Feasibility Study on Endoscopic Balloon-Assisted Laser Treatment (EBLT) of Gastroesophageal Reflux Disease (GERD) in In Vivo Porcine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laser System and Device
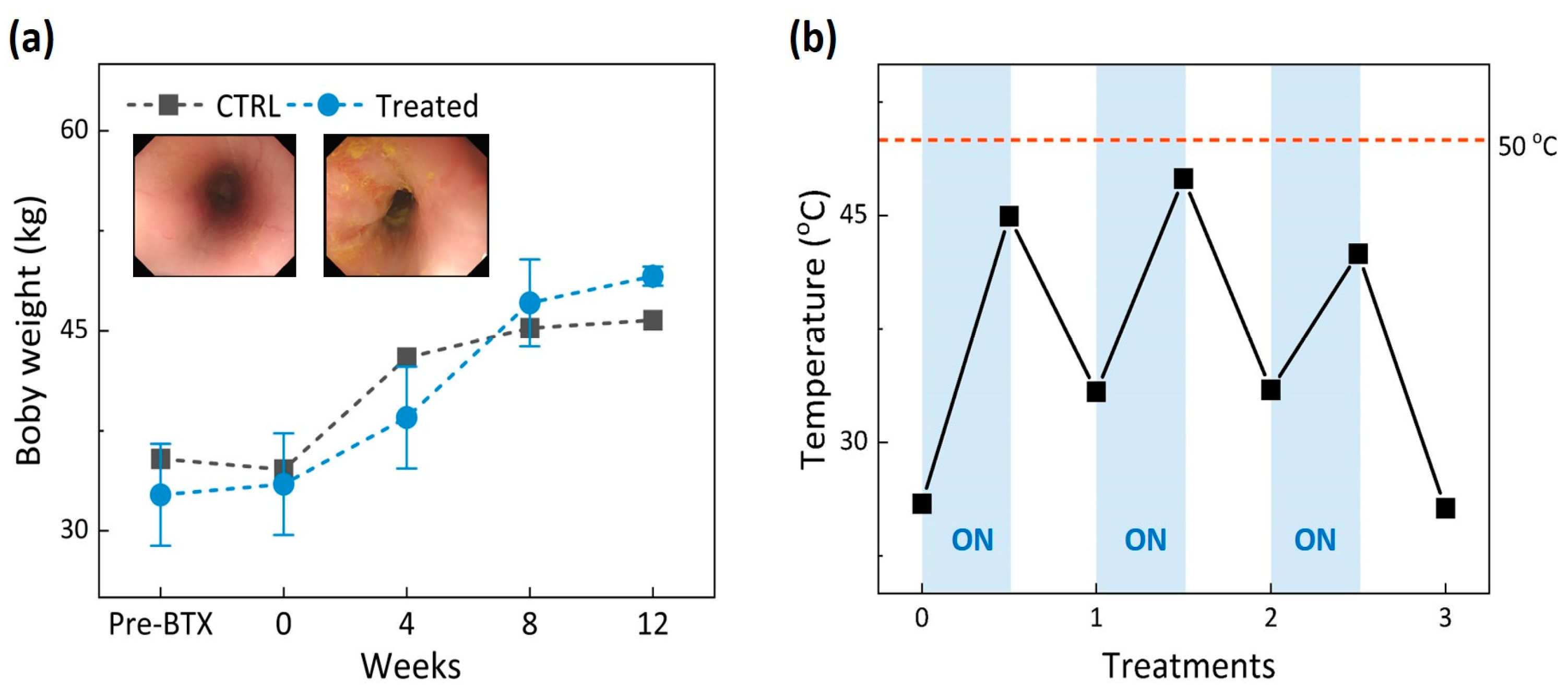
2.2. In Vivo Tests
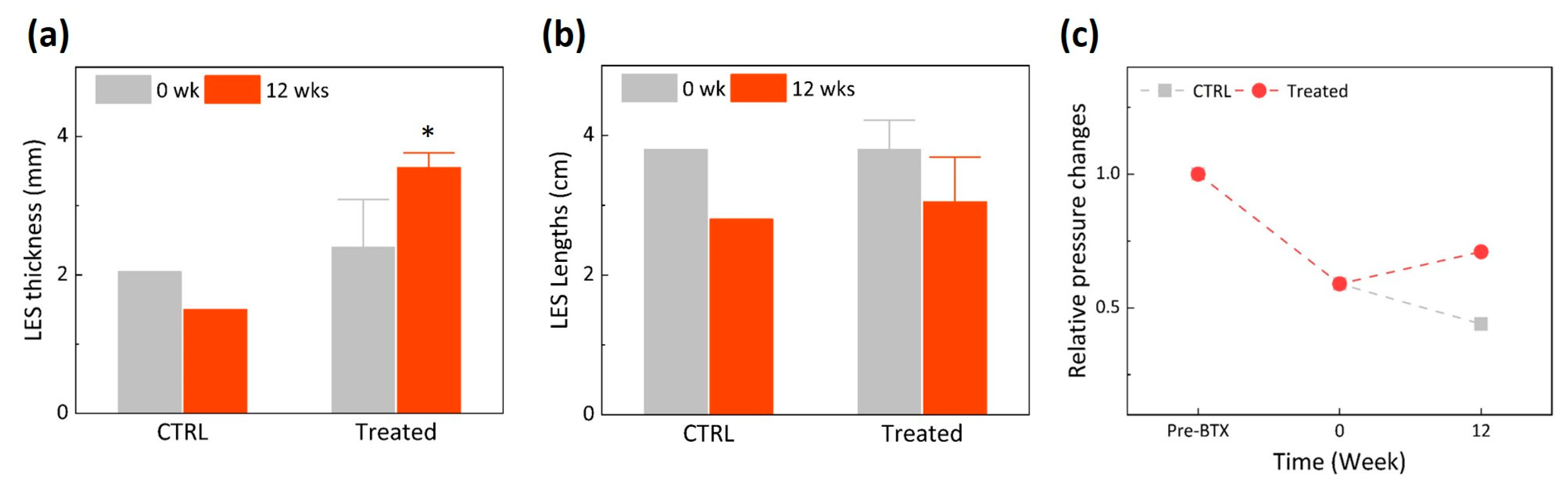
2.3. Endoscopic Ultrasound (EUS) Monitoring and Manometry
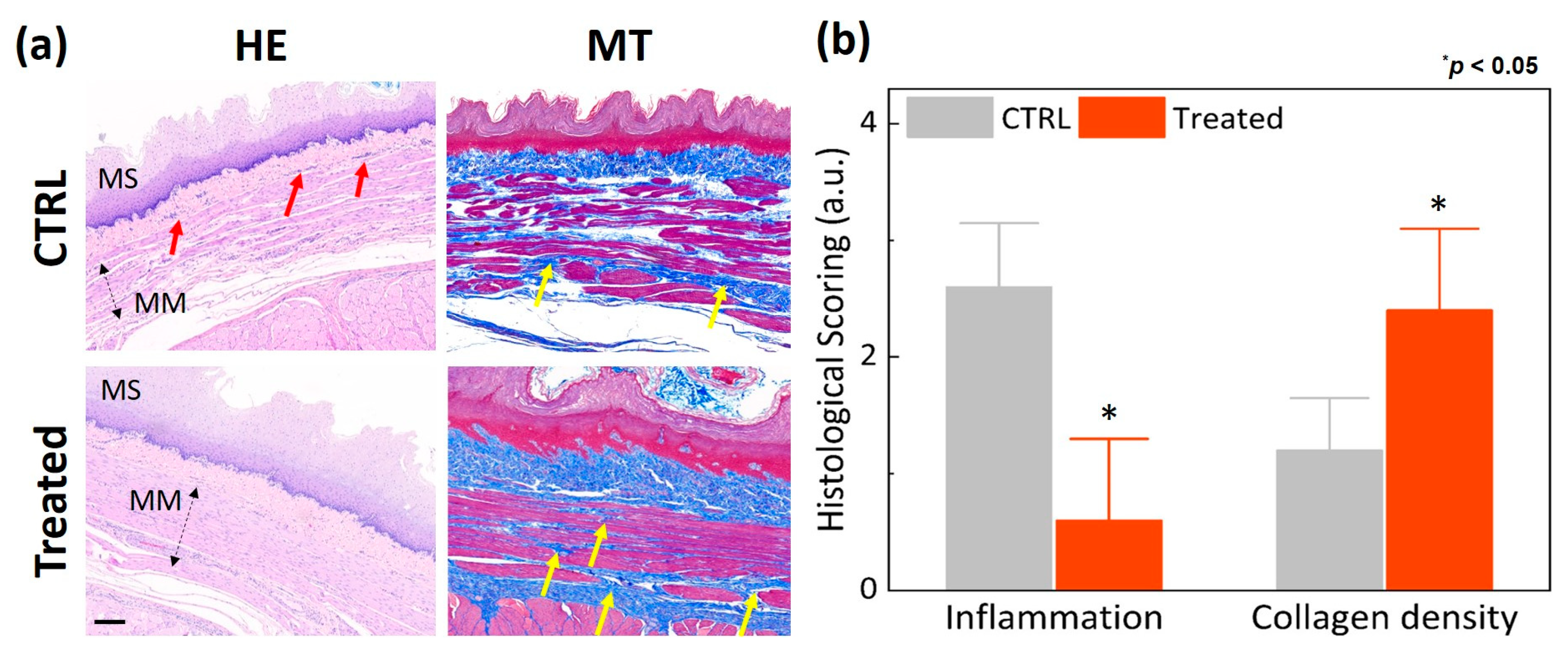
2.4. Histology Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Clarrett, D.M.; Hachem, C. Gastroesophageal reflux disease (GERD). Mo. Med. 2018, 115, 214. [Google Scholar] [PubMed]
