen and have muscle weakness | LY2495655, placebo | Muscle weakness | Change from baseline to 24 weeks endpoint in aLBM | Phase 2 | Completed | NCT01604408 | [160,161] |
| Study of the safety and efficacy of REGN1033 (SAR391786) in patients with sarcopenia | REGN1033 (SAR391786), placebo | Sarcopenia | Percent change in total lean body mass | Phase 2 | Completed | NCT01963598 | [162] |
| A study to evaluate the efficacy and safety of taldefgrobep Alfa in participants with spinal muscular atrophy (RESILIENT) | Taldefgrobep alfa, placebo | SMA, neuromuscular diseases | Efficacy of taldefgrobep alfa compared to placebo in change in the MFM-32 total score | Phase 3 | Recruiting | NCT05337553 | [163] |
| Clinical intramuscular gene transfer of rAAV1.CMV.huFollistatin344 trial to patients with Duchenne muscular dystrophy | rAAV1.CMV.huFollistin344 | Duchenne muscular dystrophy | Number of DLT adverse events as assessed by 21 CFR 312.32. | Phase 1 Phase 2 | Completed | NCT02354781 | [164] |
| Follistatin gene transfer to patients with Becker muscular dystrophy and sporadic inclusion body myositis | rAAV1.CMV.huFollistatin344 | Becker muscular dystrophy, sIBM | Safety | Phase 1 | Completed | NCT01519349 | [165,166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.Y.; Kim, D.; Kim, N.D. Pathogenesis, Intervention, and Current Status of Drug Development for Sarcopenia: A Review. Biomedicines 2023, 11, 1635. https://doi.org/10.3390/biomedicines11061635
Jang JY, Kim D, Kim ND. Pathogenesis, Intervention, and Current Status of Drug Development for Sarcopenia: A Review. Biomedicines. 2023; 11(6):1635. https://doi.org/10.3390/biomedicines11061635
Chicago/Turabian StyleJang, Jung Yoon, Donghwan Kim, and Nam Deuk Kim. 2023. "Pathogenesis, Intervention, and Current Status of Drug Development for Sarcopenia: A Review" Biomedicines 11, no. 6: 1635. https://doi.org/10.3390/biomedicines11061635
APA StyleJang, J. Y., Kim, D., & Kim, N. D. (2023). Pathogenesis, Intervention, and Current Status of Drug Development for Sarcopenia: A Review. Biomedicines, 11(6), 1635. https://doi.org/10.3390/biomedicines11061635