- Fass, R.; Boeckxstaens, G.E.; El-Serag, H.; Rosen, R.; Sifrim, D.; Vaezi, M.F. Gastro-oesophageal reflux disease. Nat. Rev. Dis. Prim. 2021, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Ratnakumaran, R.; Yuan, Y.; Solaymani-Dodaran, M.; Bazzoli, F.; Ford, A.C. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: A meta-analysis. Gut 2018, 67, 430–440. [Google Scholar] [CrossRef]
- Kim, K.M.; Cho, Y.K.; Bae, S.J.; Kim, D.S.; Shim, K.N.; Kim, J.H.; Jung, S.W.; Kim, N. Prevalence of gastroesophageal reflux disease in Korea and associated health-care utilization: A national population-based study. J. Gastroenterol. Hepatol. 2012, 27, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-H.; Huh, K.C.; Jung, H.-K.; Yoon, Y.H.; Choi, K.D.; Song, K.H.; Keum, B.; Kim, J.W. Prevalence of uninvestigated dyspepsia and gastroesophageal reflux disease in Korea: A population-based study using the Rome III criteria. Dig. Dis. Sci. 2014, 59, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J. Complications of Gastroesophageal Reflux in Adults. Available online: https://www.medilib.ir/uptodate/show/2263 (accessed on 14 January 2022).
- Sandhu, D.S.; Fass, R. Current trends in the management of gastroesophageal reflux disease. Gut Liver 2018, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Kroch, D.A.; Madanick, R.D. Medical treatment of gastroesophageal reflux disease. World J. Surg. 2017, 41, 1678–1684. [Google Scholar] [CrossRef]
- Jaynes, M.; Kumar, A.B. The risks of long-term use of proton pump inhibitors: A critical review. Ther. Adv. Drug Saf. 2019, 10, 2042098618809927. [Google Scholar] [CrossRef]
- Triadafilopoulos, G. Stretta: A valuable endoscopic treatment modality for gastroesophageal reflux disease. World J. Gastroenterol. WJG 2014, 20, 7730. [Google Scholar] [CrossRef]
- Chang, P.; Friedenberg, F. Obesity and GERD. Gastroenterol. Clin. 2014, 43, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Muthusamy, V.R.; Lightdale, J.R.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Eloubeidi, M.A.; Fanelli, R.D.; Fonkalsrud, L.; Faulx, A.L.; Khashab, M.A. The role of endoscopy in the management of GERD. Gastrointest. Endosc. 2015, 81, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.; Krause, C.; Pokala, B.; Hosein, S.; Armijo, P.R.; Mishra, T.; Kothari, S.; Oleynikov, D. Novel therapies for gastroesophageal reflux disease. Curr. Probl. Surg. 2019, 56, 100692. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.P.; Chang, K.J. Endoscopic Management of GERD. Dig. Dis. Sci. 2022, 67, 1455–1468. [Google Scholar] [CrossRef]
- Shibli, F.; Fass, R. Endoscopic Anti-Reflux Procedures: Ready for Clinical Use? Curr. Treat. Options Gastroenterol. 2021, 19, 399–420. [Google Scholar] [CrossRef]
- Fass, R. An overview of transoral incisionless fundoplication and magnetic sphincter augmentation for GERD. Gastroenterol. Hepatol. 2017, 13, 50. [Google Scholar]
- Chang, K.J.; Bell, R. Transoral incisionless fundoplication. Gastrointest. Endosc. Clin. 2020, 30, 267–289. [Google Scholar] [CrossRef]
- Ihde, G.M. The evolution of TIF: Transoral incisionless fundoplication. Ther. Adv. Gastroenterol. 2020, 13, 1756284820924206. [Google Scholar] [CrossRef]
- Fass, R. Endoscopic approaches for the treatment of gastroesophageal reflux disease. Gastroenterol. Hepatol. 2019, 15, 555. [Google Scholar]
- Zacherl, J.; Roy-Shapira, A.; Bonavina, L.; Bapaye, A.; Kiesslich, R.; Schoppmann, S.F.; Kessler, W.R.; Selzer, D.J.; Broderick, R.C.; Lehman, G.A. Endoscopic anterior fundoplication with the Medigus Ultrasonic Surgical Endostapler (MUSE™) for gastroesophageal reflux disease: 6-month results from a multi-center prospective trial. Surg. Endosc. 2015, 29, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, D.S.; Fass, R. Stretta therapy in the management of gastroesophageal reflux disease (GERD). Ann Esophagus 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Sowa, P.; Samarasena, J.B. Nonablative radiofrequency treatment for gastroesophageal reflux disease (STRETTA). Gastrointest. Endosc. Clin. 2020, 30, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.E. Gastroesophageal reflux disease treatment: Side effects and complications of fundoplication. Clin. Gastroenterol. Hepatol. 2013, 11, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, S.; Zhao, H.; Zeng, X.; Lian, J.; Tseng, Y.; Chen, J. Efficacy of transoral incisionless fundoplication (TIF) for the treatment of GERD: A systematic review with meta-analysis. Surg. Endosc. 2017, 31, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Cahn, F.; Scotti, D.J.; Gregory, D.A. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg. Endosc. 2017, 31, 4865–4882. [Google Scholar] [CrossRef]
- Zerbib, F.; Sacher-Huvelin, S.; Coron, E.; Coffin, B.; Melchior, C.; Ponchon, T.; Cholet, F.; Chabrun, E.; Vavasseur, F.; Gorbatchef, C. Randomised clinical trial: Oesophageal radiofrequency energy delivery versus sham for PPI-refractory heartburn. Aliment. Pharmacol. Ther. 2020, 52, 637–645. [Google Scholar] [CrossRef]
- Lipka, S.; Kumar, A.; Richter, J.E. No evidence for efficacy of radiofrequency ablation for treatment of gastroesophageal reflux disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 1058–1067.e1051. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Park, J.-M.; Ryu, S.; Hwang, J.; Kwon, J.-W.; Seo, K.W. Anti-reflux surgery versus proton pump inhibitors for severe gastroesophageal reflux disease: A cost-effectiveness study in Korea. J. Neurogastroenterol. Motil. 2020, 26, 215. [Google Scholar] [CrossRef] [Green Version]
- Funk, L.M.; Zhang, J.Y.; Drosdeck, J.M.; Melvin, W.S.; Walker, J.P.; Perry, K.A. Long-term cost-effectiveness of medical, endoscopic and surgical management of gastroesophageal reflux disease. Surgery 2015, 157, 126–136. [Google Scholar] [CrossRef]
- Thosani, N.; Goodman, A.; Manfredi, M.; Navaneethan, U.; Parsi, M.A.; Smith, Z.L.; Sullivan, S.A.; Banerjee, S.; Maple, J.T. Endoscopic anti-reflux devices (with videos). Gastrointest. Endosc. 2017, 86, 931–948. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Bak, J.; Kim, S.M.; Kang, H.W. Feasibility study of endoscopic thermal coagulation with circumferential laser irradiation for treating esophageal tissue. Lasers Med. Sci. 2020, 35, 893–900. [Google Scholar] [CrossRef]
- Bak, J.; Hwang, J.; Park, S.; Kang, H.W. Integration of optical applicator with balloon catheter for photothermal treatment of biliary stricture. Lasers Surg. Med. 2017, 49, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Jeong, S.; Lee, D.H.; Kim, J.M.; Kim, S.M.; Kang, H.W. The use of a 532-nm laser fitted with a balloon and a cylindrical light diffuser to treat benign biliary stricture: A pilot study. Lasers Med. Sci. 2021, 36, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.G.; Jeong, S.; Lee, Y.; Kim, S.M.; Kang, H.W. Novel endoscopic laser treatment of common bile duct stenosis using balloon catheter-integrated diffusing applicator (BCDA). In Proceedings of the SPIE Advanced Biophotonics Conference (SPIE ABC 2021), Busan, Republic of Korea, 4–6 November 2021; pp. 15–17. [Google Scholar]
- Utley, D.S.; Kim, M.; Vierra, M.A.; Triadafilopoulos, G. Augmentation of lower esophageal sphincter pressure and gastric yield pressure after radiofrequency energy delivery to the gastroesophageal junction: A porcine model. Gastrointest. Endosc. 2000, 52, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Truong, V.G.; Choi, J.; Jeong, H.J.; Oh, S.-J.; Park, J.-S.; Kang, H.W. Endoscopic ultrasound-guided laser ablation using a diffusing applicator for locally advanced pancreatic cancer treatment. Cancers 2022, 14, 2274. [Google Scholar] [CrossRef] [PubMed]
- Sipos, W.; Duvigneau, C.J.; Hartl, R.T.; Schwendenwein, I. Exploratory reference intervals on hematology and cellular immune system of multiparous Large White sows. Vet. Immunol. Immunopathol. 2011, 141, 307–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ježek, J.; Starič, J.; Nemec, M.; Plut, J.; Oven, I.G.; Klinkon, M.; Štukelj, M. The influence of age, farm, and physiological status on pig hematological profiles. J. Swine Health Prod. 2018, 26, 72–78. [Google Scholar]
- Truong, V.G.; Kim, H.; Lee, B.-I.; Cha, B.; Jeong, S.; Oh, S.-J.; Kang, H.W. Development of Novel Balloon-Integrated Optical Catheter for Endoscopic and Circumferential Laser Application. Ann. Biomed. Eng. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Herman, R.M.; Berho, M.; Murawski, M.; Nowakowski, M.; Ryś, J.; Schwarz, T.; Wojtysiak, D.; Wexner, S.D. Defining the histopathological changes induced by nonablative radiofrequency treatment of faecal incontinence–a blinded assessment in an animal model. Color. Dis. 2015, 17, 433–440. [Google Scholar] [CrossRef]
- Kim, M.S.; Holloway, R.H.; Dent, J.; Utley, D.S. Radiofrequency energy delivery to the gastric cardia inhibits triggering of transient lower esophageal sphincter relaxation and gastroesophageal reflux in dogs. Gastrointest. Endosc. 2003, 57, 17–22. [Google Scholar] [CrossRef]
- Ayazi, S.; Tamhankar, A.; DeMeester, S.R.; Zehetner, J.; Wu, C.; Lipham, J.C.; Hagen, J.A.; DeMeester, T.R. The impact of gastric distension on the lower esophageal sphincter and its exposure to acid gastric juice. Ann. Surg. 2010, 252, 57–62. [Google Scholar] [CrossRef]
- Rieder, F.; Biancani, P.; Harnett, K.; Yerian, L.; Falk, G.W. Inflammatory mediators in gastroesophageal reflux disease: Impact on esophageal motility, fibrosis, and carcinogenesis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G571–G581. [Google Scholar] [CrossRef] [Green Version]
- Noar, M.; Squires, P.; Noar, E.; Lee, M. Long-term maintenance effect of radiofrequency energy delivery for refractory GERD: A decade later. Surg. Endosc. 2014, 28, 2323–2333. [Google Scholar] [CrossRef] [PubMed]


| Normal Range | CTRL | Treated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-BTX | Post-BTX | 4 w | 8 w | 12 w | Pre-BTX | Post-BTX | 4 w | 8 w | 12 w | ||
| WBC (×103 cells/µL) | 13.6–32.2 | 16.9 | 14.97 | 25.86 | 26.56 | 18.4 | 26.24 | 22.01 | 24.59 | 19.81 | 12.61 |
| Neutrophil (%) | 8–56.9 | 36.7 | 30.2 | 43.5 | 40 | 56.4 | 44.2 | 40.4 | 30.25 | 23.65 | 32.25 |
| Eosionophil (%) | 0–6 | 0.2 | 1.4 | 1.7 | 2.2 | 0.2 | 0.15 | 0.5 | 1.1 | 1.5 | 0.15 |
| Lymphocyte (%) | 20.9–74 | 56.6 | 62.1 | 50.4 | 42.2 | 41 | 50.35 | 53.65 | 64.45 | 69.7 | 63.3 |
| meanHb (g/dL) | 10.8–14.8 | 12.6 | 10.9 | 12.9 | 14 | 12.2 | 13.25 | 11.8 | 14.05 | 14.65 | 12.1 |
| Plt (×103 cells/µL) | 155–686 | 383 | 264 | 415 | 679 | 337 | 582.5 | 434 | 378 | 366 | 355 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, B.; Kim, H.; Truong, V.G.; Oh, S.-J.; Jeong, S.; Kang, H.W. Feasibility Study on Endoscopic Balloon-Assisted Laser Treatment (EBLT) of Gastroesophageal Reflux Disease (GERD) in In Vivo Porcine Model. Biomedicines 2023, 11, 1656. https://doi.org/10.3390/biomedicines11061656
Cha B, Kim H, Truong VG, Oh S-J, Jeong S, Kang HW. Feasibility Study on Endoscopic Balloon-Assisted Laser Treatment (EBLT) of Gastroesophageal Reflux Disease (GERD) in In Vivo Porcine Model. Biomedicines. 2023; 11(6):1656. https://doi.org/10.3390/biomedicines11061656
Chicago/Turabian StyleCha, Boram, Hyejin Kim, Van Gia Truong, Sun-Ju Oh, Seok Jeong, and Hyun Wook Kang. 2023. "Feasibility Study on Endoscopic Balloon-Assisted Laser Treatment (EBLT) of Gastroesophageal Reflux Disease (GERD) in In Vivo Porcine Model" Biomedicines 11, no. 6: 1656. https://doi.org/10.3390/biomedicines11061656






